Abstract
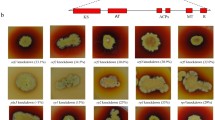
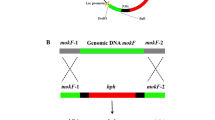
As traditional edible fungi, Monascus spp. have been widely used as folk medicine, food colorants, and fermentation starters in East Asian countries for more than a thousand years. However, the presence of citrinin, which has nephrotoxic, hepatotoxic, and carcinogenic activities, raises suspicions about the safety of Monascus products. Citrinin biosynthesis in Monascus is known to occur via a polyketide pathway and a citrinin biosynthesis gene cluster, which include the characterized polyketide synthetase pksCT. A gene, orf6, encodes a protein that shows significant similarity to glyoxalase and is located between ctnE and orf1. This study analyzed orf6 function, and successfully obtained an orf6 disruption strain (Δorf6). Citrinin production was significantly greater (3.6-fold) in the Δorf6 strain than in the wild-type Monascus purpureus YY-1, and RT-PCR analysis further revealed increased expression of numerous genes of the citrinin biosynthesis gene cluster in Δorf6. Therefore, orf6 proved to be a major inhibitor, directly involved in citrinin biosynthesis. Moreover, pigment production in Δorf6 was reduced by approximately 30%, while the transcription levels of many genes involved in Monascus pigments (MPs) biosynthesis had increased. This dichotomy indicated that MPs and citrinin yields may be improved simultaneously; however, a portion of the pigments was consumed to protect the cells from oxidative damage in the Δorf6 strain. An Δorf6 revertant restored the citrinin and pigment yields to normal levels. This study makes a contribution to explore the citrinin biosynthesis pathway and provides some theoretical guidance to improving the safety of Monascus-related products.







Similar content being viewed by others
References
Balakrishnan B, Chandran R, Park SH, Kwon HJ (2016) Delineating citrinin biosynthesis: Ctn-ORF3 dioxygenase-mediated multi-step methyl oxidation precedes a reduction-mediated pyran ring cyclization. Bioorg Med Chem Lett 26(2):392–396. doi:10.1016/j.bmcl.2015.12.001
Bito A, Haider M, Hadler I, Breitenbach M (1997) Identification and phenotypic analysis of two glyoxalase II encoding genes from Saccharomyces cerevisiae, GLO2 and GLO4, and intracellular localization of the corresponding proteins. J Biol Chem 272(34):21509–21519. doi:10.1074/jbc.272.34.21509
Chen F, Hu X (2005) Study on red fermented rice with high concentration of monacolin K and low concentration of citrinin. Int J Food Microbiol 103(3):331–337. doi:10.1016/j.ijfoodmicro.2005.03.002
Feng Y, Shao Y, Chen F (2012) Monascus pigments. Appl Microbiol Biot 96(6):1421–1440. doi:10.1007/s00253-012-4504-3
Finkgremmels J (1999) Mutagenicity of commercial Monascus fermentation products and the role of citrinin contamination. Mutat Res 444(1):7–16. doi:10.1016/S1383-5718(99)00095-9
Frank H (1992) Citrinin Z Ernahrungswiss 31(3):164–177. doi:10.1007/BF01611139
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Bioch 48(12):909–930. doi:10.1016/j.plaphy.2010.08.016
Hajjaj H, François JM, Goma G, Blanc PJ (2012) Effect of amino acids on red pigments and citrinin production in Monascus ruber. J Food Sci 77(3):M156–M159. doi:10.1111/j.1750-3841.2011.02579.x
Hajjaj H, Klaebe A, Loret MO, Goma G, Blanc PJ, Francois J (1999) Biosynthetic pathway of Citrinin in the filamentous fungus Monascus ruber as revealed by 13C nuclear magnetic resonance. Appl Environ Microbiol 65(1):311–314
Hassenfeldt TA, Lorenzi J, Scarpa A (1983) Tumorigenicity of citrinin in male F344 rats. Rev J Autism Dev Disord 17(3):281–287
He Y, Cox RJ (2016) The molecular steps of citrinin biosynthesis in fungi. Chem Sci 7(3):2119–2127. doi:10.1039/C5SC04027B
He Y, Liu Q, Shao Y, Chen F (2013) ku70 and ku80 null mutants improve the gene targeting frequency in Monascus ruber M7. Appl Microbiol Biot 97(11):4965–4976. doi:10.1007/s00253-013-4916-8
Huang CH, Shiu SM, Wu MT, Chen WL, Wang SG, Lee HM (2013) Monacolin K affects lipid metabolism through SIRT1/AMPK pathway in HepG2 cells. Arch Pharm Res 36(12):1541–1551. doi:10.1007/s12272-013-0150-2
Inoue Y, Maeta K, Nomura W (2011) Glyoxalase system in yeasts: structure, function, and physiology. Semin Cell Dev Biol 22(3):278–284. doi:10.1016/j.semcdb.2011.02.002
Inoue Y, Rhee HI, Watanabe K, Murata K, Kimura A (1988) Metabolism of 2-oxoaldehyde in mold. Eur J Biochem 171(1–2):213–218. doi:10.1111/j.1432-1033.1988.tb13778.x
Johns MR, Stuart DM (1991) Production of pigments by Monascus purpureus in solid culture. J Ind Microbiol Biot 8(1):23–28. doi:10.1007/BF01575587
Khang CH, Park SY, Rho HS, Lee YH, Kang S (2006) Filamentous fungi (Magnaporthe grisea and Fusarium oxysporum). Method Mol Biol 344:403–420. doi:10.1385/1-59745-131-2:403
Kim C, Jung H, Yong OK, Shin CS (2006) Antimicrobial activities of amino acid derivatives of Monascus pigments. FEMS Microbiol Lett 264(1):117–124. doi:10.1111/j.1574-6968.2006.00451.x
Kim J, Kim H, Hw Youn S, Choi D, Shin C (2007) Development of inhibitors against lipase and α-glucosidase from derivatives of Monascus pigment. FEMS Microbiol Lett 276(1):93–98. doi:10.1111/j.1574-6968.2007.00917.x
Kujumdzieva AV, Hallet JN, Savov VA, Rasheva TV (1997) Monascus purpureus strain producer of pigments and by-products. US Patent 5627068 A, 6 May 1997
Lee BH, Pan TM (2012a) Benefit of Monascus-fermented products for hypertension prevention: a review. Appl Microbiol Biot 94(5):1151–1161. doi:10.1007/s00253-012-4076-2
Lee CL, Hung HK, Wang JJ, Pan TM (2007) Improving the ratio of monacolin K to citrinin production of Monascus purpureus NTU 568 under dioscorea medium through the mediation of pH value and ethanol addition. J Agric Food Chem 55(16):6493–6502. doi:10.1021/jf0711946
Lee CL, Pan TM (2012b) Development of Monascus fermentation technology for high hypolipidemic effect. Appl Microbiol Biotech 4(6):1449–1459. doi:10.1007/s00253-012-4083-3
Lehua Z, Yanping L, Zhibing H, Yang X (2011) Construction of the Monascus aurantiacus As3.4384 orf7 gene deletion strains and functional analysis. China Biotechnol 31(7):79–84
Li YP, Pan YF, Zou LH, Xu Y, Huang ZB, He QH (2013) Lower citrinin production by gene disruption of ctnB involved in citrinin biosynthesis in Monascus aurantiacus Li AS3.4384. J Agric Food Chem 61(30):7397–7403. doi:10.1021/jf400879s
Li YP, Tang X, Wu W, Xu Y, Huang ZB, He QH (2014) The ctnG gene encodes carbonic anhydrase involved in mycotoxin citrinin biosynthesis from Monascus aurantiacus. Food Addit Contam A 32(4):577–583. doi:10.1080/19440049.2014.990993
Li YP, Xu Y, Huang ZB (2012) Isolation and characterization of the citrinin biosynthetic gene cluster from Monascus aurantiacus. Biotechnol Lett 34(1):131–136. doi:10.1007/s10529-011-0745-y
Liu BH, Wu TS, Su MC, Chung CP, Yu FY (2005) Evaluation of citrinin occurrence and cytotoxicity in Monascus fermentation products. J Agric Food Chem 53(1):170–175. doi:10.1021/jf048878n
Liu Q, Xie N, Yi H, Li W, Shao Y, Zhao H, Chen F (2014) MpigE, a gene involved in pigment biosynthesis in Monascus ruber M7. Appl Microbiol Biotechnol 98(1):285–296. doi:10.1007/s00253-013-5289-8
Maeta K, Izawa S, Okazaki S, Kuge S, Inoue Y (2004) Activity of the Yap1 transcription factor in Saccharomyces cerevisiae is modulated by methylglyoxal, a metabolite derived from glycolysis. Mol Cell Biol 24(19):8753–8764. doi:10.1128/MCB.24.19.8753-8764.2004
Mullins ED, Chen X, Romaine P, Raina R, Geiser DM, Kang S (2001) Agrobacterium-mediated transformation of Fusarium oxysporum: an efficient tool for insertional mutagenesis and gene transfer. Phytopathology 91(2):173–180. doi:10.1094/PHYTO.2001.91.2.173
Murata K, Fukuda Y, Watanabe K, Saikusa T, Shimosaka M, Kimura A (1985) Characterization of methylglyoxal synthase in Saccharomyces cerevisiae. Biochem BiophRes Co 131(1):190–198. doi:10.1016/0006-291X(85)91788-7
Nahar K, Hasanuzzaman M, Alam MM, Fujita M (2015) Glutathione-induced drought stress tolerance in mung bean: coordinated roles of the antioxidant defence and methylglyoxal detoxification systems. Aob Plants 7(1):1–18. doi:10.1093/aobpla/plv069
Ning ZQ, Cui H, Xu Y, Huang ZB, Tu Z, Li YP (2017) Deleting the citrinin biosynthesis-related gene, ctnE, to greatly reduce citrinin production in Monascus aurantiacus Li AS3.4384. Int J Food Microbiol 241:325–330. doi:10.1016/j.ijfoodmicro.2016.11.004
Patakova P (2013) Monascus secondary metabolites: production and biological activity. J Ind Microbiol Biot 40(2):169–181. doi:10.1007/s10295-012-1216-8
Radhika D, Bajpai VK, Kwang-Hyun B (2012) Production of gaba (γ-aminobutyric acid) by microorganisms: a review. Braz J Microbiol 43(4):1230–1241. doi:10.1590/S1517-83822012000400001
Shao Y, Ding Y, Zhao Y, Yang S, Xie B, Chen F (2009) Characteristic analysis of transformants in T-DNA mutation library of Monascus ruber. World J Microb Biot 25(6):989–995. doi:10.1007/s11274-009-9977-6
Shao Y, Lei M, Mao Z, Zhou Y, Chen F (2014) Insights into Monascus biology at the genetic level. Appl Microbiol Biot 98(9):3911–3922. doi:10.1007/s00253-014-5608-8
Shimizu T, Kinoshita H, Ishihara S, Sakai K, Nagai S, Nihira T (2005) Polyketide synthase gene responsible for citrinin biosynthesis in Monascus purpureus. Appl Environ Microbiol 71(7):3453–3457. doi:10.1128/AEM.71.7.3453-3457.2005
Shimizu T, Kinoshita H, Nihira T (2007) Identification and in vivo functional analysis by gene disruption of ctnA, an activator gene involved in citrinin biosynthesis in Monascus purpureus. Appl Environ Microbiol 73(16):5097–5103. doi:10.1128/AEM.01979-06
Srianta I (2014) Recent research and development of Monascus fermentation products. Int Food Res J 21(1):1–12
Taylor DG, Trudgill PW, Cripps RE, Harris PR (1980) The microbial metabolism of acetone. Microbiol 118(1):159–170. doi:10.1099/00221287-118-1-159
Thornalley PJ (2003) Glyoxalase I-structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc T 31(Pt 6):1343–1348. doi:10.1042/BST0311343
Vendruscolo F, Bühler RM, de Carvalho JC, De OD, Moritz DE, Schmidell W, Ninow JL (2015) Monascus: a reality on the production and application of microbial pigments. Appl Biochem Biot 178(2):211–223. doi:10.1007/s12010-015-1880-z
Yang Y, Liu B, Du X, Li P, Liang B, Cheng X, Wang S (2015) Complete genome sequence and transcriptomics analyses reveal pigment biosynthesis and regulatory mechanisms in an industrial strain, Monascus purpureus YY-1. Sci Rep-UK 5:1–8. doi:10.1038/srep08331
Zhou Y, Chen J, Dong L, Lu L, Chen F, Hu D, Wang X (2012) A study of fluorescence properties of citrinin in β-cyclodextrin aqueous solution and different solvents. J Lumin 132(6):1437–1445. doi:10.1016/j.jlumin.2012.01.005
Acknowledgements
This study was supported by the National Key Technology R&D Program (no. 2014BAD04B03) of China and the Tianjin Research Program of Application Foundation and Advanced Technology (no. 15JCQNJC44600).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 189 kb)
Rights and permissions
About this article
Cite this article
Liang, B., Du, X., Li, P. et al. Orf6 gene encoded glyoxalase involved in mycotoxin citrinin biosynthesis in Monascus purpureus YY-1. Appl Microbiol Biotechnol 101, 7281–7292 (2017). https://doi.org/10.1007/s00253-017-8462-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8462-7