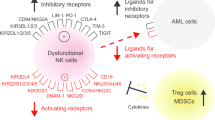
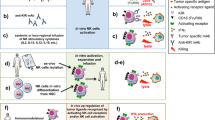
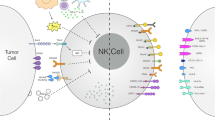
Abstract
Acute myeloid leukemia (AML) is considered as one of the most malignant conditions of the bone marrow. Over the past few decades, despite substantial progresses in the management of AML, relapse remission remains a major problem. Natural killer cells (NK cells) are known as a unique component of the innate immune system. Due to swift tumor detection, distinct cytotoxic action, and extensive immune interaction, NK cells have been used in various cancer settings for decades. It has been a growing knowledge of therapeutic magnitudes ranging from adoptive NK cell transfer to chimeric antigen receptor NK cells, aiming to achieve better therapeutic responses in patients with AML. In this article, the potentials of NK cells for treatment of AML are highlighted, and challenges for such therapeutic methods are discussed. In addition, the clinical application of NK cells, mainly in patients with AML, is pictured according to the existing evidence.


Similar content being viewed by others
Data availability
Not applicable.
References
Döhner H, Weisdorf DJ, Bloomfield CD (2015) Acute myeloid leukemia. N Engl J Med 373(12):1136–1152
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72(1):7–33
Cancer Genome Atlas Research Network (2013) Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. New Engl J Med 368(22):2059–2074
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND et al (2016) Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 374(23):2209–2221
Jongen-Lavrencic M, Grob T, Hanekamp D, Kavelaars FG, Al Hinai A, Zeilemaker A et al (2018) Molecular minimal residual disease in acute myeloid leukemia. New Engl J Med 378(13):1189–99
Creutzig U, Zimmermann M, Ritter J, Reinhardt D, Hermann J, Henze G et al (2005) Treatment strategies and long-term results in paediatric patients treated in four consecutive AML-BFM trials. Leukemia 19(12):2030–2042
Shaffer BC, Gillet J-P, Patel C, Baer MR, Bates SE, Gottesman MM (2012) Drug resistance: still a daunting challenge to the successful treatment of AML. Drug Resist Updates 15(1):62–69
Löwenberg B, Pabst T, Maertens J, Gradowska P, Biemond BJ, Spertini O et al (2021) Addition of lenalidomide to intensive treatment in younger and middle-aged adults with newly diagnosed AML: the HOVON-SAKK-132 trial. Blood Adv 5(4):1110–1121
Huntington ND, Cursons J, Rautela J (2020) The cancer–natural killer cell immunity cycle. Nat Rev Cancer 20(8):437–454
Kiessling R, Klein E, Wigzell H (1975) “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol 5(2):112–7
Moretta A, Bottino C, Mingari MC, Biassoni R, Moretta L (2002) What is a natural killer cell? Nat Immunol 3(1):6–8
Shimasaki N, Jain A, Campana D (2020) NK cells for cancer immunotherapy. Nat Rev Drug Discovery 19(3):200–218
Xu J, Niu T (2020) Natural killer cell-based immunotherapy for acute myeloid leukemia. J Hematol Oncol 13(1):167
Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S (2008) Functions of natural killer cells. Nat Immunol 9(5):503–510
Lion E, Willemen Y, Berneman ZN, Van Tendeloo VFI, Smits ELJ (2012) Natural killer cell immune escape in acute myeloid leukemia. Leukemia 26(9):2019–2026
Wang Z, Xiao Y, Guan W, Wang M, Chen J, Zhang L et al (2020) Acute myeloid leukemia immune escape by epigenetic CD48 silencing. Clin Sci 134(2):261–271
Abel AM, Yang C, Thakar MS, Malarkannan S (2018) Natural killer cells: development, maturation, and clinical utilization. Front Immunol 9:1869
Ghaemdoust F, Keshavarz-Fathi M, Rezaei N (2019) Natural killer cells and cancer therapy, what we know and where we are going. Immunotherapy 11(14):1231–1251
Sun JC, Lanier LL (2011) NK cell development, homeostasis and function: parallels with CD8+ T cells. Nat Rev Immunol 11(10):645–657
Kiessling R, Klein E, Pross H, Wigzell H (1975) “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol 5(2):117–21
Pross HF, Jondal M (1975) Cytotoxic lymphocytes from normal donors. A functional marker of human non-T lymphocytes. Clin Exp Immunol 21(2):226–35
Jondal M, Pross H (1975) Surface markers on human b and t lymphocytes. VI. Cytotoxicity against cell lines as a functional marker for lymphocyte subpopulations. Int J Cancer 15(4):596–605
Huntington ND, Vosshenrich CAJ, Di Santo JP (2007) Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol 7(9):703–714
Crinier A, Dumas P-Y, Escalière B, Piperoglou C, Gil L, Villacreces A et al (2020) Single-cell profiling reveals the trajectories of natural killer cell differentiation in bone marrow and a stress signature induced by acute myeloid leukemia. Cell Mol Immunol 18:1290–1304
Ames E, Murphy WJ (2014) Advantages and clinical applications of natural killer cells in cancer immunotherapy. Cancer Immunol Immunother 63(1):21–28
Cooper MA, Fehniger TA, Caligiuri MA (2001) The biology of human natural killer-cell subsets. Trends Immunol 22(11):633–640
Penack O, Gentilini C, Fischer L, Asemissen AM, Scheibenbogen C, Thiel E et al (2005) CD56dimCD16neg cells are responsible for natural cytotoxicity against tumor targets. Leukemia 19(5):835–840
Cullen SP, Martin SJ (2008) Mechanisms of granule-dependent killing. Cell Death Differ 15(2):251–262
Michel T, Poli A, Cuapio A, Briquemont B, Iserentant G, Ollert M et al (2016) Human CD56<sup>bright</sup> NK cells: an update. J Immunol 196(7):2923–2931
Waldhauer I, Steinle A (2008) NK cells and cancer immunosurveillance. Oncogene 27(45):5932–5943
Campbell KS, Hasegawa J (2013) Natural killer cell biology: an update and future directions. J Allergy Clin Immunol 132(3):536–544
Chester C, Fritsch K, Kohrt HE (2015) Natural killer cell immunomodulation: targeting activating, inhibitory, and co-stimulatory receptor signaling for cancer immunotherapy. Front Immunol 6:601
Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH (2011) Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol 89(2):216–224
Anfossi N, André P, Guia S, Falk CS, Roetynck S, Stewart CA et al (2006) Human NK cell education by inhibitory receptors for MHC class I. Immunity 25(2):331–342
Ljunggren H-G, Kärre K (1990) In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today 11:237–244
Kärre K, Ljunggren HG, Piontek G, Kiessling R (1986) Selective rejection of H–2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319(6055):675–678
Cornel AM, Mimpen IL, Nierkens S (2020) MHC class I downregulation in cancer: underlying mechanisms and potential targets for cancer immunotherapy. Cancers (Basel) 12(7):1760
Bessoles S, Grandclément C, Alari-Pahissa E, Gehrig J, Jeevan-Raj B, Held W (2014) Adaptations of natural killer cells to Self-MHC class I. Front Immunol 5:349
Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SEA, Yagita H et al (2005) Activation of NK cell cytotoxicity. Mol Immunol 42(4):501–510
Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK et al (2005) Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105(8):3051–3057
Mancusi A, Ruggeri L, Urbani E, Pierini A, Massei MS, Carotti A et al (2015) Haploidentical hematopoietic transplantation from KIR ligand–mismatched donors with activating KIRs reduces nonrelapse mortality. Blood 125(20):3173–3182
Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH et al (2014) Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood 123(25):3855–3863
Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM (2015) NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol 6:368
Yun HD, Schirm DK, Felices M, Miller JS, Eckfeldt CE (2019) Dinaciclib enhances natural killer cell cytotoxicity against acute myelogenous leukemia. Blood Adv 3(16):2448–2452
Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E (2005) Natural-killer cells and dendritic cells: “l’union fait la force.” Blood 106(7):2252–2258
Moretta L, Ferlazzo G, Bottino C, Vitale M, Pende D, Mingari MC et al (2006) Effector and regulatory events during natural killer–dendritic cell interactions. Immunol Rev 214(1):219–228
Degli-Esposti MA, Smyth MJ (2005) Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol 5(2):112–124
Coughlin CM, Salhany KE, Gee MS, LaTemple DC, Kotenko S, Ma X et al (1998) Tumor cell responses to IFNγ affect tumorigenicity and response to IL-12 therapy and antiangiogenesis. Immunity 9(1):25–34
Haghshenas M, Khademi B, Ashraf M, Ghaderi A, Erfani N (2016) Helper and cytotoxic T-cell subsets (Th1, Th2, Tc1, and Tc2) in benign and malignant salivary gland tumors. Oral Dis 22(6):566–572
Decot V, Voillard L, Latger-Cannard V, Aissi-Rothé L, Perrier P, Stoltz JF et al (2010) Natural-killer cell amplification for adoptive leukemia relapse immunotherapy: comparison of three cytokines, IL-2, IL-15, or IL-7 and impact on NKG2D, KIR2DL1, and KIR2DL2 expression. Exp Hematol 38(5):351–362
Cooper MA, Yokoyama WM (2010) Memory-like responses of natural killer cells. Immunol Rev 235(1):297–305
Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP et al (2012) Cytokine activation induces human memory-like NK cells. Blood 120(24):4751–4760
Fauriat C, Long EO, Ljunggren HG, Bryceson YT (2010) Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 115(11):2167–2176
Lee HM, Kim KS, Kim J (2014) A comparative study of the effects of inhibitory cytokines on human natural killer cells and the mechanistic features of transforming growth factor-beta. Cell Immunol 290(1):52–61
Park JY, Lee SH, Yoon SR, Park YJ, Jung H, Kim TD et al (2011) IL-15-induced IL-10 increases the cytolytic activity of human natural killer cells. Mol Cells 32(3):265–272
Littwitz-Salomon E, Malyshkina A, Schimmer S, Dittmer U (2018) The cytotoxic activity of natural killer cells is suppressed by IL-10(+) regulatory T cells during acute retroviral infection. Front Immunol 9:1947
Ustun C, Miller JS, Munn DH, Weisdorf DJ, Blazar BR (2011) Regulatory T cells in acute myelogenous leukemia: is it time for immunomodulation? Blood 118(19):5084–5095
Wan Y, Zhang C, Xu Y, Wang M, Rao Q, Xing H et al (2020) Hyperfunction of CD4 CD25 regulatory T cells in de novo acute myeloid leukemia. BMC Cancer 20(1):472
Mougiakakos D (2019) The Induction of a Permissive Environment to Promote T Cell Immune Evasion in Acute Myeloid Leukemia: The Metabolic Perspective. Front Oncol, 9
Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R et al (2003) Transforming growth factor β1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci 100(7):4120–4125
Street SEA, Hayakawa Y, Zhan Y, Lew AM, MacGregor D, Jamieson AM et al (2004) Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J Exp Med 199(6):879–884
Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N et al (2008) NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 28(4):571–580
Albertsson PA, Basse PH, Hokland M, Goldfarb RH, Nagelkerke JF, Nannmark U et al (2003) NK cells and the tumour microenvironment: implications for NK-cell function and anti-tumour activity. Trends Immunol 24(11):603–609
Orange JS (2013) Natural killer cell deficiency. J Allergy Clin Immunol 132(3):515–525
Burnet M (1957) Cancer: a biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applications. Br Med J. 1(5023):841–847
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD (2002) Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 3(11):991–998
Mittal D, Gubin MM, Schreiber RD, Smyth MJ (2014) New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr Opin Immunol 27:16–25
López-Soto A, Gonzalez S, Smyth MJ, Galluzzi L (2017) Control of metastasis by NK cells. Cancer Cell 32(2):135–154
Vesely MD, Schreiber RD (2013) Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Ann N Y Acad Sci 1284(1):1–5
Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ et al (2007) Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450(7171):903–907
Barrett AJ (2020) Acute myeloid leukaemia and the immune system: implications for immunotherapy. Br J Haematol 188(1):147–158
Swann JB, Smyth MJ (2007) Immune surveillance of tumors. J Clin Invest 117(5):1137–1146
Dunn GP, Old LJ, Schreiber RD (2004) The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21(2):137–148
Malhotra A, Shanker A (2011) NK cells: immune cross-talk and therapeutic implications. Immunotherapy 3(10):1143–1166
Ahmad M, Rees RC, Ali SA (2004) Escape from immunotherapy: possible mechanisms that influence tumor regression/progression. Cancer Immunol Immunother 53(10):844–854
Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MT et al (2009) Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med 361(5):478–488
Woan K, Reddy V (2007) Potential therapeutic role of natural killer cells in cancer. Expert Opin Biol Ther 7(1):17–29
Grossenbacher SK, Canter RJ, Murphy WJ (2016) Natural killer cell immunotherapy to target stem-like tumor cells. J Immunother Cancer 4(1):19
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331(6024):1565–1570
Barrett AJ, Le Blanc K (2010) Immunotherapy prospects for acute myeloid leukaemia. Clin Exp Immunol 161(2):223–232
Tratkiewicz JA, Szer J (1990) Loss of natural killer activity as an indicator of relapse in acute leukaemia. Clin Exp Immunol 80(2):241–246
Lowdell MW, Craston R, Samuel D, Wood ME, O’Neill E, Saha V et al (2002) Evidence that continued remission in patients treated for acute leukaemia is dependent upon autologous natural killer cells. Br J Haematol 117(4):821–827
Pross HF, Lotzová E (1993) Role of natural killer cells in cancer. Nat Immun 12(4–5):279–292
Tajima F, Kawatani T, Endo A, Kawasaki H (1996) Natural killer cell activity and cytokine production as prognostic factors in adult acute leukemia. Leukemia 10(3):478–482
Bryceson YT, March ME, Ljunggren H-G, Long EO (2006) Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 107(1):159–166
Stringaris K, Sekine T, Khoder A, Alsuliman A, Razzaghi B, Sargeant R et al (2014) Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica 99(5):836–847
Costello RGT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci M-J, Reviron D et al (2002) Defective expression and function of natural killer cell–triggering receptors in patients with acute myeloid leukemia. Blood 99(10):3661–7
Szczepanski MJ, Szajnik M, Welsh A, Foon KA, Whiteside TL, Boyiadzis M (2010) Interleukin-15 enhances natural killer cell cytotoxicity in patients with acute myeloid leukemia by upregulating the activating NK cell receptors. Cancer Immunol Immunother CII 59(1):73–79
Sanchez-Correa B, Gayoso I, Bergua JM, Casado JG, Morgado S, Solana R et al (2012) Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol Cell Biol 90(1):109–115
Pende D, Spaggiari GM, Marcenaro S, Martini S, Rivera P, Capobianco A et al (2005) Analysis of the receptor-ligand interactions in the natural killer–mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112). Blood 105(5):2066–2073
Hilpert J, Grosse-Hovest L, Grünebach F, Buechele C, Nuebling T, Raum T et al (2012) Comprehensive analysis of NKG2D ligand expression and release in leukemia: implications for NKG2D-mediated NK cell responses. J Immunol 189(3):1360–1371
Fauriat C, Just-Landi S, Mallet FO, Arnoulet C, Sainty D, Olive D et al (2006) Deficient expression of NCR in NK cells from acute myeloid leukemia: evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood 109(1):323–30
Verheyden S, Bernier M, Demanet C (2004) Identification of natural killer cell receptor phenotypes associated with leukemia. Leukemia 18(12):2002–2007
Sandoval-Borrego D, Moreno-Lafont MC, Vazquez-Sanchez EA, Gutierrez-Hoya A, López-Santiago R, Montiel-Cervantes LA et al (2016) Overexpression of CD158 and NKG2A inhibitory receptors and underexpression of NKG2D and NKp46 activating receptors on NK cells in acute myeloid leukemia. Arch Med Res 47(1):55–64
Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T et al (2007) Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood 110(1):433–440
Farag SS, Bacigalupo A, Eapen M, Hurley C, Dupont B, Caligiuri MA et al (2006) The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant 12(8):876–884
Verheyden S, Demanet C (2008) NK cell receptors and their ligands in leukemia. Leukemia 22(2):249–257
Nguyen S, Beziat V, Dhedin N, Kuentz M, Vernant JP, Debre P et al (2009) HLA-E upregulation on IFN-γ-activated AML blasts impairs CD94/NKG2A-dependent NK cytolysis after haplo-mismatched hematopoietic SCT. Bone Marrow Transplant 43(9):693–699
Nowbakht P, Ionescu M-CS, Rohner A, Kalberer CP, Rossy E, Mori L et al (2005) Ligands for natural killer cell–activating receptors are expressed upon the maturation of normal myelomonocytic cells but at low levels in acute myeloid leukemias. Blood 105(9):3615–3622
Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee H-G et al (2003) Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood 102(4):1389–1396
Baragaño Raneros A, Martín-Palanco V, Fernandez AF, Rodriguez RM, Fraga MF, Lopez-Larrea C et al (2015) Methylation of NKG2D ligands contributes to immune system evasion in acute myeloid leukemia. Genes Immun 16(1):71–82
Kearney CJ, Ramsbottom KM, Voskoboinik I, Darcy PK, Oliaro J (2016) Loss of DNAM-1 ligand expression by acute myeloid leukemia cells renders them resistant to NK cell killing. OncoImmunology 5(8):e1196308
Baessler T, Charton JE, Schmiedel BJ, Grünebach F, Krusch M, Wacker A et al (2010) CD137 ligand mediates opposite effects in human and mouse NK cells and impairs NK-cell reactivity against human acute myeloid leukemia cells. Blood 115(15):3058–3069
Baessler T, Krusch M, Schmiedel BJ, Kloss M, Baltz KM, Wacker A et al (2009) Glucocorticoid-induced tumor necrosis factor receptor-related protein ligand subverts immunosurveillance of acute myeloid leukemia in humans. Can Res 69(3):1037–1045
Coles SJ, Wang ECY, Man S, Hills RK, Burnett AK, Tonks A et al (2011) CD200 expression suppresses natural killer cell function and directly inhibits patient anti-tumor response in acute myeloid leukemia. Leukemia 25(5):792–799
Lim SH, Worman CP, Jewell A, Goldstone AH (1991) Production of tumour-derived suppressor factor in patients with acute myeloid leukaemia. Leuk Res 15(4):263–268
Zamai L, Ponti C, Mirandola P, Gobbi G, Papa S, Galeotti L et al (2007) NK cells and cancer. J Immunol 178(7):4011–4016
Min YJ, Lee J-H, Choi S-J, Chi H-S, Lee J-S, Kim W-K et al (2004) Prognostic significance of Fas (CD95) and TRAIL receptors (DR4/DR5) expression in acute myelogenous leukemia. Leuk Res 28(4):359–365
Fauriat C, Moretta A, Olive D, Costello RGT (2005) Defective killing of dendritic cells by autologous natural killer cells from acute myeloid leukemia patients. Blood 106(6):2186–8
Ebata K, Shimizu Y, Nakayama Y, Minemura M, Murakami J, Kato T et al (2006) Immature NK cells suppress dendritic cell functions during the development of leukemia in a mouse model. J Immunol 176(7):4113–4124
Wang X, Zheng J, Liu J, Yao J, He Y, Li X et al (2005) Increased population of CD4+CD25high regulatory T cells with their higher apoptotic and proliferating status in peripheral blood of acute myeloid leukemia patients. Eur J Haematol 75(6):468–476
Shenghui Z, Yixiang H, Jianbo W, Kang Y, Laixi B, Yan Z et al (2011) Elevated frequencies of CD4+CD25+CD127lo regulatory T cells is associated to poor prognosis in patients with acute myeloid leukemia. Int J Cancer 129(6):1373–1381
Szczepanski MJ, Szajnik M, Czystowska M, Mandapathil M, Strauss L, Welsh A et al (2009) Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clin Cancer Res 15(10):3325–3332
Hallett WHD, Ames E, Álvarez M, Barao I, Taylor PA, Blazar BR et al (2008) Combination therapy using IL-2 and Anti-CD25 results in augmented natural killer cell-mediated antitumor responses. Biol Blood Marrow Transplant 14(10):1088–1099
Carlsten M, Järås M (2019) Natural killer cells in myeloid malignancies: immune surveillance, NK cell dysfunction, and pharmacological opportunities to bolster the endogenous NK cells. Front Immunol 10:2357
Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T et al (2016) Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med 8(357):357ra123
Chiossone L, Dumas P-Y, Vienne M, Vivier E (2018) Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol 18(11):671–688
Sakamoto N, Ishikawa T, Kokura S, Okayama T, Oka K, Ideno M et al (2015) Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J Transl Med 13(1):277
Wang Y, Zhang Y, Hughes T, Zhang J, Caligiuri MA, Benson DM et al (2018) Fratricide of NK Cells in daratumumab therapy for multiple myeloma overcome by <em>Ex Vivo</em>–expanded autologous NK cells. Clin Cancer Res 24(16):4006–4017
Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE et al (1985) Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 313(23):1485–1492
Myers JA, Miller JS (2021) Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol 18(2):85–100
Handgretinger R, Lang P, André MC (2016) Exploitation of natural killer cells for the treatment of acute leukemia. Blood 127(26):3341–3349
Veluchamy JP, Kok N, van der Vliet HJ, Verheul HMW, de Gruijl TD, Spanholtz J (2017) The rise of allogeneic natural killer cells as a platform for cancer immunotherapy: recent innovations and future developments. Front Immunol 8:631
Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA (2011) Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res Off J Am Assoc Cancer Res 17(19):6287–6297
Rezvani K, Rouce RH (2015) The application of natural killer cell immunotherapy for the treatment of cancer. Front Immunol 6:578
Fang F, Xiao W, Tian Z (2017) NK cell-based immunotherapy for cancer. Semin Immunol 31:37–54
Malmberg K-J, Schaffer M, Ringdén O, Remberger M, Ljunggren H-G (2005) KIR-ligand mismatch in allogeneic hematopoietic stem cell transplantation. Mol Immunol 42(4):531–534
Guillerey C, Huntington ND, Smyth MJ (2016) Targeting natural killer cells in cancer immunotherapy. Nat Immunol 17(9):1025–1036
Kärre K (2002) Immunology. A perfect mismatch. Science 295(5562):2029–2031
Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A et al (2002) Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295(5562):2097–2100
Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS (2010) NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood 115(21):4293–4301
Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK et al (2005) Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105(8):3051–3057
Mills CD, North RJ (1983) Expression of passively transferred immunity against an established tumor depends on generation of cytolytic T cells in recipient. Inhibition by suppressor T cells. J Exp Med 157(5):1448–60
Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH et al (2014) Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood 123(25):3855–3863
Lupo KB, Matosevic S (2019) Natural killer cells as allogeneic effectors in adoptive cancer immunotherapy. Cancers (Basel) 11(6):769
Franks SE, Wolfson B, Hodge JW (2020) Natural born killers: NK cells in cancer therapy. Cancers (Basel) 12(8):2131
Granzin M, Wagner J, Köhl U, Cerwenka A, Huppert V, Ullrich E (2017) Shaping of natural killer cell antitumor activity by ex vivo cultivation. Front Immunol 8:458
Romee R, Cooley S, Berrien-Elliott MM, Westervelt P, Verneris MR, Wagner JE et al (2018) First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 131(23):2515–2527
Huang J, Liu Y, Au BC, Barber DL, Arruda A, Schambach A et al (2016) Preclinical validation: LV/IL-12 transduction of patient leukemia cells for immunotherapy of AML. Mol Ther Methods Clin Dev 3:16074
Shi Y, Dincheva-Vogel L, Ayemoba CE, Fung JP, Bergamaschi C, Pavlakis GN et al (2018) IL-15/IL-15Rα/CD80-expressing AML cell vaccines eradicate minimal residual disease in leukemic mice. Blood Adv 2(22):3177–3192
Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL et al (1999) Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285(5428):727–729
Gasser S, Orsulic S, Brown EJ, Raulet DH (2005) The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 436(7054):1186–1190
Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL et al (1999) An activating immunoreceptor complex formed by NKG2D and DAP10. Science 285(5428):730–732
Garrity D, Call ME, Feng J, Wucherpfennig KW (2005) The activating NKG2D receptor assembles in the membrane with two signaling dimers into a hexameric structure. Proc Natl Acad Sci USA 102(21):7641–7646
Wu Z, Zhang H, Wu M, Peng G, He Y, Wan N et al (2021) Targeting the NKG2D/NKG2D-L axis in acute myeloid leukemia. Biomed Pharmacother 137:111299
Chang YH, Connolly J, Shimasaki N, Mimura K, Kono K, Campana D (2013) A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res 73(6):1777–1786
Kamiya T, Chang YH, Campana D (2016) Expanded and activated natural killer cells for immunotherapy of hepatocellular carcinoma. Cancer Immunol Res 4(7):574–581
Parihar R, Rivas C, Huynh M, Omer B, Lapteva N, Metelitsa LS et al (2019) NK cells expressing a chimeric activating receptor eliminate MDSCs and rescue impaired CAR-T cell activity against solid tumors. Cancer Immunol Res 7(3):363–375
Kamiya T, Seow SV, Wong D, Robinson M, Campana D (2019) Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J Clin Invest 129(5):2094–2106
Venton G, Labiad Y, Colle J, Fino A, Afridi S, Torres M et al (2016) Natural killer cells in acute myeloid leukemia patients: from phenotype to transcriptomic analysis. Immunol Res 64(5):1225–1236
van der Ploeg K, Le Luduec J-B, Stevenson PA, Park S, Gooley TA, Petersdorf EW et al (2020) HLA-A alleles influencing NK cell function impact AML relapse following allogeneic hematopoietic cell transplantation. Blood Adv 4(19):4955–4964
Tsirogianni M, Grigoriou E, Dagla K, Pappa V, Konsta E, Kapsimali-Vaiopoulou V et al (2016) NK cytotoxicity in vitro and NK/ MDSCs/Tcell subpopulations in AML/MDS patients on 5-azacytidine treatment. Blood 128(22):5123
Arvindam US, van Hauten P, Hallstrom C, Vallera DA, Dolstra H, Miller JS et al (2018) CD16-IL15-CLEC12A trispecific killer engager (TriKE) drives NK cell expansion, activation, and antigen specific killing of cancer stem cells in acute myeloid leukemia. Blood 132(Supplement 1):1454
Imamura M, Shook D, Kamiya T, Shimasaki N, Chai SM, Coustan-Smith E et al (2014) Autonomous growth and increased cytotoxicity of natural killer cells expressing membrane-bound interleukin-15. Blood 124(7):1081–1088
Liu S, Galat V, Galat Y, Lee YKA, Wainwright D, Wu J (2021) NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol 14(1):7
Porter DL, Levine BL, Kalos M, Bagg A, June CH (2011) Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365(8):725–733
Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A et al (2011) T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 3(95):95ra73
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ et al (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371(16):1507–1517
Ritchie DS, Neeson PJ, Khot A, Peinert S, Tai T, Tainton K et al (2013) Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol Ther 21(11):2122–2129
Wang Q-S, Wang Y, Lv H-Y, Han Q-W, Fan H, Guo B et al (2015) Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther 23(1):184–91
Cummins KD, Gill S (2019) Chimeric antigen receptor T-cell therapy for acute myeloid leukemia: how close to reality? Haematologica 104(7):1302–1308
Yáñez L, Sánchez-Escamilla M, Perales M-A (2019) CAR T cell toxicity: current management and future directions. Hemasphere 3(2):e186-e
Mardiana S, Gill S (2020) CAR T cells for acute myeloid leukemia: state of the art and future directions. Front Oncol 10:697
Klingemann H (2014) Are natural killer cells superior CAR drivers? OncoImmunology 3(4):e28147
Morvan MG, Lanier LL (2016) NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer 16(1):7–19
Hope KJ, Jin L, Dick JE (2004) Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol 5(7):738–743
Sutherland H, Blair A, Zapf R (1996) Characterization of a hierarchy in human acute myeloid leukemia progenitor cells. Blood 87(11):4754–4761
Hu Y, Tian Z-G, Zhang C (2018) Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy. Acta Pharmacol Sin 39(2):167–76
Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R et al (2020) Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med 382(6):545–553
Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J (2020) CAR-NK cells: a promising cellular immunotherapy for cancer. eBioMedicine, 59
Naeimi Kararoudi M, Likhite S, Elmas E, Schwartz M, Sorathia K, Yamamoto K et al (2020) CD33 targeting primary CAR-NK cells generated by CRISPR mediated gene insertion show enhanced anti-AML activity. Blood 136(Supplement 1):3
Hauswirth AW, Florian S, Printz D, Sotlar K, Krauth M-T, Fritsch G et al (2007) Expression of the target receptor CD33 in CD34+/CD38−/CD123+ AML stem cells. Eur J Clin Invest 37(1):73–82
Sperr WR, Florian S, Hauswirth AW, Valent P (2005) CD33 as a target of therapy in acute myeloid leukemia: current status and future perspectives. Leuk Lymphoma 46(8):1115–1120
Sievers EL, Appelbaum FR, Spielberger RT, Forman SJ, Flowers D, Smith FO et al (1999) Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: a phase I study of an Anti-CD33 calicheamicin immunoconjugate: presented in part at the 1997 annual meeting of the American Society of clinical oncology, Denver, CO; the 1997 European cancer conference, hamburg, germany; and the 1997 Annual meeting of the American Society of hematology, San Diego, CA. Blood 93(11):3678–3684
Schirrmann T, Pecher G (2005) Specific targeting of CD33+ leukemia cells by a natural killer cell line modified with a chimeric receptor. Leuk Res 29(3):301–306
Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu T et al (2018) First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res 8(6):1083–1089
Salman H, Pinz KG, Wada M, Shuai X, Yan LE, Petrov JC et al (2019) Preclinical targeting of human acute myeloid leukemia using CD4-specific chimeric antigen receptor (CAR) T cells and NK cells. J Cancer 10(18):4408–4419
Albinger N, Hartmann J, Ullrich E (2021) Current status and perspective of CAR-T and CAR-NK cell therapy trials in Germany. Gene Therapy
Marofi F, Rahman HS, Thangavelu L, Dorofeev A, Bayas-Morejón F, Shirafkan N et al (2021) Renaissance of armored immune effector cells, CAR-NK cells, brings the higher hope for successful cancer therapy. Stem Cell Res Ther 12(1):200
Basar R, Daher M, Rezvani K (2020) Next-generation cell therapies: the emerging role of CAR-NK cells. Blood Adv 4(22):5868–5876
Kloess S, Oberschmidt O, Dahlke J, Vu XK, Neudoerfl C, Kloos A et al (2019) Preclinical assessment of suitable natural killer cell sources for chimeric antigen receptor natural killer-based “Off-the-Shelf” acute myeloid leukemia immunotherapies. Hum Gene Ther 30(4):381–401
Feldman EJ, Brandwein J, Stone R, Kalaycio M, Moore J, O’Connor J et al (2005) Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia. J Clin Oncol 23(18):4110–4116
He SZ, Busfield S, Ritchie DS, Hertzberg MS, Durrant S, Lewis ID et al (2015) A Phase 1 study of the safety, pharmacokinetics and anti-leukemic activity of the anti-CD123 monoclonal antibody CSL360 in relapsed, refractory or high-risk acute myeloid leukemia. Leuk Lymphoma 56(5):1406–1415
Kayser S, Heitmann JS, Dörfel D, Thol F, Heuser M, Märklin M et al (2019) Interim results of a first in man study with the Fc-optimized FLT3 antibody flysyn for treatment of acute myeloid leukemia with minimal residual disease. Blood 134:3928
Riether C, Pabst T, Höpner S, Bacher U, Hinterbrandner M, Banz Y et al (2020) Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents. Nat Med 26(9):1459–1467
Ågerstam H, Karlsson C, Hansen N, Sandén C, Askmyr M, Palffy SV et al (2015) Antibodies targeting human IL1RAP (IL1R3) show therapeutic effects in xenograft models of acute myeloid leukemia. Proc Natl Acad Sci 112(34):10786–91
Koerner SP, André MC, Leibold JS, Kousis PC, Kübler A, Pal M et al (2017) An Fc-optimized CD133 antibody for induction of NK cell reactivity against myeloid leukemia. Leukemia 31(2):459–469
Vasu S, He S, Cheney C, Gopalakrishnan B, Mani R, Lozanski G et al (2016) Decitabine enhances anti-CD33 monoclonal antibody BI 836858–mediated natural killer ADCC against AML blasts. Blood 127(23):2879–2889
Venditti A, Buccisano F, Maurillo L, Del Principe MI, Coppola A, Palomba P et al (2015) MEN1112/OBT357, an anti Bst1/CD157 humanized antibody inducing acute myelogenous leukemia (AML) blast depletion in an autologous ex vivo assay: a potential new targeted therapy for AML. Blood 126(23):788
Krupka C, Lichtenegger FS, Köhnke T, Bögeholz J, Bücklein V, Roiss M et al (2017) Targeting CD157 in AML using a novel. Fc-Eng Antibody Const Oncotarget 8(22):35707–35717
Mani R, Rajgolikar G, Nunes J, Zapolnik K, Wasmuth R, Mo X et al (2020) Fc-engineered anti-CD33 monoclonal antibody potentiates cytotoxicity of membrane-bound interleukin-21 expanded natural killer cells in acute myeloid leukemia. Cytotherapy 22(7):369–376
Daver NG, Erba HP, Papadantonakis N, DeAngelo DJ, Wang ES, Konopleva MY et al (2018) A phase I, first-in-human study evaluating the safety and preliminary antileukemia activity of IMGN632, a novel CD123-targeting antibody-drug conjugate, in patients with relapsed/refractory acute myeloid leukemia and other CD123-positive hematologic malignancies. Blood 132:27
Jurcic JG, Ravandi F, Pagel JM, Park JH, Smith BD, Douer D et al (2015) Phase I trial of α-particle therapy with actinium-225 (225Ac)-lintuzumab (anti-CD33) and low-dose cytarabine (LDAC) in older patients with untreated acute myeloid leukemia (AML). J Clin Oncol 33(15_suppl):7050
Narayan R, Blonquist TM, Emadi A, Hasserjian RP, Burke M, Lescinskas C et al (2020) A phase 1 study of the antibody-drug conjugate brentuximab vedotin with re-induction chemotherapy in patients with CD30-expressing relapsed/refractory acute myeloid leukemia. Cancer 126(6):1264–1273
Castaigne S, Pautas C, Terré C, Raffoux E, Bordessoule D, Bastie J-N et al (2012) Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet 379(9825):1508–1516
Taksin AL, Legrand O, Raffoux E, de Revel T, Thomas X, Contentin N et al (2007) High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia 21(1):66–71
Anami Y, Deng M, Gui X, Yamaguchi A, Yamazaki CM, Zhang N et al (2020) LILRB4-targeting antibody-drug conjugates for the treatment of acute myeloid leukemia. Mol Cancer Ther 19(11):2330–2339
Jiang Y-P, Liu BY, Zheng Q, Panuganti S, Chen R, Zhu J et al (2018) CLT030, a leukemic stem cell–targeting CLL1 antibody-drug conjugate for treatment of acute myeloid leukemia. Blood Adv 2(14):1738–1749
Kovtun Y, Noordhuis P, Whiteman KR, Watkins K, Jones GE, Harvey L et al (2018) IMGN779, a novel CD33-targeting antibody-drug conjugate with DNA-alkylating activity, exhibits potent antitumor activity in models of AML. Mol Cancer Ther 17(6):1271–1279
Pereira DS, Guevara CI, Jin L, Mbong N, Verlinsky A, Hsu SJ et al (2015) AGS67E, an Anti-CD37 monomethyl Auristatin E antibody-drug conjugate as a potential therapeutic for B/T-cell malignancies and AML: a new role for CD37 in AML. Mol Cancer Ther 14(7):1650–1660
Zheng B, Yu S-F, del Rosario G, Leong SR, Lee GY, Vij R et al (2019) An anti–CLL-1 antibody-drug conjugate for the treatment of acute myeloid leukemia. Clin Cancer Res 25(4):1358–1368
Khan M, Arooj S, Wang H (2020) NK Cell-Based Immune Checkpoint Inhibition. Frontiers in Immunology, 11
Vey N, Bourhis J-H, Boissel N, Bordessoule D, Prebet T, Charbonnier A et al (2012) A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood 120(22):4317–4323
Vey N, Bourhis J-H, Recher C, Etienne A, Charbonnier A, Andre P et al (2013) Repeated dosing Of anti-KIR (IPH2101) as maintenance therapy in ederly patients with acute myeloid leukemia. Blood 122(21):2696
Vey N, Dumas P-Y, Recher C, Gastaud L, Lioure B, Bulabois C-E et al (2017) Randomized phase 2 trial of lirilumab (anti-KIR monoclonal antibody, mAb) as maintenance treatment in elderly patients (pts) with acute myeloid leukemia (AML): results of the effikir trial. Blood 130:889
Daver N, Boddu P, Garcia-Manero G, Ravandi F, Jabbour EJ, Borthakur G et al (2017) Phase IB/II study of lirilumab with azacytidine (AZA) in relapsed AML. Blood 130:2634
Godal R, Bachanova V, Gleason M, McCullar V, Yun GH, Cooley S et al (2010) Natural killer cell killing of acute myelogenous leukemia and acute lymphoblastic leukemia blasts by killer cell immunoglobulin-like receptor–negative natural killer cells after NKG2A and LIR-1 blockade. Biol Blood Marrow Transplant 16(5):612–621
Loredana R, Elena U, Pascale A, Antonella M, Antonella T, Fabiana T et al (2016) Effects of anti-NKG2A antibody administration on leukemia and normal hematopoietic cells. Haematologica 101(5):626–633
Gleason MK, Verneris MR, Todhunter DA, Zhang B, McCullar V, Zhou SX et al (2012) Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol Cancer Ther 11(12):2674–2684
Wiernik A, Foley B, Zhang B, Verneris MR, Warlick E, Gleason MK et al (2013) Targeting natural killer cells to acute myeloid leukemia in vitro with a CD16 × 33 bispecific killer cell engager and ADAM17 inhibition. Clin Cancer Res 19(14):3844–3855
Vallera DA, Felices M, McElmurry R, McCullar V, Zhou X, Schmohl JU et al (2016) IL15 trispecific killer engagers (TriKE) make natural killer cells specific to CD33+ targets while also inducing persistence, in vivo expansion, and enhanced function. Clin Cancer Res 22(14):3440–3450
Kügler M, Stein C, Kellner C, Mentz K, Saul D, Schwenkert M et al (2010) A recombinant trispecific single-chain Fv derivative directed against CD123 and CD33 mediates effective elimination of acute myeloid leukaemia cells by dual targeting. Br J Haematol 150(5):574–586
Singer H, Kellner C, Lanig H, Aigner M, Stockmeyer B, Oduncu F et al (2010) Effective elimination of acute myeloid leukemic cells by recombinant bispecific antibody derivatives directed against CD33 and CD16. J Immunother 33(6):599–608
Märklin M, Hagelstein I, Koerner SP, Rothfelder K, Pfluegler MS, Schumacher A et al (2019) Bispecific NKG2D-CD3 and NKG2D-CD16 fusion proteins for induction of NK and T cell reactivity against acute myeloid leukemia. J Immunother Cancer 7(1):143
Arvindam US, van Hauten PMM, Schirm D, Schaap N, Hobo W, Blazar BR et al (2021) A trispecific killer engager molecule against CLEC12A effectively induces NK-cell mediated killing of AML cells. Leukemia 35(6):1586–1596
Sanchez-Correa B, Bergua JM, Pera A, Campos C, Arcos MJ, Bañas H, et al. (2017) In vitro culture with interleukin-15 leads to expression of activating receptors and recovery of natural killer cell function in acute myeloid leukemia patients. Front Immunol, 8
Baer MR, George SL, Caligiuri MA, Sanford BL, Bothun SM, Mrózek K et al (2008) Low-dose interleukin-2 immunotherapy does not improve outcome of patients age 60 years and older with acute myeloid leukemia in first complete remission: cancer and leukemia group b study 9720. J Clin Oncol 26(30):4934–4939
Pautas C, Merabet F, Thomas X, Raffoux E, Gardin C, Corm S et al (2010) Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol 28(5):808–814
Petit A, Ducassou S, Leblanc T, Pasquet M, Rousseau A, Ragu C et al (2018) Maintenance therapy with interleukin-2 for childhood AML: results of ELAM02 phase III randomized trial. Hemasphere 2(6):e159-e
Brune M, Castaigne S, Catalano J, Gehlsen K, Ho AD, Hofmann W-K et al (2006) Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood 108(1):88–96
Nilsson MS, Hallner A, Brune M, Nilsson S, Thorén FB, Martner A et al (2020) Immunotherapy with HDC/IL-2 may be clinically efficacious in acute myeloid leukemia of normal karyotype. Hum Vaccin Immunother 16(1):109–111
Cuapio A, Post M, Cerny-Reiterer S, Gleixner KV, Stefanzl G, Basilio J et al (2016) Maintenance therapy with histamine plus IL-2 induces a striking expansion of two CD56 bright NK cell subpopulations in patients with acute myeloid leukemia and supports their activation. Oncotarget 7:29
Rosario M, Romee R, Schneider SE, Leong JW, Sullivan RP, Fehniger TA (2014) Human cytokine-induced memory-like (CIML) NK cells are active against myeloid leukemia in vitro and in vivo. Blood 124(21):1117
Berrien-Elliott MM, Cashen AF, Cubitt CC, Neal CC, Wong P, Wagner JA et al (2020) Multidimensional analyses of donor memory-like NK cells reveal new associations with response after adoptive immunotherapy for leukemia. Cancer Discov 10(12):1854–1871
Bednarski JJ, Zimmerman C, Cashen AF, Desai S, Foster M, Schappe T et al (2019) Adoptively transferred donor-derived cytokine induced memory-like NK cells persist and induce remission in pediatric patient with relapsed acute myeloid leukemia after hematopoietic cell transplantation. Blood 134(Supplement_1):3307
Foltz JA, Berrien-Elliott MM, Neal C, Foster M, McClain E, Schappe T, et al. (2019) Cytokine-induced memory-like (ML) NK cells persist for > 2 months following adoptive transfer into leukemia patients with a MHC-compatible hematopoietic cell transplant (HCT). Blood, 134(Supplement1), 1954
Mastaglio S, Wong E, Perera T, Ripley J, Blombery P, Smyth MJ et al (2018) Natural killer receptor ligand expression on acute myeloid leukemia impacts survival and relapse after chemotherapy. Blood Adv 2(4):335–346
Rohner A, Langenkamp U, Siegler U, Kalberer CP, Wodnar-Filipowicz A (2007) Differentiation-promoting drugs up-regulate NKG2D ligand expression and enhance the susceptibility of acute myeloid leukemia cells to natural killer cell-mediated lysis. Leuk Res 31(10):1393–1402
Baragaño Raneros A, Minguela A, Rodriguez RM, Colado E, Bernal T, Anguita E et al (2017) Increasing TIMP3 expression by hypomethylating agents diminishes soluble MICA, MICB and ULBP2 shedding in acute myeloid leukemia, facilitating NK cell-mediated immune recognition. Oncotarget 8:19
Diermayr S, Himmelreich H, Durovic B, Mathys-Schneeberger A, Siegler U, Langenkamp U et al (2008) NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK-cell lines with single KIR-HLA class I specificities. Blood 111(3):1428–1436
Poggi A, Catellani S, Garuti A, Pierri I, Gobbi M, Zocchi MR (2009) Effective in vivo induction of NKG2D ligands in acute myeloid leukaemias by all-trans-retinoic acid or sodium valproate. Leukemia 23(4):641–648
Lu X, Ohata K, Kondo Y, Luis Espinoza J, Qi Z, Nakao S (2010) Hydroxyurea upregulates NKG2D ligand expression in myeloid leukemia cells synergistically with valproic acid and potentially enhances susceptibility of leukemic cells to natural killer cell-mediated cytolysis. Cancer Sci 101(3):609–615
Le Roy A, Prébet T, Castellano R, Goubard A, Riccardi F, Fauriat C, et al. (2018) Immunomodulatory drugs exert anti-leukemia effects in acute myeloid leukemia by direct and immunostimulatory activities. Front Immunol, 9
Lai K-C, Lu H-F, Chen K-B, Hsueh S-C, Chung J-G, Huang W-W et al (2019) Casticin promotes immune responses, enhances macrophage and NK cell activities, and increases survival rates of leukemia BALB/c mice. Am J Chin Med 47(01):223–236
Yu F-S, Yang J-S, Yu C-S, Chiang J-H, Lu C-C, Chung H-K et al (2013) Safrole suppresses murine myelomonocytic leukemia WEHI-3 cells in vivo, and stimulates macrophage phagocytosis and natural killer cell cytotoxicity in leukemic mice. Environ Toxicol 28(11):601–608
Shih Y-L, Shang H-S, Chen Y-L, Hsueh S-C, Chou H-M, Lu H-F et al (2019) Ouabain promotes immune responses in WEHI-3 cells to generate leukemia mice through enhancing phagocytosis and natural killer cell activities in vivo. Environ Toxicol 34(5):659–665
Lin J-J, Lu K-W, Ma Y-S, Tang N-Y, Wu P-P, Wu C-C et al (2014) Alpha-phellandrene, a natural active monoterpene, influences a murine WEHI-3 leukemia model in vivo by enhancing macrophague phagocytosis and natural killer cell activity. In Vivo 28(4):583–588
Lee JY, Park S, Kim DC, Yoon J-H, Shin SH, Min W-S et al (2013) A VEGFR-3 antagonist increases IFN-γ expression on low functioning NK cells in acute myeloid leukemia. J Clin Immunol 33(4):826–837
Lee JY, Park S, Min W-S, Kim H-J (2014) Restoration of natural killer cell cytotoxicity by VEGFR-3 inhibition in myelogenous leukemia. Cancer Lett 354(2):281–289
Otegbeye F, Ojo E, Moreton S, Mackowski N, Lee DA, de Lima M et al (2018) Inhibiting TGF-beta signaling preserves the function of highly activated, in vitro expanded natural killer cells in AML and colon cancer models. PLoS ONE 13(1):e0191358
Acknowledgements
Not applicable.
Funding
The authors did not receive fund, grant, and support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
SR conceptualized the title, collected the data, designed the figures, and prepared the first draft of the manuscript. NY edited and revised the manuscript and finalized the draft. NR critically revised the manuscript, edited and finalized the draft, and supervised the project. All the authors have read and approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rahmani, S., Yazdanpanah, N. & Rezaei, N. Natural killer cells and acute myeloid leukemia: promises and challenges. Cancer Immunol Immunother 71, 2849–2867 (2022). https://doi.org/10.1007/s00262-022-03217-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03217-1