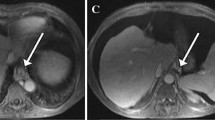
Abstract
Purpose
To assess and compare the performance of liver surface nodularity (LSN) quantification using Gd-BOPTA-enhanced MRI and contrast-enhanced CT for the diagnosis of clinically significant portal hypertension (CSPH) in patients with cirrhosis.
Methods
This retrospective study included 30 patients with compensated histologically proven cirrhosis who underwent hepatic venous pressure gradient (HVPG), abdominal CT and Gd-BOPTA-MRI within a 60-day interval during pre-surgery workup for hepatocellular carcinoma (HCC) between January 2016 and August 2018. LSN score was derived from CT portal venous phase (PVP), axial T2- and T1-weighted PVP and hepatobiliary phase (HBP). Accuracy for the detection of CSPH was evaluated for each set of images by ROC curve analysis. Intra-observer, inter-observer and inter-method reproducibilities were assessed by the intraclass correlation coefficient (ICC) and coefficient of variation (CV).
Results
Thirty patients were analysed (23 men [77%], mean age 60 ± 11 years old), including 15 (50%) with CSPH. All CT- and MRI-derived LSN quantifications were correlated to HVPG (CT-PVP: r = 0.63, p = 0.001, AUROC = 0.908 ± 0.06; T1-w-PVP: r = 0.43, p = 0.028, AUROC = 0.876 ± 0.07; T1-w-HBP: r = 0.50, p = 0.012, AUROC = 0.823 ± 0.08; T2-w: r = 0.51, p = 0.007, AUROC = 0.801 ± 0.09). There was no significant difference in AUROC pairwise comparisons (p = 0.12–0.88). Patients with CSPH had higher LSN than those without (CT-PVP: 3.2 ± 0.6 vs 2.4 ± 0.5, p < 0.001; T1-w-PVP: 2.7 ± 0.4 vs 2.2 ± 0.4, p = 0.002; T1-w-HBP: 3.0 ± 0.6 vs 2.3 ± 0.3, p < 0.001; T2-w: 3.0 ± 0.6 vs 2.2 ± 0.3, p = 0.001) and 86%, 82%, 85% and 82% of patients were correctly classified, respectively. Reproducibility of inter-image set comparisons was excellent (ICC = 0.84–0.96 and CV = 8.3–14.2%).
Conclusion
The diagnostic performance of MRI-based LSN for detecting CSPH is strong and similar to that of CT-based LSN.



Similar content being viewed by others
References
Berzigotti A, Reig M, Abraldes JG, Bosch J, Bruix J (2015) Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology 61(2):526-536. https://doi.org/10.1002/hep.27431.
Liu J, Zhang H, Xia Y, et al. (2019) Impact of clinically significant portal hypertension on outcomes after partial hepatectomy for hepatocellular carcinoma: a systematic review and meta-analysis. HPB 21(1):1-13. https://doi.org/10.1016/j.hpb.2018.07.005.
De Franchis R (2000) Updating consensus in portal hypertension: report of the Baveno III Consensus Workshop on definitions, methodology and therapeutic strategies in portal hypertension. J. Hepatol. 33(5):846-822.
Cucchetti A, Cescon M, Golfieri R, et al. (2016) Hepatic venous pressure gradient in the preoperative assessment of patients with resectable hepatocellular carcinoma. J. Hepatol. 64:79-86. https://doi.org/10.1016/j.jhep.2015.08.025.
Augustin S, Millan L, Gonzalez A, et al. (2014) Detection of early portal hypertension with routine data and liver stiffness in patients with asymptomatic liver disease: a prospective study. J. Hepatol. 60(3):561-569. https://doi.org/10.1016/j.jhep.2013.10.027.
de Franchis R, Baveno VI Faculty (2015) Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 63(3):743-752. https://doi.org/10.1016/j.jhep.2015.05.022.
Elkrief L, Ronot M, Andrade F, et al. (2018) Non-invasive evaluation of portal hypertension using shear-wave elastography: analysis of two algorithms combining liver and spleen stiffness in 191 patients with cirrhosis. Aliment. Pharmacol. Ther. 47(5):621-630. https://doi.org/10.1111/apt.14488.
Motosugi U, Roldán-Alzate A, Bannas P, et al. (2019) Four-dimensional Flow MRI as a Marker for Risk Stratification of Gastroesophageal Varices in Patients with Liver Cirrhosis. Radiology 290(1):101-107. https://doi.org/10.1148/radiol.2018180230.
Gouya H, Grabar S, Vignaux O, et al. (2016) Portal hypertension in patients with cirrhosis: indirect assessment of hepatic venous pressure gradient by measuring azygos flow with 2D-cine phase-contrast magnetic resonance imaging. Eur. Radiol. 26(7):1981-1990. https://doi.org/10.1007/s00330-015-3991-x.
Ronot M, Lambert S, Elkrief L, et al. (2014) Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. Eur. Radiol.24(6):1394-1402. https://doi.org/10.1007/s00330-014-3124-y.
Smith AD, Branch CR, Zand K, et al. (2016) Liver Surface Nodularity Quantification from Routine CT Images as a Biomarker for Detection and Evaluation of Cirrhosis. Radiology 280(3):771-81. https://doi.org/10.1148/radiol.2016151542.
) Pickhardt PJ, Malecki K, Kloke J, Lubner MG (2016) Accuracy of Liver Surface Nodularity Quantification on MDCT as a Noninvasive Biomarker for Staging Hepatic Fibrosis. AJR Am. J. Roentgenol. 207(6):1194-1199. https://doi.org/10.2214/ajr.16.16514
Pickhardt PJ, Graffy PM, Said A, et al. (2019) Multiparametric CT for Noninvasive Staging of Hepatitis C Virus-Related Liver Fibrosis: Correlation with the histopathologic Fibrosis score. AJR Am. J. Roentgenol. 212(3):547-553. https://doi.org/10.2214/AJR.18.20284.
Sartoris R, Rautou PE, Elkrief L, et al. (2018) Quantification of Liver Surface Nodularity at CT: Utility for Detection of Portal Hypertension. Radiology 289(3):698-707. https://doi.org/10.1148/radiol.2018181131.
Lo GC, Besa C, King M, et al. (2017) Feasibility and reproducibility of liver surface nodularity quantification for the assessment of liver cirrhosis using CT and MRI. Eur J Radiol Open 21(4):95-100. https://doi.org/10.1016/j.ejro.2017.07.001.
Neri E, Bali MA, Ba-Ssalamah A, et al. (2016) ESGAR consensus statement on liver MR imaging and clinical use of liver-specific contrast agents. Eur. Radiol. 26(4):921-931. https://doi.org/10.1007/s00330-015-3900-3.
Lubner MG, Jones D, Said A, et al. (2018) Accuracy of liver surface nodularity quantification on MDCT for staging hepatic fibrosis in patients with hepatitis C virus. Abdom. Radiol. 43(11):2980-2986. https://doi.org/10.1016/j.gtc.2018.04.012.
Sethasine S, Jain D, Groszmann RJ, Garcia-Tsao G. (2012) Quantitative histological-hemodynamic correlations in cirrhosis. Hepatology 55(4):1146-1153. https://doi.org/10.1002/hep.24805.
Besa C, Wagner M, Lo G, et al. (2018) Detection of liver fibrosis using qualitative and quantitative MR elastography compared to liver surface nodularity measurement, gadoxetic acid uptake, and serum markers. J. Magn. Reson. Imaging 47(6):1552-1561. https://doi.org/10.1002/jmri.25911.
Khouri Chalouhi C, Vernuccio F, Rini F, et al. (2019) Hepatobiliary phase in cirrhotic patients with different Model for End-stage Liver Disease score: comparison of the performance of gadoxetic acid to gadobenate dimeglumine. Eur. Radiol. 29(6):3090-3099. https://doi.org/10.1007/s00330-018-5884-2.
Ciolina M, Di Martino M, Bruno O, et al. (2018) Peritoneal and pleural fluids may appear hyperintense on hepatobiliary phase using hepatobiliary MR contrast agents. Eur. Radiol. 28(7)3020-3031. https://doi.org/10.1007/s00330-017-5261-6.
Bonatti M, Valletta R, Zamboni G, et al. (2019) Ascites relative enhancement during hepatobiliary phase after Gd-BOPTA administration: a new promising tool for characterising abdominal free fluid of unknown origin. Eur. Radiol. 29(6):2830-2836. https://doi.org/10.1007/s00330-018-5932-y.
Smith AD, Zand KA, Florez E, et al. (2017) Liver Surface Nodularity Score Allows Prediction of Cirrhosis Decompensation and Death. Radiology, 283(3)711-722. https://doi.org/10.1148/radiol.2016160799
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
De Vos, N., Sartoris, R., Cauchy, F. et al. Performance of liver surface nodularity quantification for the diagnosis of portal hypertension in patients with cirrhosis: comparison between MRI with hepatobiliary phase sequences and CT. Abdom Radiol 45, 365–372 (2020). https://doi.org/10.1007/s00261-019-02355-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-02355-y