Abstract
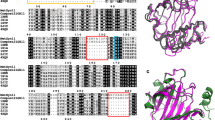
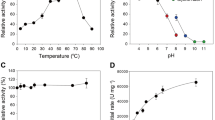
Lignocellulose feedstock constitutes the most abundant carbon source in the biosphere; however, its recalcitrance remains a challenge for microbial conversion into biofuel and bioproducts. Bacillus licheniformis is a microbial mesophilic bacterium capable of secreting a large number of glycoside hydrolase (GH) enzymes, including a glycoside hydrolase from GH family 9 (BlCel9). Here, we conducted biochemical and biophysical studies of recombinant BlCel9, and its low-resolution molecular shape was retrieved from small angle X-ray scattering (SAXS) data. BlCel9 is an endoglucanase exhibiting maximum catalytic efficiency at pH 7.0 and 60 °C. Furthermore, it retains 80% of catalytic activity within a broad range of pH values (5.5–8.5) and temperatures (up to 50 °C) for extended periods of time (over 48 h). It exhibits the highest hydrolytic activity against phosphoric acid swollen cellulose (PASC), followed by bacterial cellulose (BC), filter paper (FP), and to a lesser extent carboxymethylcellulose (CMC). The HPAEC-PAD analysis of the hydrolytic products demonstrated that the end product of the enzymatic hydrolysis is primarily cellobiose, and also small amounts of glucose, cellotriose, and cellotetraose are produced. SAXS data analysis revealed that the enzyme adopts a monomeric state in solution and has a molecular mass of 65.8 kDa as estimated from SAXS data. The BlCel9 has an elongated shape composed of an N-terminal family 3 carbohydrate-binding module (CBM3c) and a C-terminal GH9 catalytic domain joined together by 20 amino acid residue long linker peptides. The domains are closely juxtaposed in an extended conformation and form a relatively rigid structure in solution, indicating that the interactions between the CBM3c and GH9 catalytic domains might play a key role in cooperative cellulose biomass recognition and hydrolysis.





Similar content being viewed by others
References
de Araújo EA, Manzine LR, Piyadov V, Kadowaki MAS, Polikarpov I (2017) Biochemical characterization, low-resolution SAXS structure and an enzymatic cleavage pattern of BlCel48 from Bacillus licheniformis. Int J Biol Macromol 111:302–310. https://doi.org/10.1016/J.IJBIOMAC.2017.12.138
Artzi L, Bayer EA, Moraïs S (2016) Cellulosomes: bacterial nanomachines for dismantling plant polysaccharides. Nat Rev Microbiol 15:83–95. https://doi.org/10.1038/nrmicro.2016.164
Badalato N, Guillot A, Sabarly V, Dubois M, Pourette N, Pontoire Bruno, Robert P, Bridier A, Monnet V, Sousa DZ, Durand S, Mazéas L, Buléon A, Bouchez T, Mortha G, Bize A, Yang Shihui S (2017) Whole proteome analyses on Ruminiclostridium cellulolyticum show a modulation of the cellulolysis machinery in response to cellulosic materials with subtle differences in chemical and structural properties. PLOS ONE 12:1–22. https://doi.org/10.1371/journal.pone.0170524
Batista PR, Costa MG de S, Pascutti PG, Bisch PM, de Souza W (2011) High temperatures enhance cooperative motions between CBM and catalytic domains of a thermostable cellulase: mechanism insights from essential dynamics. Phys Chem Chem Phys 13:13709–13720. https://doi.org/10.1039/c0cp02697b
Berlemont R, Martiny AC (2015) Genomic potential for polysaccharide deconstruction in Bacteria. Appl Environ Microbiol 81:1513–1519. https://doi.org/10.1128/AEM.03718-14
Bhattacharya AS, Bhattacharya A, Pletschke BI (2015) Synergism of fungal and bacterial cellulases and hemicellulases: a novel perspective for enhanced bio-ethanol production. Biotechnol Lett 37:1117–1129. https://doi.org/10.1007/s10529-015-1779-3
Brunecky R, Alahuhta M, Xu Q, Donohoe BS, Crowley MF, Kataeva IA, Yang S-J, Resch MG, Adams MWW, Lunin VV, Himmel ME, Bomble YJ (2013) Revealing nature’s cellulase diversity: the digestion mechanism of Caldicellulosiruptor bescii CelA. Science 342:1513–1516. https://doi.org/10.1126/science.1244273
Brunecky R, Donohoe BS, Yarbrough JM, Mittal A, Scott BR, Ding H, Taylor LE II, Russell JF, Chung D, Westpheling J, Teter SA, Himmel ME, Bomble YJ (2017) The multi domain Caldicellulosiruptor bescii CelA cellulase excels at the hydrolysis of crystalline cellulose. Sci Rep 7:9622. https://doi.org/10.1038/s41598-017-08985-w
Burger VM, Arenas DJ, Stultz CM (2016) A structure-free method for quantifying conformational flexibility in proteins. Sci Rep 6:29040. https://doi.org/10.1038/srep29040
Burstein T, Shulman M, Jindou S, Petkun S, Frolow F, Shoham Y, Bayer EA, Lamed R (2009) Physical association of the catalytic and helper modules of a family-9 glycoside hydrolase is essential for activity. FEBS Lett 583:879–884. https://doi.org/10.1016/j.febslet.2009.02.013
Camilo CM, Polikarpov I (2014) High-throughput cloning, expression and purification of glycoside hydrolases using ligation-independent cloning (LIC). Protein Expr Purif 99C:35–42. https://doi.org/10.1016/j.pep.2014.03.008
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The carbohydrate-active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res 37:D233–D238. https://doi.org/10.1093/nar/gkn663
Chen Y, Stipanovic AJ, Winter WT, Wilson DB, Kim Y-J (2007) Effect of digestion by pure cellulases on crystallinity and average chain length for bacterial and microcrystalline celluloses. Cellulose 14:283–293. https://doi.org/10.1007/s10570-007-9115-2
Cosgrove DJ (2014) Re-constructing our models of cellulose and primary cell wall assembly. Curr Opin Plant Biol 22:122–131. https://doi.org/10.1016/j.pbi.2014.11.001
Cosgrove DJ, Jarvis MC (2012) Comparative structure and biomechanics of plant primary and secondary cell walls. Front Plant Sci 3:204. https://doi.org/10.3389/fpls.2012.00204
Dassa B, Borovok I, Lombard V, Henrissat B, Lamed R, Bayer E, Moraïs S (2017) Pan-cellulosomics of mesophilic Clostridia: variations on a theme. Microorganisms 5:74. https://doi.org/10.3390/microorganisms5040074
Davison A, Blaxter M (2005) Ancient origin of glycosyl hydrolase family 9 cellulase genes. Mol Biol Evol 22:1273–1284. https://doi.org/10.1093/molbev/msi107
Ding S-Y, Liu Y-S, Zeng Y, Himmel ME, Baker JO, Bayer EA (2012) How does plant cell wall nanoscale architecture correlate with enzymatic digestibility? Science 338:1055–1060. https://doi.org/10.1126/science.1227491
Donohoe BS, Resch MG (2015) Mechanisms employed by cellulase systems to gain access through the complex architecture of lignocellulosic substrates. Curr Opin Chem Biol 29:100–107. https://doi.org/10.1016/j.cbpa.2015.08.014
Eckert K, Vigouroux A, Lo Leggio L, Moréra S (2009) Crystal structures of A. acidocaldarius endoglucanase Cel9A in complex with cello-oligosaccharides: strong −1 and −2 subsites mimic cellobiohydrolase activity. J Mol Biol 394:61–70. https://doi.org/10.1016/j.jmb.2009.08.060
El Kaoutari A, Armougom F, Gordon JI, Raoult D, Henrissat B (2013) The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol 11:497–504. https://doi.org/10.1038/nrmicro3050
Elshaghabee FMF, Rokana N, Gulhane RD, Sharma C, Panwar H (2017) Bacillus as potential probiotics: status, concerns, and future perspectives. Front Microbiol 8:1490. https://doi.org/10.3389/fmicb.2017.01490
Erickson RJ (1976) Industrial applications of the bacilli: a review and prospectus. Microbiol Am Soc Microbiol Washington, DC 406–419
Evangelista DE, De Araújo EA, Oliveira Neto M, Antonio M, Kadowaki S, Polikarpov I (2017) Biochemical characterization and low-resolution SAXS structure of an exo- polygalacturonase from Bacillus licheniformis. New Biotechnol 40:268–274. https://doi.org/10.1016/j.nbt.2017.10.001
Fischer H, de Oliveira Neto M, Napolitano HB, Polikarpov I, Craievich AF (2010) Determination of the molecular weight of proteins in solution from a single small-angle X-ray scattering measurement on a relative scale. J Appl Crystallogr 43:101–109. https://doi.org/10.1107/S0021889809043076
Franke D, Svergun DI (2009) DAMMIF , a program for rapid ab-initio shape determination in small-angle scattering. J Appl Crystallogr 42:342–346. https://doi.org/10.1107/S0021889809000338
Gal L, Gaudin C, Belaich A, Pages S, Tardif C, Belaich JP (1997) CelG from Clostridium cellulolyticum: a multidomain endoglucanase acting efficiently on crystalline cellulose. J Bacteriol 179:6595–6601. https://doi.org/10.1128/JB.179.21.6595-6601.1997
Ghani M, Ansari A, Aman A, Zohra RR, Siddiqui NN, Qader SAU (2013) Isolation and characterization of different strains of Bacillus licheniformis for the production of commercially significant enzymes. Pak J Pharm Sci 26:691–697
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268. https://doi.org/10.1351/pac198759020257
Gilad R, Rabinovich L, Yaron S, Bayer EA, Lamed R, Gilbert HJ, Shoham Y (2003) CelI, a noncellulosomal family 9 enzyme from Clostridium thermocellum, is a processive endoglucanase that degrades crystalline cellulose. J Bacteriol 185:391–398. https://doi.org/10.1128/JB.185.2.391-398.2003
Gilbert HJ (2010) The biochemistry and structural biology of plant cell wall deconstruction. Plant Physiol 153:444–455. https://doi.org/10.1104/pp.110.156646
Gilbert HJ (2014) Developing novel enzyme repertoires for the efficient deconstruction of plant biomass tailored for the bioenergy industry. In: McCann M, Buckeridge M, Carpita N (eds) Plants and BioEnergy. Advances in plant biology, vol 4. Springer, New York, pp 197–209. https://doi.org/10.1007/978-1-4614-9329-7
Glatter O, Kratky O (1982) Small angle x-ray scattering. Academic Press, London, p 515
Guinier A (1939) La diffraction des rayons X aux très petits angles : application à l’étude de phénomènes ultramicroscopiques. Ann Phys (Paris) 11:161–237. https://doi.org/10.1051/anphys/193911120161
Hammel M (2012) Validation of macromolecular flexibility in solution by small-angle X-ray scattering (SAXS). Eur Biophys J 41:789–799. https://doi.org/10.1007/s00249-012-0820-x
Henrissat B, Davies G (1997) Structural and sequence-based classification of glycoside hydrolases. Curr Opin Struct Biol 7:637–644
Himmel ME, Ding S-Y, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315:804–807. https://doi.org/10.1126/science.1137016
Honda Y, Arai S, Suzuki K, Kitaoka M, Fushinobu S (2016) The crystal structure of an inverting glycoside hydrolase family 9 exo-β-D-glucosaminidase and the design of glycosynthase. Biochem J 473:463–472. https://doi.org/10.1042/BJ20150966
Igarashi K (2013) Cellulases: cooperative biomass breakdown. Nat Chem Biol 9:350–351. https://doi.org/10.1038/nchembio.1237
Irwin D, Shin D-H, Zhang S, Barr BK, Sakon J, Karplus PA, Wilson DB (1998) Roles of the catalytic domain and two cellulose binding domains of Thermomonospora fusca E4 in cellulose hydrolysis. J Bacteriol 180:1709–1714
Kafle K, Shin H, Lee CM, Park S, Kim SH (2015) Progressive structural changes of Avicel, bleached softwood, and bacterial cellulose during enzymatic hydrolysis. Sci Rep 5:15102. https://doi.org/10.1038/srep15102
Kataeva I, Li X-L, Chen H, Choi S-K, Ljungdahl LG (1999) Cloning and sequence analysis of a new cellulase gene encoding CelK, a major cellulosome component of Clostridium thermocellum: evidence for gene duplication and recombination. J Bacteriol 181:5288–5295
Kataeva IA, Brewer JM, Uversky VN, Ljungdahl LG (2005) Domain coupling in a multimodular cellobiohydrolase CbhA from Clostridium thermocellum. FEBS Lett 579:4367–4373. https://doi.org/10.1016/j.febslet.2005.06.074
Keegstra K (2010) Plant cell walls. Plant Physiol 154:483–486. https://doi.org/10.1104/pp.110.161240
Kim SJ, Joo JE, Jeon SD, Hyeon JE, Kim SW, Um YS, Han SO (2016) Enhanced thermostability of mesophilic endoglucanase Z with a high catalytic activity at active temperatures. Int J Biol Macromol 86:269–276. https://doi.org/10.1016/j.ijbiomac.2016.01.068
Klein-Marcuschamer D, Oleskowicz-Popiel P, Simmons BA, Blanch HW (2012) The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol Bioeng 109:1083–1087. https://doi.org/10.1002/bit.24370
Kostylev M, Wilson D (2014) A distinct model of synergism between a processive endocellulase (TfCel9A) and an exocellulase (TfCel48A) from Thermobifida fusca. Appl Environ Microbiol 80:339–344. https://doi.org/10.1128/AEM.02706-13
Kostylev M, Moran-Mirabal JM, Walker LP, Wilson DB (2012) Determination of the molecular states of the processive endocellulase Thermobifida fusca Cel9A during crystalline cellulose depolymerization. Biotechnol Bioeng 109:295–299. https://doi.org/10.1002/bit.23299
Kozin MB, Svergun DI (2001) Automated matching of high- and low-resolution structural models. J Appl Crystallogr 34:33–41
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess 4:7. https://doi.org/10.1186/s40643-017-0137-9
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Leis B, Held C, Bergkemper F, Dennemarck K, Steinbauer R, Reiter A, Mechelke M, Moerch M, Graubner S, Liebl W, Schwarz WH, Zverlov VV (2017) Comparative characterization of all cellulosomal cellulases from Clostridium thermocellum reveals high diversity in endoglucanase product formation essential for complex activity. Biotechnol Biofuels 10:240. https://doi.org/10.1186/s13068-017-0928-4
Li Y, Irwin DC, Wilson DB (2007) Processivity, substrate binding, and mechanism of cellulose hydrolysis by Thermobifida fusca Cel9A. Appl Environ Microbiol 73:3165–3172. https://doi.org/10.1128/AEM.02960-06
Liberato MV, Silveira RL, Prates ÉT, de Araujo EA, Pellegrini VOA, Camilo CM, Kadowaki MA, Neto M de O, Popov A, Skaf MS, Polikarpov I (2016) Molecular characterization of a family 5 glycoside hydrolase suggests an induced-fit enzymatic mechanism. Sci Rep 6:23473. https://doi.org/10.1038/srep23473
Liu Y, Zhang J, Liu Q, Zhang C, Ma Q (2004) Molecular cloning of novel cellulase genes cel9a and cel12a from Bacillus licheniformis GXN151 and synergism of their encoded polypeptides. Curr Microbiol 49:234–238. https://doi.org/10.1007/s00284-004-4291-x
Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B (2014) The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res 42:D490–D495. https://doi.org/10.1093/nar/gkt1178
Lopez-Contreras AM, Martens AA, Szijarto N, Mooibroek H, Claassen PAM, van der Oost J, de Vos WM (2003) Production by Clostridium acetobutylicum ATCC 824 of CelG, a cellulosomal glycoside hydrolase belonging to family 9. Appl Environ Microbiol 69:869–877. https://doi.org/10.1128/AEM.69.2.869-877.2003
López-Mondéjar R, Zühlke D, Becher D, Riedel K, Baldrian P (2016) Cellulose and hemicellulose decomposition by forest soil bacteria proceeds by the action of structurally variable enzymatic systems. Sci Rep 6:25279. https://doi.org/10.1038/srep25279
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577. https://doi.org/10.1128/MMBR.66.3.506-577.2002
Mandelman D, Belaich A, Belaich JP, Aghajari N, Driguez H, Haser R (2003) X-ray crystal structure of the multidomain endoglucanase Cel9G from Clostridium cellulolyticum complexed with natural and synthetic cello-oligosaccharides. J Bacteriol 185:4127–4135. https://doi.org/10.1128/JB.185.14.4127-4135.2003
Marriott PE, Gómez LD, McQueen-Mason SJ (2016) Unlocking the potential of lignocellulosic biomass through plant science. New Phytol 209:1366–1381. https://doi.org/10.1111/nph.13684
Master ER, Rudsander UJ, Zhou W, Henriksson H, Divne C, Denman S, Wilson DB, Teeri TT (2004) Recombinant expression and enzymatic characterization of PttCel9A, a KOR homologue from Populus tremula x tremuloides. Biochemistry 43:10080–10089. https://doi.org/10.1021/bi049453x
Okano H, Kanaya E, Ozaki M, Angkawidjaja C, Kanaya S (2015) Structure, activity, and stability of metagenome-derived glycoside hydrolase family 9 endoglucanase with an N-terminal Ig-like domain. Protein Sci 24:408–419. https://doi.org/10.1002/pro.2632
Oliveira OV, Freitas LCG, Straatsma TP, Lins RD (2009) Interaction between the CBM of Cel9A from Thermobifida fusca and cellulose fibers. J Mol Recognit 22:38–45. https://doi.org/10.1002/jmr.925
Parks TD, Leuther KK, Howard ED, Johnston SA, Dougherty WG (1994) Release of proteins and peptides from fusion proteins using a recombinant plant virus proteinase. Anal Biochem 216:413–417. https://doi.org/10.1006/abio.1994.1060
Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786. https://doi.org/10.1038/nmeth.1701
Petkun S, Rozman Grinberg I, Lamed R, Jindou S, Burstein T, Yaniv O, Shoham Y, Shimon LJW, Bayer EA, Frolow F (2015) Reassembly and co-crystallization of a family 9 processive endoglucanase from its component parts: structural and functional significance of the intermodular linker. PeerJ 3:e1126. https://doi.org/10.7717/peerj.1126
Piiadov V, de Araújo EA, Oliveira Neto M, Craievich A, Polikarpov I (2018) SAXSMoW 2.0: Online calculator of the molecular weight of proteins in dilute solution from experimental SAXS data measured on a relative scale. Protein Sci. https://doi.org/10.1002/pro.3528
Putnam CD, Hammel M, Hura GL, Tainer JA (2007) X-ray solution scattering (SAXS) combined with crystallography and computation: defining accurate macromolecular structures, conformations and assemblies in solution. Q Rev Biophys 40:191–285. https://doi.org/10.1017/S0033583507004635
Rambo RP, Tainer JA (2011) Characterizing flexible and intrinsically unstructured biological macromolecules by SAS using the Porod-Debye law. Biopolymers 95:559–571. https://doi.org/10.1002/bip.21638
Ramírez-Ramírez N, Romero-García E, Calderón V, Avitia C, Téllez-Valencia A, Pedraza-Reyes M (2008) Expression, characterization and synergistic interactions of Myxobacter Sp. AL-1 Cel9 and Cel48 glycosyl hydrolases. Int J Mol Sci 9:247–257. https://doi.org/10.3390/ijms9030247
Ravachol J, Borne R, Tardif C, de Philip P, Fierobe H-P (2014) Characterization of all family-9 glycoside hydrolases synthesized by the cellulosome-producing bacterium Clostridium cellulolyticum. J Biol Chem 289:7335–7348. https://doi.org/10.1074/jbc.M113.545046
Ravachol J, de Philip P, Borne R, Mansuelle P, Maté MJ, Perret S, Fierobe H-P (2016) Mechanisms involved in xyloglucan catabolism by the cellulosome-producing bacterium Ruminiclostridium cellulolyticum. Sci Rep 6:22770. https://doi.org/10.1038/srep22770
Receveur-Brechot V, Durand D (2012) How random are intrinsically disordered proteins? A small angle scattering perspective. Curr Protein Pept Sci 13:55–75
Rey MW, Ramaiya P, Nelson BA, Brody-Karpin SD, Zaretsky EJ, Tang M, Lopez de Leon A, Xiang H, Gusti V, Clausen IG, Olsen PB, Rasmussen MD, Andersen JT, Jørgensen PL, Larsen TS, Sorokin A, Bolotin A, Lapidus A, Galleron N, Ehrlich SD, Berka RM (2004) Complete genome sequence of the industrial bacterium Bacillus licheniformis and comparisons with closely related Bacillus species. Genome Biol 5:R77. https://doi.org/10.1186/gb-2004-5-10-r77
Rudsander UJ, Sandstrom C, Piens K, Master ER, Wilson DB, Brumer III H, Kenne L, Teeri TT (2008) Comparative NMR analysis of cellooligosaccharide hydrolysis by GH9 bacterial and plant endo-1,4-beta-glucanases. Biochemistry 47:5235–5241. https://doi.org/10.1021/bi702193e
Sakon J, Irwin D, Wilson DB, Karplus PA (1997) Structure and mechanism of endo/exocellulase E4 from Thermomonospora fusca. Nat Struct Biol 4:810–818. https://doi.org/10.1038/nsb1097-810
Schallmey M, Singh A, Ward OP (2004) Developments in the use of Bacillus species for industrial production. Can J Microbiol 50:1–17. https://doi.org/10.1139/w03-076
Schneidman-Duhovny D, Hammel M, Sali A (2010) FoXS: a web server for rapid computation and fitting of SAXS profiles. Nucleic Acids Res 38:W540–W544. https://doi.org/10.1093/nar/gkq461
Schubot FD, Kataeva IA, Chang J, Shah AK, Ljungdahl LG, Rose JP, Wang B-C (2004) Structural basis for the exocellulase activity of the cellobiohydrolase CbhA from Clostridium thermocellum. Biochemistry 43:1163–1170. https://doi.org/10.1021/bi030202i
Soccol CR, Vandenberghe LPDS, Medeiros ABP, Karp SG, Buckeridge M, Ramos LP, Pitarelo AP, Ferreira-Leitão V, Gottschalk LMF, Ferrara MA, da Silva Bon EP, de Moraes LMP, Araújo JDA, Torres FAG (2010) Bioethanol from lignocelluloses: status and perspectives in Brazil. Bioresour Technol 101:4820–4825. https://doi.org/10.1016/j.biortech.2009.11.067
Somerville C, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, Osborne E, Paredez A, Persson S, Raab T, Vorwerk S, Youngs H (2004) Toward a systems approach to understanding plant cell walls. Science 306:2206–2211. https://doi.org/10.1126/science.1102765
Svergun DI (1999) Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing. Biophys J 76:2879–2886. https://doi.org/10.1016/S0006-3495(99)77443-6
Svergun DI, Petoukhov MV, Koch MH (2001) Determination of domain structure of proteins from X-ray solution scattering. Biophys J 80:2946–2953. https://doi.org/10.1016/S0006-3495(01)76260-1
Talamantes D, Biabini N, Dang H, Abdoun K, Berlemont R (2016) Natural diversity of cellulases, xylanases, and chitinases in bacteria. Biotechnol Biofuels 9:133. https://doi.org/10.1186/s13068-016-0538-6
Telke AA, Ghatge SS, Kang SH, Thangapandian S, Lee KW, Shin HD, Um Y, Kim SW (2012) Construction and characterization of chimeric cellulases with enhanced catalytic activity towards insoluble cellulosic substrates. Bioresour Technol 112:10–17. https://doi.org/10.1016/j.biortech.2012.02.066
Tuukkanen AT, Kleywegt GJ, Svergun DI (2016) Resolution of ab initio shapes determined from small-angle scattering. IUCrJ 3:440–447. https://doi.org/10.1107/S2052252516016018
Urbanowicz BR, Bennett AB, Del Campillo E, Catalá C, Hayashi T, Henrissat B, Höfte H, McQueen-Mason SJ, Patterson SE, Shoseyov O, Teeri TT, Rose JKC (2007a) Structural organization and a standardized nomenclature for plant endo-1,4-beta-glucanases (cellulases) of glycosyl hydrolase family 9. Plant Physiol 144:1693–1696. https://doi.org/10.1104/pp.107.102574
Urbanowicz BR, Catalá C, Irwin D, Wilson DB, Ripoll DR, Rose JKC (2007b) A tomato endo-beta-1,4-glucanase, SlCel9C1, represents a distinct subclass with a new family of carbohydrate binding modules (CBM49). J Biol Chem 282:12066–12074. https://doi.org/10.1074/jbc.M607925200
Vazana Y, Moraïs S, Barak Y, Lamed R, Bayer EA (2010) Interplay between Clostridium thermocellum family 48 and family 9 cellulases in cellulosomal versus noncellulosomal states. Appl Environ Microbiol 76:3236–3243. https://doi.org/10.1128/AEM.00009-10
Volkov VV, Svergun DI (2003) Uniqueness of ab initio shape determination in small-angle scattering. J Appl Crystallogr 36:860–864. https://doi.org/10.1107/S0021889803000268
Wang H-J, Hsiao Y-Y, Chen Y-P, Ma T-Y, Tseng C-P (2016) Polarity alteration of a calcium site induces a hydrophobic interaction network and enhances Cel9A endoglucanase thermostability. Appl Environ Microbiol 82:1662–1674. https://doi.org/10.1128/AEM.03326-15
Wilson DB (2011) Microbial diversity of cellulose hydrolysis. Curr Opin Microbiol 14:259–263. https://doi.org/10.1016/j.mib.2011.04.004
Yang M, Zhang K-D, Zhang P-Y, Zhou X, Ma X-Q, Li F-L (2016) Synergistic cellulose hydrolysis dominated by a multi-modular processive endoglucanase from Clostridium cellulosi. Front Microbiol 7:932. https://doi.org/10.3389/fmicb.2016.00932
Yarbrough JM, Zhang R, Mittal A, Vander Wall T, Bomble YJ, Decker SR, Himmel ME, Ciesielski PN (2017) Multifunctional cellulolytic enzymes outperform processive fungal cellulases for coproduction of nanocellulose and biofuels. ACS Nano 11:3101–3109. https://doi.org/10.1021/acsnano.7b00086
Yi Z, Su X, Revindran V, Mackie RI, Cann I (2013) Molecular and biochemical analyses of CbCel9A/Cel48A, a highly secreted multi-modular cellulase by Caldicellulosiruptor bescii during growth on crystalline cellulose. PLoS One 8:e84172. https://doi.org/10.1371/journal.pone.0084172
Younesi FS, Pazhang M, Najavand S, Rahimizadeh P, Akbarian M, Mohammadian M, Khajeh K (2016) Deleting the Ig-like domain of Alicyclobacillus acidocaldarius endoglucanase Cel9a causes a simultaneous increase in the activity and stability. Mol Biotechnol 58:12–21. https://doi.org/10.1007/s12033-015-9900-3
Zhang Y-HP, Cui J, Lynd LR, Kuang LR (2006) A transition from cellulose swelling to cellulose dissolution by o-phosphoric acid: evidence from enzymatic hydrolysis and supramolecular structure. Biomacromolecules 7:644–648. https://doi.org/10.1021/bm050799c
Zhang F, Chen JJ, Ren WZ, Nie GX, Ming H, Tang SK, Li WJ (2011) Cloning, expression and characterization of an alkaline thermostable GH9 endoglucanase from Thermobifida halotolerans YIM 90462 T. Bioresour Technol 102:10143–10146. https://doi.org/10.1016/j.biortech.2011.08.019
Zhang C, Zhang W, Lu X (2015) Expression and characteristics of a Ca2+−dependent endoglucanase from Cytophaga hutchinsonii. Appl Microbiol Biotechnol 99:9617–9623. https://doi.org/10.1007/s00253-015-6746-3
Acknowledgments
The authors acknowledge the Diamond Light Source for Synchrotron beam time and staff scientists of the B21 beamline for their assistance. They are also grateful to Maria Auxiliadora Morim Santos, Ph.D., who conducted gene cloning, and Marco Antonio Seiki Kadowaki, Ph.D., for HPAEC-PAD data collection.
Funding
This research was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) via grant 2015/13684-0, and by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) via grants 405191/2015-4, 140667/2015-6, 158752/2015-5, 303988/2016-9, and 440977/2016-9.
Author information
Authors and Affiliations
Contributions
E.A.A. and I.P. conceived and designed the study. M.O.N. collected SAXS data. E.A.A. performed enzyme production, enzyme characterization, and SAXS data processing; also carried out data analyses. E.A.A. and I.P wrote the manuscript with the assistance of all authors. I.P conducted the overall supervision. All the authors approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animals participants.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 215 kb)
Rights and permissions
About this article
Cite this article
de Araújo, E.A., de Oliveira Neto, M. & Polikarpov, I. Biochemical characterization and low-resolution SAXS structure of two-domain endoglucanase BlCel9 from Bacillus licheniformis. Appl Microbiol Biotechnol 103, 1275–1287 (2019). https://doi.org/10.1007/s00253-018-9508-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9508-1