Abstract
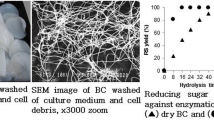
In this study we employed Size Exclusion Chromatography (SEC) and X-ray diffraction to monitor the molecular weight and crystallinity of bacterial cellulose I and II (BC-I, BC-II) and microcrystalline cellulose (MCC) digested with three “pure” Thermobifida fusca cellulases (Cel6A, Cel6B, and Cel9A ). For each enzyme, cellulose crystallinity was found to increase modestly with treatment time. The digestion rate of BC-II was higher than that of BC-I for Cel6A and Cel9A, both endocellulases. SEC results show that the endocellulases create a very rapid decrease in cellulose molecular weight while a slower molecular weight loss was observed with Cel6B, an exocellulase. This work suggests that conversion of native cellulose I to cellulose II by mercerization may beneficially impact the rate of sugar release by cellulases from biomass. In general, lower conversion rates are observed for MCC compared to BC, possibly due to a higher initial crystallinity for MCC. Surface area effects may also be important.







Similar content being viewed by others
References
Amano Y, Nozaki K, Araki T, Shibasaki H, Kuga S (2001) Reactivities of cellulases from fungi towards ribbon-type bacterial cellulose and band-shaped bacterial cellulose. Cellulose 8(4):267–274
Bertoniere NR, Zeronian S, Haig S (1987) Chemical characterization of cellulose. ACS Symposium Series 340, Washington D.C., pp 255–271
Bredereck K, Bader E, Schmitt U (1989) Determination of the pore structure of water-swollen cellulosic fibers. Textilveredlung 24(4):142–150
Chen Y (2005) The effect of cellulose crystal structure and solid-state morphology on the activity of cellulases, M.S. Thesis, SUNY-College of Environmental Science and Forestry, Syracuse, NY
Gama FM, Teixeira JA, Mota M (1994) Cellulose morphology and enzymatic reactivity: a modified solute exclusion technique. Enzyme Microb Technol 43(5):381–387
Goodrich JD, Winter WT (2007) α-Chitin nanocrystals prepared from shrimp shells and their specific surface area measutements. Biomacromolecules 8(1):252–257
Grous WR, Converse AO, Grethlein HE (1986) Effect of steam explosion pretreatment on pore size and enzymatic hydrolysis of poplar. Enzyme Microb Technol 8(5):274–280
Heinze T, Dicke R, Koschella A, Kull AH, Klohr E-K, Kock W (2000) Effective preparation of cellulose derivatives in a new simple cellulose solvent. Macromol Chem Phys 201:627–631
Heux L, Dinand E, Vignon MR (1999) Structural aspects in ultrathin cellulose microfibrills followed by 13C CP-MAS NMR. Carbohydr Polym 40:115–124
Huang AA (1975) Kinetic studies on insoluble cellulose-cellulase system. Biotechnol Bioeng 17(10):1421–1433
Hult E-L, Larsson PT, Iversen T (2000) A comparative CP/MAS 13C-NMR study of cellulose strtcture in spruce wood and kraft pulp. Cellulose 7:35–55
Igarashi J, Wada M, Hori R, Samejima M (2006) Surface density of cellobiohydrolase on crystalline celluloses. Eur J Biochem (FEBS J) 273:2869–2878
Irwin DC, Spezio M, Walker LP, Wilson DB (1993) Activity studies of eight purified cellulases: specificity, synergism, and binding domain effects. Biotechnol Bioeng 42:1002–1013
Inglesby MK, Zeronian SH (1996) The accessibility of cellulose as determined by dye adsorption. Cellulose 3:165–181
Kim N-H, Imai T, Wada M, Sugiyama J (2006) Molecular directionality in cellulose polymorphs. Biomacromolecules 7:274–280
Laszkiewicz B (1998) Solubility of bacterial cellulose and its structural properties. J Appl Polym Sci 67:1871–1876
Lee SB, Kim IH, Dewey DY, Ryu H, Taguchi H (1983) Structural properties of cellulose and cellulase reaction. Biotechnol Bioeng 25(1):33–51
Lever M (1972) A new reaction for colorimetric determination of carbohydrates. Anal Biochem 47:273–279
Mansfield SD, Meder R (2003) Cellulose hydrolysis—the role of monocomponent cellulases in crystalline cellulose degradation. Cellulose 10:159–169
Marshall K, Sixsmith D, Stanley-Wood NG (1972) Surface geometry of some microcrystalline celluloses. J Pharm Pharmacol 24(Suppl):138P
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladish M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686
National Research Council (2000) Biobased industrial products—priorities for research and commercialization. National Academy Press, Washington, DC
Neuman RP, Walker LP (1992) Solute exclusion from cellulose in packed columns: experimental investigation and pore volume measurements. Biotechnol Bioeng 40(2):218–225
Ougiya H (1998) Relationship between the physical properties and surface area of cellulose derived from adsorbates of various molecular sizes. Biosci Biotechnol Biochem 62:1880–1884
Rousselle M-A (2002) Determining the molecular weight distribution of cotton cellulose: a new GPC solvent. Textile Res J 72(2):131–134
Schult T, Hjerde T, Optun OI, Kleppe PJ, Moe S (2002) Characterization of cellulose by SEC-MALLS. Cellulose 9(2):149–158
Sottys J, Lisowski Z, Knapczyky J (1984) X-Ray diffraction study on the crystallinity index and the structure of the microcrystalline cellulose. Acta Pharm Technol 30(2):174–180
Srisodšuk M, Klemam-Leyer K, Keranen S, Kirk TK, Teeri TT (1998). Modes of action on cotton and bacterial cellulose of a homologous endoglucanase-exoglucanase pair from Trichoderma reesei. Eur Biochem J 251:885–892
Stone JE, Scallan AM, Donefer E, Ahlgren E (1969) Digestibility as a simple function of a molecule of similar size to a cellulase enzyme. Advances in Chemistry Series 95, American Chemical Society, Washington D.C., pp 219–241
Timell TE (1963) Title, journal of polymer science, part C. Polym Symp 2:109–115
Timpa JD (1991) Application of universal calibration in gel permeation chromatography for molecular weight determinations of plant cell wall polymers: cotton fiber. J Agric Food Chem 39(2):270–275
Timpa JD (1993) Size-exclusion chromatography to characterize cotton fiber. ACS Symposium Series 521—Chromatography of Polymers, Washington D.C., pp 302–313
US-DOE/USDA (2005) A billion-ton feedstock supply for a bioenergy and bioproducts industry—technical feasibility of annually supplying 1 billion dry tons of biomass, a joint study sponsored by the US Dept. of Energy and US Dept. of Agriculture, 2005
VanderHart DL, Atalla RH (1984) Strudies of microstruicture in native cellulose using solid state 13-NMR. Macromolecules 17:1465–1472
Wilson DB, Irwin DC (1999) Genetics and properties of cellulases Adv Biochem Eng 65:1–21
Wilson DB (2004) Studies of Thermobifida fusca plant cell wall degrading enzymes. Chem Rec 4:72–82
Wyman C, Dale B, Elander R, Holtzapple M, Ladish M, Lee YY (2005a) Coordinated development of leading biomass pretreatment technologies. Bioresour Technol 96:1959–1966
Wyman C, Dale B, Elander R, Holtzapple M, Ladish M, Lee YY (2005b) Comparative sugar recovery data from laboratory scale application of leading pretreatment technologies to corn stover. Bioresour Technol 96:2026–2032
Yanagisawa M, Shibata I, Isogai A (2004) SEC-MALLS analysis of cellulose using LiCL/1,3-dimethyl-2-imidazolidinone as an eluent. Cellulose 11(2):169–176
Acknowledgements
This work was financially supported by U.S. Department of Energy, Energy Biosciences Division Grant No. DE-FG02-02ER15356. The authors also acknowledge the technical support provided by Mr. Cory Hauke in determining the surface area of BC-I and II.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Stipanovic, A.J., Winter, W.T. et al. Effect of digestion by pure cellulases on crystallinity and average chain length for bacterial and microcrystalline celluloses. Cellulose 14, 283–293 (2007). https://doi.org/10.1007/s10570-007-9115-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-007-9115-2