Abstract
The emergence of malignant ascites (MA) indicates poor prognoses in patients with ovarian, gastrointestinal, breast, and pancreatic cancer. Interleukin-10 (IL-10) is a pleiotropic cytokine with immunoregulatory effects in tumor microenvironment. The level of IL-10 in MA varied across cancer types and patients, influencing cancer progression and outcomes. Originating from various immune and cancer cells, IL-10 contributes to complex signaling pathways in MA. Systemic IL-10 administration, although the evidence of its efficacy on MA is limited, still emerges as a promising therapeutic strategy because it can increase CD8+ T cells cytotoxicity and invigorate exhausted CD8+ tumor infiltration lymphocytes (TILs) directly. IL-10 signaling blockade also demonstrates great potential when combined with other immunotherapies in MA treatment. We reviewed the levels, origins, and functions of IL-10 in malignant ascites and overviewed the current IL-10 signaling targeting therapies, aiming to provide insights for MA treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant ascites (MA) is defined as the peritoneal fluid collection containing malignant cells in a cancer patient. Substantial amount of ascites can lead to abdominal swelling, nausea, early satiety, edema, pain, and even dyspnea, decreasing quality of life and usually indicating a unfavorable prognosis [1,2,3]. MA is commonly associated with primary abdomen or pelvic malignancies such as liver, colon, or ovarian cancers; it can also manifest in metastatic diseases. The precise molecular mechanisms underlying MA remain elusive, but increased vascular permeability and peritoneal lymphatic obstruction are recognized as key pathophysiological processes involving multiple cytokines. Notably, vascular endothelial growth factors (VEGF), a prominent vascular permeability factor, exhibit heightened expression in MA [4,5,6,7]. Additionally, other cytokines like tumor necrosis factor-\(\alpha\), interleukin-6, interleukin-8, and interleukin-10 (IL-10) are elevated in the context of ovarian cancer (OC) patients with MA [8,9,10]. The intricate interplay of these cytokines may contribute to the impediment of fluid absorption and drainage, as tumor cells disseminate and proliferate within the peritoneal cavity, potentially obstructing lymphatic vessels [5, 11]. In addition to these primary mechanisms, neovascularization induced by matrix metalloproteinase (MMPs) and the retention of hypertonic fluids secreted by tumor may also play pivotal roles in the formation of MA [12,13,14,15,16] (Figure S1).
There is no clear guidance on managing MA, but the treatments mainly fall into either relieving symptoms or controlling the primary diseases. Intermittent paracentesis is the most widely used intervention for controlling MA clinically, and its modified technique, cell-free and concentrated ascites reinfusion therapy is applied in Japan, which is thought to avoid protein loss compared with conventional paracentesis [17]. However, paracentesis relieves symptoms temporarily, with the ascites rapidly reaccumulating, increasing the risks of rehospitalization and deteriorating the quality of life (QOL) [16]. Long-term drainage approaches include indwelling catheters, indwelling peritoneal ports, and peritoneovenous shunts (PVSs). Palliation rates of long-term drainage approaches achieve 97%, while their complication rates all exceed paracentesis, and PVSs had the highest complication rate (26–55%) [18]. Since the presence of MA usually indicates a more advanced disease status, targeting the primary diseases can be quite challenging. Intraperitoneal chemotherapy (i.p. chemotherapy) is considered to be effective for controlling MA, and the efficacy and safety of various agents have been investigated. Catumaxomab is a mouse/rat monoclonal antibody targeting epithelial cell-adhesion molecule (EpCAM) and has been approved for MA treatment by European Union in 2009 [19]. A phase II/III trial found that paracentesis plus catumaxomab could increase progress-free survival and improve QOL in patients with MA compared to paracentesis alone [20, 21]. A phase II clinical trial results demonstrated the safety and efficacy of the combination chemotherapy comprising of intravenous paclitaxel (PTX) and intraperitoneal S-1 in pancreatic cancer patients with MA [22]. Moreover, bevacizumab, a VEGF inhibitor, is considered as an option for recurrent OC patients with ascites, but with increased risk of GI perforation [23, 24]. A multicenter double-blind, placebo-controlled phase II trial explored intraperitoneally applied bevacizumab in patients with advanced gastrointestinal cancer and MA, and the results showed that bevacizumab was well tolerated but without efficacy in controlling ascites-related symptoms [25]. Hyperthermic intraperitoneal chemotherapy (HIPEC) is another i.p. chemotherapy technique which is thought to increase chemotherapy penetration and enhance cancer cells sensitivity to chemotherapy throughout the peritoneal cavity by heated solution delivery. It is recommended by the NCCN guidelines to use in FIGO stage III OC patients who undertook neoadjuvant chemotherapy (NACT) and interval debulking surgery (IDS) [23, 26]. Moreover, cytoreductive surgery with HIPEC is recommended for patients with gastric cancer and limited peritoneal metastases [27]. However, the application of HIPEC in colon cancer patients with peritoneal metastases remains controversial as several trials found the technique increased morbidity and mortality [28]. Both palliation therapies and methods targeting primary diseases have shown improvements in QOL, but did not demonstrate obvious advantages in survival of patients with MA. As a result, therapies with less complications and higher efficacy are warranted.
IL-10, a pleiotropic cytokine, plays a crucial role in both inflammation and immunity within tumor microenvironment. It is primarily produced by innate immune cells, including monocytes, dendritic cells (DCs), macrophages, and natural killer cells (NK cells), and adaptive immune cells such as CD4+ T cells, CD8+ T cells, Th17 cells, and B cells [29, 30]. Studies also indicate that tumor cells can secrete IL-10 [31, 32]. Cancer patients, in comparison with healthy people and those with benign tumors, exhibit elevated levels of IL-10 in both serum and ascites, with this correlating with advanced clinical staging and poor outcomes [33,34,35,36]. Notably, IL-10 in OC contributes to ascites-mediated apoptosis resistance and transcriptomic data identifying IL-10 in ascites as a robust indicator of poor outcomes for OC patients [37, 38]. Nevertheless, the precise role of IL-10 in oncogenesis remains controversial, with reported anti- and pro-tumor effects observed in various malignancies such as thyroid cancer, non-small cell lung cancer, breast cancer, and colorectal cancer [39,40,41,42,43,44,45,46,47]. IL-10 predominantly exerts its inhibitory function on macrophages, while its impact on T cells is complex, involving both the inhibition of T cell responses and the stimulation of CD8+ T cell proliferation [48,49,50]. Despite extensive research on MA, a comprehensive review focused on IL-10 in MA is notably absent. Consequently, this review aims to summarize the role of IL-10, spanning its levels to therapeutic targeting in MA, with the intent of providing evidence for the potential application of IL-10 in MA from bench to bedside.
Levels of IL-10 in malignant ascites
Numerous studies have appraised IL-10 concentrations in diverse specimens obtained from cancer patients, consistently reporting elevated IL-10 levels in MA compared to non-malignant ascites (Table 1). The association between IL-10 levels in MA and patients’ survival outcomes remains controversial, with IL-10 not yet acknowledged as a prognostic biomarker for MA patients [10, 51,52,53]. Notably, a positive correlation between serum IL-10 levels and ascitic IL-10 concentrations in OC patients has been established, with significantly higher IL-10 levels detected in MA than in corresponding serum samples [10, 54]. Post-debulking surgery and/or chemotherapy interventions led to reduction in IL-10 levels in the in serum samples of OC patients [34, 55,56,57]. Furthermore, Antoneeva et al. revealed that IL-10 levels in the serum of OC patients peaked upon disease progression after NACT, suggesting its potential as a marker for predicting patients’ response after NACT [58]. Yigit et al. reported undetectable IL-10 levels in MA following NACT; interestingly, they also found minimal IL-10 levels in recurrent patients [52]. Explorations of IL-10 level change in MA after chemotherapy, using ID8 tumor-bearing mice, indicating a decreasing trend, though statistical significance was not achieved [59]. Additionally, Park et al. disclosed higher IL-10 levels in MA of metastatic gastric cancer (mGC) patients compared to healthy volunteers’ body fluids. Elevated levels of at least two cytokines (VEGF-A, IL-10, or TGF-beta) in MA correlated with shorter overall survival (OS) in mGC patients, yet IL-10 alone did not emerge as an independent risk factor for shorter OS in mGC patients [60]. Atta et al. demonstrated elevated IL-10 levels in ascites of patients with hepatocellular carcinoma (HCC) compared to those with cirrhosis [61]. Given the disparate outcomes and limited sample sizes of prior studies, a comprehensive evaluation of IL-10 levels in MA before and after treatment, particularly within a sizable population, is warranted. Despite the potential of IL-10 level changes during treatment to shed light on the prognostic value of IL-10 in MA, a dearth of data currently exists on this subject.
Most studies focus on cytokines in MA as prognostic markers. IL-6, IL-10, TNF-α, IFN-γ, TNF-α have been substantiated as directly linked to poor outcomes in epithelial ovarian cancer (EOC) patients. [51, 66] However, research into the interplay among these cytokines remains limited. Reinartz et al. established transcriptome-derived datasets from ascites cells of 29 EOC patients, revealing that IL-10, IL-6, and leukemia inhibitory factor (LIF) activate downstream STAT3 signaling pathway, and these three cytokines are associated with early relapse. IL-10 and IL-6 played pivotal yet insufficient roles in inducing the proliferation of Myeloid-derived suppressor cells (MDSCs) [68]. Additionally, a robust association was observed between high IL-10 levels and elevated Arachidonic acid (AA) level [37]. Soluble human leukocyte antigen-G (sHLA-G) and IL-10 were also deemed functionally related [10, 53]. Certain tumor cells were found to produce IL-10, thereby upregulating the expression of HLA-G, which, in turn, induced immune cells to produce IL-10, IL-4 and IL-3 [69]. Notably, limited evidence exists regarding the association between IL-10 and other cytokines, necessitating further studies to explore these correlations.
IL-10-producing cells and the possible functions
Myeloid cells
Myeloid cells, derived from hematopoietic stem cells in the bone marrow, comprise granulocytes and monocytes, exerting intricate roles in the tumor microenvironment [70, 71]. Inflammation is closely linked to the cancer progression, with neutrophils considered a source of IL-10. In the context of OC, infiltrated neutrophils secrete various cytokines, including IL-2, IL-6, IL-10, TNF-α, and VEGF, promoting cancer progression in the tumor microenvironment [6]. Hart et al. identified a CD11b+ population of MDSCs in OC, which significantly produces IL-10. IL-10, along with other immunosuppressive molecules, induces myeloid maturation, activates MDSCs, alters T cells phenotypes, and reduces their IFN-γ production [72]. Subsequent studies revealed that the CX3CR1-negative subset is the primary source of IL-10 in early oncogenesis, while the positive subset becomes dominant in later stages [73]. IL-10 neutralization in ascites reverses the immunosuppressive effects of MDSCs, improving survival and alleviating tumor burden in OC patients [74]. Additionally, IL-10, IL-6, and STAT3 activation in OC ascites induces MDSCs accumulation, inhibiting autologous T cell proliferation and effector function [68].
Dendritic cells (DCs), as antigen-presenting cells, act as messengers between innate and adaptive immune responses. High IL-10 levels are secreted by immature DCs accumulating around tumor foci [75]. Plasmacytoid DCs (pDCs), a subset plasma cell morphology, migrate to OC microenvironment due to OC cells secreting CXCR4/SDF-1, upregulating VLA-5 expression [76]. Precursor pDCs in OC microenvironment induce regulatory T cells (Tregs), suppressing immune function by producing IL-10 [77]. Furthermore, DCs isolated from OC patients’ ascites induce IL-10 production in CD3+ CD4+ T cells [78].
Tumor-associated macrophages (TAMs) are another source of IL-10 in MA [79]. Contrary to immunosuppressive aspects, IL-10 secreted by TAMs induces Tregs by activating Foxp3 expression, leading to tumor progression [63]. IL-10, coupled with N-acetylaspartate (NAA) released by OC cells, induces glutamine synthetase (GS) expression in macrophages which acquired M2-like phenotype in OC ascites [80].
In MA, a monocyte subset could produce IL-10, contributing to antitumor immune response by inhibiting T cell proliferation and cytokine production [14]. MA of OC patient induces strong IL-10 production and decreases INF-γ and IL-2 in resting and activated peripheral blood mononuclear cells (PBMCs), suggesting that OC MA favored Th2 inhibitory immune response [65]. Freedman et al. identified a subset of CD14+ HLA-DR- monocytes secreting IL-10 with suppressive activity against effector T cells [81] (Fig. 1).
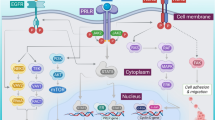
IL-10-producing cells and biological effects after IL-10 stimulation. Many cells in MA can produce IL-10, such as myeloid cells (including neutrophils, MDSCs, DCs and TAMs, etc.), lymphoid cells including T cells and B cells, and cancer cells. IL-10 in MA, in turn, can induce the maturation and activation of MDSCs, alter the phenotypes of T cells and stimulate IL-10 secretion in T cells and TAMs. The red arrows signal the IL-10-secreting process and the green arrows signal the promotion and proliferation process. Bregs, regulatory B cells. CSCs, cancer stem cells. MDSCs, myeloid-derived suppressor cells. pDCs, plasmacytoid dendritic cells. TAMs, tumor-associated macrophages. TRAPs, tumor cell-released autophagosomes. Tregs, regulatory T cells
Lymphoid cells
Lymphoid cells, predominant in the lymph system, include NK cells, T cells, and B cells, playing crucial roles in immune response. T cells and B cells both produce IL-10 in MA. Tregs are a significant source of IL-10 in MA, inducing inhibitory receptor expression on both CD4+ T cells and CD8+ T cells along with IL-35 [67, 82]. Regulatory B cells (Bregs), a subset restraining excessive inflammatory responses, also produce IL-10 [83]. Several studies indicate increased IL-10-producing Bregs correlate with poor prognosis in gastric cancer patients. Evaluated tumor-infiltrating Bregs are associated with increased Tregs in patients with gastric cancer, OC, and tongue squamous cell carcinoma, indicating potential interactions between Tregs and Breg in immune evasion [84,85,86,87]. Zhou et al. isolated tumor cells-released autophagosomes (TRAPs) from malignant effusion and ascites, demonstrating TRAPs induce Bregs differentiation through the TLR2/MyD88/NF-κB signaling pathway, inhibiting CD4+ T cells and CD8+ T cells through IL-10 secretion [88]. The levels and functions of Bregs in MA require further exploration (Fig. 1).
Cancer cells
Whether cancer cells could produce IL-10 is controversial. Gotlieb et al. reported undetectable levels of IL-10 in five OC cell lines (CAOV-3, SKOV-3, CC194, OC222, and OC 494) [33]. However, Berger et al. did not detect IL-10 in SKOV-3, CAOV-3, and OAW42 cell lines, but they found IL-10 expression in OVCAR-3 cell line [89]. Carr et al. found significant IL-10 production only in SW626 among several OC cell lines (including CAOV-3, CAOV-4, ES-2, OV-90, OVCAR-3, SKOV-3, TOV-21G, TOV-112D, TTB-6, COV413) [90]. Zhou et al. identified IL-10 production in SKOV-3, CAOV-3, and 3AO cell lines [32]. Nowak et al. isolated OC cells from patients’ tumor tissue and found both unstimulated and LPS-stimulated OC cells produce high levels of IL-10, with the FIGO staging correlated with IL-10 levels, while cells from benign ovarian tumors and normal tissue produced low IL-10 levels [91]. Recent studies identified cancer stem cells as a possible source of IL-10. Gening et al. found a strongly positive relationship between stem-like tumor cells (CD45-CD44+CD133-) and IL-10 levels in MA [64]. Raghavan et al. established a hanging drop spheroid model and found that hetero-spheroids containing cancer stem cells and M2-macrophages (CSC/M2 hetero-spheroids) could secrete IL-10, indicating CSCs from MA can produce IL-10 under M2-macrophage interaction. [92] Beyond OC cells, IL-10 has been found in several human carcinoma cell lines and cells isolated directly from tumor tissues, with pancreatic cancer cell-derived IL-10 suppressing CTL lytic function of T cells [93,94,95]. The metastatic ability of EOC cells correlates with IL-10 and TGF-β in ascites, associated with altered MMP-2 and TIMP-2 levels [96] (Fig. 1).
Possible IL-10 signaling pathways in malignant ascites
The IL-10 signaling pathway is presumed to play a role in the development of immunosuppressive malignant ascites within the tumor microenvironment. Nevertheless, there is a scarcity of studies focusing on the downstream reactions of IL-10. A comprehensive understanding of IL-10 signal transduction in the development of MA can contribute to the integration of immunotherapeutic strategies. The receptor for IL-10 forms a functional complex consisting of two ligand-binding subunits, IL-10 R1 and IL-10 R2. Ligand engagement and binding are primarily facilitated by IL-10 R1, which exhibits high affinity, while IL-10R2 is responsible for transducing subsequent signals. In addition to being expressed on immune cells, Rabinovich et al. observed elevated IL-10 receptor expression on both OC tumor tissue and cells [31]. At the cell membrane surface, IL-10 binding to IL-10R1 and IL-10R2 activates Janus Kinase 1 (JAK1) and Tyrosine Kinase 2 (TYK2), phosphorylating the receptor complexes Y446 and Y496 located at the cytoplasmic tail. Subsequently, Signal Transducer and Activator of Transcription 3 (STAT3), a key transcription factor downstream of the IL-10 signaling pathway, is assembled and phosphorylated. It then translocates into the nucleus, binding to the promoter elements of relevant target genes and ultimately participating in the expression of pro-tumor and anti-apoptotic genes such as BCL2, TWIST, thereby exerting tumor-promoting effects [97,98,99]. Notably, IL-10 can also phosphorylate STAT1 and STAT5, perpetuating downstream signaling [100, 101]. Apart from the JAK1-STAT3 cascade, IL-10 is hypothesized to boost cancer stemness through the JAK1-STAT1-NF-\(\kappa\) B pathway, thereby promoting tumor progression and metastasis [102]. However, contradictory findings exist, as some studies suggest that IL-10-mediated STAT1 activation induces pro-apoptotic and anti-proliferative effects in macrophages pre-treated with IFN-γ or IFN-α, contradicting IL-10-mediated STAT3 activation. The mechanism and switches of IL-10-mediated STAT pathway in MA remain unclear, with ongoing investigations into the involved mechanisms [103]. Additionally, Zhou et al. demonstrated that IL-10 could activate the Phosphatidylinositol 3-Kinase (PI3K) -AKT pathway, thereby regulating cell apoptosis [104]. Furthermore, Reitamo et al. discovered that under the influence of IL-10, the expression of MMPs, associated with neovascularization in MA, increased, suggesting a potential mechanisms by which IL-10 contributes to the formation of MA [105] (Figure S2).
IL-10 as a prospective therapeutic target in malignant ascites
To harness IL-10 signaling, both recombinant IL-10 protein and the blockade of IL-10 and/or IL-10R are being explored for the MA treatment [106, 107]. The potential anti-tumor effects of IL-10 involve upregulating CD8+ T cells cytotoxicity, thereby reducing macrophage infiltration, increasing NK cell infiltration, resisting angiogenesis, and stabilizing intrinsic apoptosis [108]. Guo et al. demonstrated that a half-life-extended IL-10/Fc fusion protein could directly invigorate terminally exhausted CD8+ tumor-infiltrating lymphocytes (TILs) via oxidative phosphorylation and the process is in absence of the progenitor cells, making it a promising complementary therapy to immune checkpoint blockade in the treatment of patients with poor or none tumor infiltration of progenitor-exhausted CD8+ TILs [109]. Consequently, pegilodecakin, a pegylated form of recombinant IL-10 sustaining high concentration, has entered clinical trials. In a phase 1/1b trial (NCT02009449), one ovarian cancer patient and 22 pancreatic cancer patients were recruited to evaluate the safety and efficacy of pegilodecakin (pegylated IL-10) as monotherapy, and the results showed that patients did not have obvious clinical responses [110]. The result of SEQUOIA (NCT02923921), a phase 3 study evaluating pegilodecakin combined with folinic acid, fluorouracil, and oxaliplatin (FOLFOX) in patients with metastasis pancreatic ductal adenocarcinoma (PDAC), demonstrated that the addition of pegilodecakin did not increase efficacy of FOLFOX [111]. Recombinant IL-10 protein exerted promising anti-tumor effects on solid tumors such as renal cell carcinoma and melanoma, but ideal therapeutic effects did not be observed on patients with MA-associated cancer types such as ovarian cancer and pancreatic cancer, and the agent’s direct efficacy on MA remains unknown, impeding its further clinical application [107]. The complex tumor microenvironment and small sample size may account for these limited effects. Future research should specifically investigate recombinant IL-10 protein efficacy on MA.
Blocking IL-10 signaling also presents an immunotherapy strategy due to IL-10’s tumor immunosuppressive effect. Direct blockade via anti-IL-10 antibodies or indirectly use of anti-IL-10R antibodies can interfere with IL-10 function. IL-10/IL-10R neutralization has demonstrated more anti-tumor effects when combined with other immunotherapeutic strategies [112, 113]. Lamichhane et al. found that the combination of PD-1 blocking and IL-10 (R) neutralization activated more T and B cells, decreasing MDSCs in the ascites of ID8 tumor-bearing mice, leading to tumor burden alleviation and survival prolongation [114]. Adams et al. engineered a tumor vaccine by isolating ascites-derived monocytes from ID8 tumor-bearing mice and stimulating with Toll-like receptor (TLR) 4 lipopolysaccharide, TLR 9 CpG-oligonucleotides, and IL-10R antibody. The vaccine suppressed tumor growth and ascites generation in mice, and the proposed mechanism was macrophage activation and increased T cell response [115]. Additionally, IL-10 antibody enhanced the efficacy of OK-432’s locoregional immunotherapy for mouse breast cancer ascites [94]. Hu et al. showed that arsenic trioxide exerted a potential therapeutic response by decreasing IL-10 and TGF-β levels in TILs from ascites of gastric cancer patients, with the proportion of CD4+CD25+CD127-Tregs decreasing and the CD8+ T cells frequency and IFN-γ level increasing [116].
Furthermore, Martincuks et al. found that PARP inhibitors induced their therapy resistance against OC by activating STAT3 pathway, which closely related to the downstream pathway of IL-10. Thus, neutralization of IL-10/IL-10R in combination with PARP inhibitors could reduce therapeutic resistance [117]. Chimeric antigen receptor-T cell (CAR-T) therapy is defined as administrating reengineered chimeric T cells that can recognize tumor cells from cancer patients, enhancing T cell proliferation and reducing apoptosis. CAR-T therapy is different with conventional therapies as it can directly bypass the processing and presentation of tumor antigens by DCs, so it can avoid part of the immunosuppressive effect in the tumor microenvironment. In vitro experiments found that mesothelin CAR-T cells reacted with SKOV-3 cells, promoting the secretion of granzyme B and IFN-γ, which have important roles in producing cytotoxicity and killing OC cells. Blocking IL-10 signaling pathway can further increase granzyme B and IFN-γ levels. Therefore, blocking IL-10 signaling pathway has the potential to reverse the immunosuppressive effect in MA and enable stronger cytotoxicity produced by mesothelin CAR-T therapy [118, 119].
In addition to conventional therapies, emerging medical materials show promise in cancer treatments. Methotrexate-loaded gold nanoparticles (MTX-AuNP) inhibited ascites formation in mice with LL2 ascites tumors [120]. Silver nanoparticles (AgNPs) decreased ascites fluid volume in a lymphoma ascites mice model [121]. α-Tocopheryl succinate nanoparticles (TS-NP) prevented peritoneal dissemination, reducing tumor nodules, tumor weights, and ascites volume via VEGF-A and IL-10 levels decreasing and M2-like TAMs polarization decreasing [122]. Except for nanoparticles, peptide hydrogels stand for another promising drug-loading candidate. Dai et al. created a melittin hydrogel loaded with KN93 (CAMKII inhibitor), resulting in decreased ascites volume and reprogramming the ascites microenvironment in mice with hepatoma and MA [123]. Shamskhou et al. established a hyaluronan and heparin-based system delivery recombinant IL-10 for the treatment of lung fibrosis in vivo [124]. The above results indicate that it is realistic and pragmatic for novel materials like hydrogel system to load with IL-10 protein or IL-10/IL-10R antibody, enhancing their efficacy of immunoregulatory function in MA (Fig. 2).
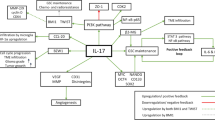
Therapeutic approaches mediated by IL-10 signaling blockade. APCs, antigen-presenting cells. GC, gastric cancer. IL-10 Ab, IL-10 antibody. IL-10R Ab, IL-10 receptor antibody. PD-1 Ab, PD-1 antibody. MDSCs, myeloid-derived suppressor cells. TALs, tumor-associated lymphocytes. TAMs, tumor-associated macrophages. TS-NP, α-Tocopheryl succinate nanoparticles
IL-10 is a pleiotropic cytokine exerting both immunosuppression and immunostimulatory effects within the cancer microenvironment. The levels of IL-10 in MA exhibit considerable variability across different cancer types and even among patients with the same cancer type. Notably, IL-10 levels surpass those found in non-malignant ascites and exceed the serum IL-10 levels within the same patient. Several studies have proposed its potential as a marker for predicting patients' response following neoadjuvant chemotherapy. The cellular origins of IL-10 in MA are diverse, encompassing neutrophils, MDSCs, DCs, TAMs, T cells, B cells, and even cancer cells. Functionally, IL-10, secreted by MA cells, elicits multifaceted effects such as phenotypic alterations and the regulation of cytokines secretion. It is hypothesized that IL-10 could phosphorylate and activate STAT1, STAT3, STAT5, and PI3K pathways, exerting apoptosis regulation and tumor progression functions on immune cells. Systemic administration of IL-10 holds promise as an immunotherapy, as it can enhance CD8+ T cell cytotoxicity and invigorate terminally exhausted CD8+ TILs. Despite the therapeutic efficacy demonstrated by PEGylated human IL-10 (pegilodecakin) in certain solid tumors like renal cell carcinoma and melanoma, its effects on patients with MA remain unclear. Strategies including the blockade of IL-10 signaling, driven by its immunosuppressive effects, have shown therapeutic promise in both in vitro and in vivo experiments, particularly when combined with other immunotherapies. Effectively harnessing IL-10 pleiotropy is challenging yet essential in the treatment of MA. Further research is warranted in the following directions: (1) Explore cytokines interacting with IL-10 in MA to elucidate the cytokine network in microenvironment of MA. (2) Investigate the IL-10 signaling pathway, with a focus on the STAT1/STAT3 switches in MA. (3) Evaluate the therapeutic effects of recombinant IL-10 protein on patients with MA. (4) Develop appropriate IL-10 blocking agents and delivery systems, assessing their efficacy and safety in preclinical trials.
Data availability
N/A.
References
Becker GGD, Blum HE (2006) Malignant ascites: systematic review and guideline for treatment. Eur J Cancer 42(5):589–597
Woopen HSJ (2009) Current and future options in the treatment of malignant ascites in ovarian cancer. Anticancer Res 29(8):3353–3359
van Baal JO, Van de Vijver KK, Nieuwland R, van Noorden CJ, van Driel WJ, Sturk A et al (2017) The histophysiology and pathophysiology of the peritoneum. Tissue Cell 49(1):95–105
Senger DR, de Van Water L, Brown LF, Nagy JA, Yeo KT, Yeo TK et al (1993) Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev 12(3–4):303–324
Cavazzoni E, Bugiantella W, Graziosi L, Franceschini MS, Donini A (2013) Malignant ascites: pathophysiology and treatment. Int J Clin Oncol 18(1):1–9
Rickard BP, Conrad C, Sorrin AJ, Ruhi MK, Reader JC, Huang SA et al (2021) Malignant ascites in ovarian cancer: cellular, acellular, and biophysical determinants of molecular characteristics and therapy response. Cancers (Basel) 13(17):4318
Liang B, Guo Z, Li Y, Liu C (2013) Elevated VEGF concentrations in ascites and serum predict adverse prognosis in ovarian cancer. Scand J Clin Lab Invest 73(4):309–314
Fang X, Yu S, Bast RC, Liu S, Xu HJ, Hu SX et al (2004) Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J Biol Chem 279(10):9653–9661
Moradi MM, Carson LF, Weinberg B, Haney AF, Twiggs LB, Ramakrishnan S (1993) Serum and ascitic fluid levels of interleukin-1, interleukin-6, and tumor necrosis factor-alpha in patients with ovarian epithelial cancer. Cancer 72(8):2433–2440
Sipak-Szmigiel O, Wlodarski P, Ronin-Walknowska E, Niedzielski A, Karakiewicz B, Sluczanowska-Glabowska S et al (2017) Serum and peritoneal fluid concentrations of soluble human leukocyte antigen, tumor necrosis factor alpha and interleukin 10 in patients with selected ovarian pathologies. J Ovarian Res 10(1):25
Adam RA, Adam YG (2004) Malignant ascites: past, present, and future. J Am Coll Surg 198(6):999–1011
Wojtowicz-Praga S, Low J, Marshall J, Ness E, Dickson R, Barter J et al (1996) Phase I trial of a novel matrix metalloproteinase inhibitor batimastat (BB-94) in patients with advanced cancer. Invest New Drugs 14(2):193–202
Parsons SL, Watson SA, Steele RJ (1997) Phase I/II trial of batimastat, a matrix metalloproteinase inhibitor, in patients with malignant ascites. Eur J Surg Oncol 23(6):526–531
Smith EM, Jayson GC (2003) The current and future management of malignant ascites. Clin Oncol (R Coll Radiol) 15(2):59–72
Miyoshi A, Miyatake T, Hara T, Tanaka A, Komura N, Komiya S et al (2015) Etiology of ascites and pleural effusion associated with ovarian tumors: literature review and case reports of three ovarian tumors presenting with massive ascites, but without peritoneal dissemination. Case Rep Obstet Gynecol 2015:414019
Hodge C, Badgwell BD (2019) Palliation of malignant ascites. J Surg Oncol 120(1):67–73
Chen HIM, Horita N, Tanzawa S, Kazahari H, Ochiai R, Sakamoto T, Honda T, Ichikawa Y, Watanabe K, Seki N (2021) Effectiveness of cell-free and concentrated ascites reinfusion therapy in the treatment of malignancy-related ascites: a systematic review and meta-analysis. Cancers (Basel) 13(19):4873
Stukan M (2017) Drainage of malignant ascites: patient selection and perspectives. Cancer Manag Res 9:115–130
Seimetz D (2011) Novel monoclonal antibodies for cancer treatment: the trifunctional antibody catumaxomab (removab). J Cancer Res Clin Oncol 2:309–316
Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV et al (2010) The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer 127(9):2209–2221
Wimberger P, Gilet H, Gonschior AK, Heiss MM, Moehler M, Oskay-Oezcelik G et al (2012) Deterioration in quality of life (QoL) in patients with malignant ascites: results from a phase II/III study comparing paracentesis plus catumaxomab with paracentesis alone. Ann Oncol 23(8):1979–1985
Takahara N, Isayama H, Nakai Y, Ishigami H, Satoi S, Mizuno S et al (2016) Intravenous and intraperitoneal paclitaxel with S-1 for treatment of refractory pancreatic cancer with malignant ascites. Invest New Drugs 34(5):636–642
NCCN (2022) The NCCN ovarian cancer clinical practice guidelines in oncology (version 5.2022). https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1453
Ferriss JS, Java JJ, Bookman MA, Fleming GF, Monk BJ, Walker JL et al (2015) Ascites predicts treatment benefit of bevacizumab in front-line therapy of advanced epithelial ovarian, fallopian tube and peritoneal cancers: an NRG Oncology/GOG study. Gynecol Oncol 139(1):17–22
Jordan KLT, Gog C, Killing B, Arnold D, Hinke A, Stahl M, Freier W, Rüssel J, Atanackovic D, Hegewisch-Becker S (2016) Intraperitoneal bevacizumab for control of malignant ascites due to advanced-stage gastrointestinal cancers: a multicentre double-blind, placebo-controlled phase II study—AIO SUP-0108. Eur J Cancer 63:127–134
Ba MC, Long H, Cui SZ, Tang YQ, Wu YB, Zhang XL et al (2013) Multivariate comparison of B-ultrasound guided and laparoscopic continuous circulatory hyperthermic intraperitoneal perfusion chemotherapy for malignant ascites. Surg Endosc 27(8):2735–2743
NCCN (2022) The NCCN gastric cancer clinical practice guidelines in oncology (version 2.2022). https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1434
NCCN (2022) The NCCN colon cancer clinical practice guidelines in oncology (version 2.2022). https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1428
Landskron JHØ, Torgersen KM, Aandahl EM, Gjertsen BT, Bjørge L, Taskén K (2015) Activated regulatory and memory T-cells accumulate in malignant ascites from ovarian carcinoma patients. Cancer Immunol Immunother 64(3):337–347
Mannino MHZZ, Xiao H, Bai Q, Wakefield MR, Fang Y (2015) The paradoxical role of IL-10 in immunity and cancer. Cancer Lett 367(2):103–107
Rabinovich A, Medina L, Piura B, Huleihel M (2010) Expression of IL-10 in human normal and cancerous ovarian tissues and cells. Eur Cytokine Netw 21(2):122–128
Zhou JYF, Chen H, Lv W, Gan N (2007) The expression of interleukin-10 in patients with primary ovarian epithelial carcinoma and in ovarian carcinoma cell lines. J Int Med Res 35(3):290–300
Gotlieb WHAJ, Watson JM, Velu TJ, Berek JS, Martínez-Maza O (1992) Presence of interleukin 10 (IL-10) in the ascites of patients with ovarian and other intra-abdominal cancers. Cytokine 4(5):385–390
Mustea A, Braicu EI, Koensgen D, Yuan S, Sun PM, Stamatian F et al (2009) Monitoring of IL-10 in the serum of patients with advanced ovarian cancer: results from a prospective pilot-study. Cytokine 45(1):8–11
Nowak MGE, Szpakowski M, Szyllo K, Malinowski A, Kulig A, Tchorzewski H, Wilczynski J (2010) Proinflammatory and immunosuppressive serum, ascites and cyst fluid cytokines in patients with early and advanced ovarian cancer and benign ovarian tumors. Neuro Endocrinol Lett 31(3):375–383
Zeni EML, Miotto D, Lo Cascio N, Maestrelli P, Querzoli P, Pedriali M, De Rosa E, Fabbri LM, Mapp CE, Boschetto P (2007) Macrophage expression of interleukin-10 is a prognostic factor in nonsmall cell lung cancer. Eur Respir J 30(4):627–632
Reinartz S, Finkernagel F, Adhikary T, Rohnalter V, Schumann T, Schober Y et al (2016) A transcriptome-based global map of signaling pathways in the ovarian cancer microenvironment associated with clinical outcome. Genome Biol 17(1):108
Matte ILD, Laplante C, Rancourt C, Piché A (2012) Profiling of cytokines in human epithelial ovarian cancer ascites. Am J Cancer Res 2(5):566–580
Lech-Maranda EBJ, Michallet AS, Houot R, Robak T, Coiffier B, Salles G (2006) Elevated IL-10 plasma levels correlate with poor prognosis in diffuse large B-cell lymphoma. Eur Cytokine Netw 17(1):60–66
Lu ZW, Hu JQ, Liu WL, Wen D, Wei WJ, Wang YL et al (2020) IL-10 restores MHC class I expression and interferes with immunity in papillary thyroid cancer with hashimoto thyroiditis. Endocrinology 161(10):bqaa062
Cunha LL, Morari EC, Nonogaki S, Marcello MA, Soares FA, Vassallo J et al (2017) Interleukin 10 expression is related to aggressiveness and poor prognosis of patients with thyroid cancer. Cancer immunol immunother CII 66(2):141–148
Hatanaka HAY, Kamiya T, Morino F, Nagata J, Tokunaga T, Oshika Y, Suemizu H, Kijima H, Tsuchida T, Yamazaki H, Inoue H, Nakamura M, Ueyama Y (2000) Clinical implications of interleukin (IL)-10 induced by non-small-cell lung cancer. Ann Oncol 11(7):815–819
Soria JCMC, Kemp BL, Liu DD, Feng L, Tang X, Chang YS, Mao L, Khuri FR (2003) Lack of interleukin-10 expression could predict poor outcome in patients with stage I non-small cell lung cancer. Clin Cancer Res 9(5):1785–1791
Ahmad N, Ammar A, Storr SJ, Green AR, Rakha E, Ellis IO et al (2018) IL-6 and IL-10 are associated with good prognosis in early stage invasive breast cancer patients. Cancer immunol immunother CII 67(4):537–549
Valle Oseguera CA, Spencer JV (2017) Human cytomegalovirus interleukin-10 enhances matrigel invasion of MDA-MB-231 breast cancer cells. Cancer Cell Int 17:24
Toiyama Y, Miki C, Inoue Y, Minobe S, Urano H, Kusunoki M (2010) Loss of tissue expression of interleukin-10 promotes the disease progression of colorectal carcinoma. Surg Today 40(1):46–53
Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G et al (1996) Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Investig 98(4):1010–1020
Zigmond EBB, Friedlander G, Walker CR, Yona S, Kim KW, Brenner O, Krauthgamer R, Varol C, Müller W, Jung S (2014) Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity 40(5):720–733
Macatonia SEDT, Knight SC, O’Garra A (1993) Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. J Immunol 150(9):3755–3765
Naing AIJ, Papadopoulos KP, Chan IH, Shen C, Ratti NP, Rojo B, Autio KA, Wong DJ, Patel MR, Ott PA, Falchook GS, Pant S, Hung A, Pekarek KL, Wu V, Adamow M, McCauley S, Mumm JB, Wong P, Van Vlasselaer P, Leveque J, Tannir NM, Oft M (2018) PEGylated IL-10 (pegilodecakin) induces systemic immune activation, CD8+ T cell invigoration and polyclonal T cell expansion in cancer patients. Cancer Cell Int 34(5):775–791
Fahmi MN, Pradjatmo H, Astuti I, Nindrea RD (2021) Cytokines as prognostic biomarkers of epithelial ovarian cancer (EOC): a systematic review and meta-analysis. Asian Pac J Cancer Prev 22(2):315–323
Yigit R, Figdor CG, Zusterzeel PL, Pots JM, Torensma R, Massuger LF (2011) Cytokine analysis as a tool to understand tumour-host interaction in ovarian cancer. Eur J Cancer 47(12):1883–1889
Cho KRSI (2009) Ovarian cancer. Annu Rev Pathol 4:287–313
Giuntoli RL, Webb TJ, Zoso A, Rogers O, Diaz-Montes TP, Bristow RE, Oelke M (2009) Ovarian cancer-associated ascites demonstrates altered immune environment: implications for antitumor immunity. Anticancer Res 29(8):2875–2884
Coosemans A, Decoene J, Baert T, Laenen A, Kasran A, Verschuere T et al (2016) Immunosuppressive parameters in serum of ovarian cancer patients change during the disease course. Oncoimmunology 5(4):e1111505
Micheli DC, Jammal MP, Martins-Filho A, Cortes J, Souza CN, Nomelini RS et al (2020) Serum cytokines and CXCR2: potential tumour markers in ovarian neoplasms. Biomarkers 25(6):474–482
Zhang L, Liu W, Wang X, Wang X, Sun H (2019) Prognostic value of serum IL-8 and IL-10 in patients with ovarian cancer undergoing chemotherapy. Oncol Lett 17(2):2365–2369
Antoneeva II, Abakumova TV, Dolgova DR, Gening TP, Pirmamedova SS, Myasnikova DF et al (2017) Cytokine status of serum in ovarian cancer patients with different tumor neoadjuvant chemotherapy response. Anticancer Agents Med Chem 17(9):1251–1255
Hopkins D, Sanchez H, Berwin B, Wilkinson-Ryan I (2021) Cisplatin increases immune activity of monocytes and cytotoxic T-cells in a murine model of epithelial ovarian cancer. Transl Oncol 14(12):101217
Park HS, Kwon WS, Park S, Jo E, Lim SJ, Lee CK et al (2019) Comprehensive immune profiling and immune-monitoring using body fluid of patients with metastatic gastric cancer. J Immunother Cancer 7(1):268
Atta S, Kamel M, Mansour W, Hussein T, Maher K, Elrefaiy MA (2021) Ascitic fluid cytokines in chronic liver disease: a possible prognostic tool. Dig Dis 39(5):534–539
Santin ADBS, Ravaggi A, Roman J, Smith CV, Pecorelli S, Cannon MJ, Parham GP (2001) Increased levels of interleukin-10 and transforming growth factor-beta in the plasma and ascitic fluid of patients with advanced ovarian cancer. BJOG 108(8):804–808
Zhu Q, Wu X, Wu Y, Wang X (2016) Interaction between Treg cells and tumor-associated macrophages in the tumor microenvironment of epithelial ovarian cancer. Oncol Rep 36(6):3472–3478
Gening SOAT, Antoneeva II, Rizvanov AA, Gening TP, Gafurbaeva DU (2021) Stem-like tumor cells and proinflammatory cytokines in the ascitic fluid of ovarian cancer patients. Klin Lab Diagn 66(5):297–303
Naldini A, Morena E, Belotti D, Carraro F, Allavena P, Giavazzi R (2011) Identification of thrombin-like activity in ovarian cancer associated ascites and modulation of multiple cytokine networks. Thromb Haemost 106(4):705–711
Candido EB, Silva LM, Carvalho AT, Lamaita RM, Filho RM, Cota BD et al (2013) Immune response evaluation through determination of type 1, type 2, and type 17 patterns in patients with epithelial ovarian cancer. Reprod Sci 20(7):828–837
Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A (2003) CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer 98(5):1089–1099
Wu LDZ, Peng Y, Han L, Liu J, Wang L, Li B, Zhao J, Jiao S, Wei H (2017) Ascites-derived IL-6 and IL-10 synergistically expand CD14+HLA-DR-/low myeloid-derived suppressor cells in ovarian cancer patients. Oncotarget 8(44):76843–76856
Khong HTRN (2002) Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol 3:999–1005
Kawamoto H, Minato N (2004) Myeloid cells. Int J Biochem Cell Biol 36(8):1374–1379
Dou AFJ (2021) Heterogeneous myeloid cells in tumors. Cancers (Basel) 13(15):3772
Hart KM, Byrne KT, Molloy MJ, Usherwood EM, Berwin B (2011) IL-10 immunomodulation of myeloid cells regulates a murine model of ovarian cancer. Front Immunol 2:29
Hart KM, Usherwood EJ, Berwin BL (2014) CX3CR1 delineates temporally and functionally distinct subsets of myeloid-derived suppressor cells in a mouse model of ovarian cancer. Immunol Cell Biol 92(6):499–508
Zhang B, Chen F, Xu Q, Han L, Xu J, Gao L et al (2018) Revisiting ovarian cancer microenvironment: a friend or a foe? Protein Cell 9(8):674–692
Liu CZ, Zhang L, Chang XH, Cheng YX, Cheng HY, Ye X et al (2012) Overexpression and immunosuppressive functions of transforming growth factor 1, vascular endothelial growth factor and interleukin-10 in epithelial ovarian cancer. Chin J Cancer Res 24(2):130–137
Zou WMV, Coulomb-L’Hermin A, Borvak J, Nome F, Isaeva T, Wei S, Krzysiek R, Durand-Gasselin I, Gordon A, Pustilnik T, Curiel DT, Galanaud P, Capron F, Emilie D, Curiel TJ (2001) Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med 12(7):1339–1346
Wei SKI, Zou L, Daniel B, Cheng P, Mottram P, Curiel T, Lange A, Zou W (2005) Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res 65(12):5020–5026
Labidi-Galy SI, Sisirak V, Meeus P, Gobert M, Treilleux I, Bajard A et al (2011) Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer. Cancer Res 71(16):5423–5434
Colvin EK (2014) Tumor-associated macrophages contribute to tumor progression in ovarian cancer. Front Oncol 4:137
Menga A, Favia M, Spera I, Vegliante MC, Gissi R, De Grassi A et al (2021) N-acetylaspartate release by glutaminolytic ovarian cancer cells sustains protumoral macrophages. EMBO Rep 22(9):e51981
Loercher AENM, Kavanagh JJ, Platsoucas CD, Freedman RS (1999) Identification of an IL-10-producing HLA-DR-negative monocyte subset in the malignant ascites of patients with ovarian carcinoma that inhibits cytokine protein expression and proliferation of autologous T cells. J Immunol 163(11):6251–6260
Sawant DV, Yano H, Chikina M, Zhang Q, Liao M, Liu C et al (2019) Adaptive plasticity of IL-10(+) and IL-35(+) T(reg) cells cooperatively promotes tumor T cell exhaustion. Nat Immunol 20(6):724–735
Mauri C, Bosma A (2012) Immune regulatory function of B cells. Annu Rev Immunol 30:221–241
Wei XJY, Tian Y, Zhang H, Wu J, Lu W, Lu X (2016) Regulatory B cells contribute to the impaired antitumor immunity in ovarian cancer patients. Tumour Biol 37(5):6581–6588
Murakami Y, Saito H, Shimizu S, Kono Y, Shishido Y, Miyatani K et al (2019) Increased regulatory B cells are involved in immune evasion in patients with gastric cancer. Sci Rep 9(1):13083
Qian LBG, Zhou Y, Wang Y, Hu J, Liu X, Xu Y (2015) Clinical significance of regulatory B cells in the peripheral blood of patients with oesophageal cancer. Cent Eur J Immunol 40(2):263–265
Zhou XSY, Lao XM, Liang YJ, Liao GQ (2016) CD19(+)IL-10(+) regulatory B cells affect survival of tongue squamous cell carcinoma patients and induce resting CD4(+) T cells to CD4(+)Foxp3(+) regulatory T cells. Oral Oncol 53:27–35
Zhou M, Wen Z, Cheng F, Ma J, Li W, Ren H et al (2016) Tumor-released autophagosomes induce IL-10-producing B cells with suppressive activity on T lymphocytes via TLR2-MyD88-NF-kappaB signal pathway. Oncoimmunology 5(7):e1180485
Berger SSA, Denkert C, Köbel M, Hauptmann S (2001) Interleukin-10 in serous ovarian carcinoma cell lines. Cancer Immunol Immunother 50(6):328–333
Carr TM, Adair SJ, Fink MJ, Hogan KT (2008) Immunological profiling of a panel of human ovarian cancer cell lines. Cancer Immunol Immunother 57(1):31–42
Nowak M, Klink M, Glowacka E, Sulowska Z, Kulig A, Szpakowski M et al (2010) Production of cytokines during interaction of peripheral blood mononuclear cells with autologous ovarian cancer cells or benign ovarian tumour cells. Scand J Immunol 71(2):91–98
Raghavan S, Mehta P, Xie Y, Lei YL, Mehta G (2019) Ovarian cancer stem cells and macrophages reciprocally interact through the WNT pathway to promote pro-tumoral and malignant phenotypes in 3D engineered microenvironments. J Immunother Cancer 7(1):190
Gastl GAAJ, Nanus DM, Oosterkamp R, Silver J, Liu F, Chen M, Albino AP, Bander NH (1993) Interleukin-10 production by human carcinoma cell lines and its relationship to interleukin-6 expression. Int J Cancer 55(1):96–101
Hihara JYY, Minami K, Noma K, Toge T (1999) Down-regulation of IL-10 enhances the efficacy of locoregional immunotherapy using OK-432 against malignant effusion. Anticancer Res 19(2A):1077–1084
Mukherjee PGA, Madsen CS, Tinder TL, Jacobs F, Parker J, Agrawal B, Longenecker BM, Gendler SJ (2001) MUC1-specific CTLs are non-functional within a pancreatic tumor microenvironment. Glycoconj J 18(11–12):931–942
Ke X, Shen L (2019) Targeting cytokines secreted by CD4(+) CD25(high) CD127(low) regulatory T cells inhibits ovarian cancer progression. Scand J Immunol 89(2):e12736
Santner-Nanan B, Straubinger K, Hsu P, Parnell G, Tang B, Xu B et al (2013) Fetal-maternal alignment of regulatory T cells correlates with IL-10 and Bcl-2 upregulation in pregnancy. J Immunol 191(1):145–153
Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y et al (2007) Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res 67(19):9066–9076
Donnelly RP, Dickensheets H, Finbloom DS (1999) The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J Interf Cytokine Res 19(6):563–573
Finbloom DS, Winestock KD (1995) IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. J Immunol 155(3):1079–1090
Wehinger J, Gouilleux F, Groner B, Finke J, Mertelsmann R, Weber-Nordt RM (1996) IL-10 induces DNA binding activity of three STAT proteins (Stat1, Stat3, and Stat5) and their distinct combinatorial assembly in the promoters of selected genes. FEBS Lett 394(3):365–370
Yang L, Dong Y, Li Y, Wang D, Liu S, Wang D et al (2019) IL-10 derived from M2 macrophage promotes cancer stemness via JAK1/STAT1/NF-kappaB/Notch1 pathway in non-small cell lung cancer. Int J Cancer 145(4):1099–1110
Regis G, Pensa S, Boselli D, Novelli F, Poli V (2008) Ups and downs: the STAT1:STAT3 seesaw of Interferon and gp130 receptor signalling. Semin Cell Dev Biol 19(4):351–359
Zhou JH, Broussard SR, Strle K, Freund GG, Johnson RW, Dantzer R et al (2001) IL-10 inhibits apoptosis of promyeloid cells by activating insulin receptor substrate-2 and phosphatidylinositol 3′-kinase. J Immunol 167(8):4436–4442
Reitamo S, Remitz A, Tamai K, Uitto J (1994) Interleukin-10 modulates type I collagen and matrix metalloprotease gene expression in cultured human skin fibroblasts. J Clin Invest 94(6):2489–2492
Carlini V, Noonan DM, Abdalalem E, Goletti D, Sansone C, Calabrone L et al (2023) The multifaceted nature of IL-10: regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front Immunol 14:1161067
Salkeni MA, Naing A (2023) Interleukin-10 in cancer immunotherapy: from bench to bedside. Trends Cancer 9(9):716–725
Rallis KSCA, Dadah H, Stanislovas J, Zamani P, Makker S, Szabados B, Sideris M (2022) IL-10 in cancer: an essential thermostatic regulator between homeostatic immunity and inflammation—a comprehensive review. Future Oncol 18(29):3349–3365
Guo Y, Xie YQ, Gao M, Zhao Y, Franco F, Wenes M et al (2021) Metabolic reprogramming of terminally exhausted CD8(+) T cells by IL-10 enhances anti-tumor immunity. Nat Immunol 22(6):746–756
Naing A, Papadopoulos KP, Autio KA, Ott PA, Patel MR, Wong DJ et al (2016) Safety, antitumor activity, and immune activation of pegylated recombinant human interleukin-10 (AM0010) in patients with advanced solid tumors. J Clin Oncol Off J Am Soc Clin Oncol 34(29):3562–3569
Hecht JRLS, Bendell J, Sim HW, Macarulla T, Lopez CD, Van Cutsem E, Muñoz Martin AJ, Park JO, Greil R, Wang H, Hozak RR, Gueorguieva I, Lin Y, Rao S, Ryoo BY (2021) Randomized phase III study of FOLFOX alone or with pegilodecakin as second-line therapy in patients with metastatic pancreatic cancer that progressed after gemcitabine (SEQUOIA). J Clin Oncol 39(10):1108–1118
Autio K, Oft M (2019) Pegylated interleukin-10: clinical development of an immunoregulatory cytokine for use in cancer therapeutics. Curr Oncol Rep 21(2):19
Asadullah K, Sterry W, Volk HD (2003) Interleukin-10 therapy–review of a new approach. Pharmacol Rev 55(2):241–269
Lamichhane P, Karyampudi L, Shreeder B, Krempski J, Bahr D, Daum J et al (2017) IL10 release upon PD-1 blockade sustains immunosuppression in ovarian cancer. Cancer Res 77(23):6667–6678
Adams SFGA, Chiang CL, Mookerjee A, Flies D, Jean S, McCann GA, Michaux J, Pak H, Huber F, Neal C, Dangaj D, Bassani-Sternberg M, Rusakiewicz S, Facciabene A, Coukos G, Gimotty PA, Kandalaft LE (2020) Rapid tumor vaccine using Toll-like receptor-activated ovarian cancer ascites monocytes. J Immunother Cancer 8(2):e000875
Hu Z, Hu S, Wu Y, Li S, He C, Xing X et al (2018) Accumulation and suppressive function of regulatory T cells in malignant ascites: reducing their suppressive function using arsenic trioxide in vitro. Oncol Lett 15(4):5384–5390
Martincuks A, Song J, Kohut A, Zhang C, Li YJ, Zhao Q et al (2021) PARP inhibition activates STAT3 in both tumor and immune cells underlying therapy resistance and immunosuppression in ovarian cancer. Front Oncol 11:724104
Gruzdyn OV, Gruber SA, Batchu RB, Mahmud EM, Chukr FK, Dachepalli R (2017) Mesothelin chimeric antigen receptor (car)-mediated therapy for ovarian cancer. J Am Coll Surg 225(4):e47
Batchu RBGO, Kolli BK, Dachepalli R, Umar PS, Rai SK, Singh N, Tavva PS, Weaver DW, Gruber SA (2021) IL-10 signaling in the tumor microenvironment of ovarian cancer. Adv Exp Med Biol 1290:51–65
Chen YHTC, Huang PY, Chang MY, Cheng PC, Chou CH, Chen DH, Wang CR, Shiau AL, Wu CL (2007) Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model. Mol Pharm 4(5):713–722
Sriram MI, Kanth SB, Kalishwaralal K, Gurunathan S (2010) Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. Int J Nanomed 5:753–762
Hama S, Nishi T, Isono E, Itakura S, Yoshikawa Y, Nishimoto A et al (2022) Intraperitoneal administration of nanoparticles containing tocopheryl succinate prevents peritoneal dissemination. Cancer Sci 113(5):1779–1788
Dai X, Meng J, Deng S, Zhang L, Wan C, Lu L et al (2020) Targeting CAMKII to reprogram tumor-associated macrophages and inhibit tumor cells for cancer immunotherapy with an injectable hybrid peptide hydrogel. Theranostics 10(7):3049–3063
Shamskhou EA, Kratochvil MJ, Orcholski ME, Nagy N, Kaber G, Steen E et al (2019) Hydrogel-based delivery of Il-10 improves treatment of bleomycin-induced lung fibrosis in mice. Biomaterials 203:52–62
Funding
This work was supported by The Science and Technology Project of the Health Planning Committee of Sichuan (21PJ050).
Author information
Authors and Affiliations
Contributions
YH and KNZ wrote the main manuscript text and prepared Figs. 1–4 and Table 1. HJ critically edited the main manuscript and helped prepare Figs. 1–4. ZYL concepted the study and edited the main manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
N/A.
Informed consent
N/A
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, Y., Zou, K., Jiang, H. et al. The complex role of IL-10 in malignant ascites: a review. Cancer Immunol Immunother 73, 32 (2024). https://doi.org/10.1007/s00262-023-03616-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00262-023-03616-y