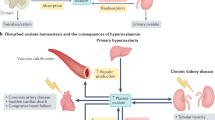
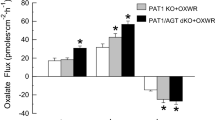
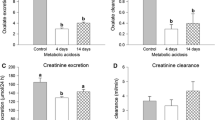
Abstract
The intestine exerts a considerable influence over urinary oxalate in two ways, through the absorption of dietary oxalate and by serving as an adaptive extra-renal pathway for elimination of this waste metabolite. Knowledge of the mechanisms responsible for oxalate absorption and secretion by the intestine therefore have significant implications for understanding the etiology of hyperoxaluria, as well as offering potential targets for future treatment strategies for calcium oxalate kidney stone disease. In this review, we present the recent developments and advances in this area over the past 10 years, and put to the test some of the new ideas that have emerged during this time, using human and mouse models. A key focus for our discussion are the membrane-bound anion exchangers, belonging to the SLC26 gene family, some of which have been shown to participate in transcellular oxalate absorption and secretion. This has offered the opportunity to not only examine the roles of these specific transporters, revealing their importance to oxalate homeostasis, but to also probe the relative contributions made by the active transcellular and passive paracellular components of oxalate transport across the intestine. We also discuss some of the various physiological stimuli and signaling pathways which have been suggested to participate in the adaptation and regulation of intestinal oxalate transport. Finally, we offer an update on research into Oxalobacter formigenes, alongside recent investigations of other oxalate-degrading gut bacteria, in both laboratory animals and humans.






Similar content being viewed by others
References
Osswald H, Hautmann R (1979) Renal elimination kinetics and plasma half-life of oxalate in man. Urol Int 34(6):440–450
Prenen JAC, Boer P, Mees EJD, Endeman HJ, Spoor SM, Oei HY (1982) Renal clearance of C-14-labeled oxalate—comparison of constant-infusion with single-injection techniques. Clin Sci 63(1):47–51
Costello JF, Smith M, Stolarski C, Sadovnic MJ (1992) Extrarenal clearance of oxalate increases with progression of renal-failure in the rat. J Am Soc Nephrol 3(5):1098–1104
Worcester EM (2002) Stones from bowel disease. Endocrinol Metab Clin North Am 31(4):979–999. doi:10.1016/s0889-8529(02)00035-x
Hatch M, Freel RW, Vaziri ND (1994) Intestinal excretion of oxalate in chronic-renal-failure. J Am Soc Nephrol 5(6):1339–1343
Hatch M, Freel RW (2003) Angiotensin II involvement in adaptive enteric oxalate excretion in rats with chronic renal failure induced by hyperoxaluria. Urol Res 31(6):426–432. doi:10.1007/s00240-003-0367-5
Hatch M, Cornelius J, Allison M, Sidhu H, Peck A, Freel RW (2006) Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int 69(4):691–698. doi:10.1038/sj.ki.5000162
Hatch M, Gjymishka A, Salido EC, Allison MJ, Freel RW (2011) Enteric oxalate elimination is induced and oxalate is normalized in a mouse model of primary hyperoxaluria following intestinal colonization with Oxalobacter. Am J Physiol Gastrointest Liver Physiol 300(3):G461–G469. doi:10.1152/ajpgi.00434.2010
Hatch M, Freel RW (2013) A human strain of Oxalobacter (HC-1) promotes enteric oxalate secretion in the small intestine of mice and reduces urinary oxalate excretion. Urolithiasis 41(5):379–384. doi:10.1007/s00240-013-0601-8
Freel RW, Hatch M, Earnest DL, Goldner AM (1980) Oxalate transport across the isolated rat colon—a reexamination. Biochim Biophys Acta 600(3):838–843. doi:10.1016/0005-2736(80)90486-1
Hatch M, Freel RW, Goldner AM, Earnest DL (1984) Oxalate and chloride absorption by the rabbit colon: sensitivity to metabolic and anion transport inhibitors. Gut 25(3):232–237
Hatch M, Freel RW, Vaziri ND (1993) Characteristics of the transport of oxalate and other ions across rabbit proximal colon. Pflug Archiv Eur J Physiol 423(3–4):206–212. doi:10.1007/bf00374396
Hatch M, Freel RW, Vaziri ND (1994) Mechanisms of oxalate absorption and secretion across the rabbit distal colon. Pflug Archiv Eur J Physiol 426(1–2):101–109. doi:10.1007/bf00374677
Dawson KA, Allison MJ, Hartman PA (1980) Isolation and some characteristics of anaerobic oxalate-degrading bacteria from the rumen. Appl Environ Microbiol 40(4):833–839
Allison MJ, Dawson KA, Mayberry WR, Foss JG (1985) Oxalobacter formigenes gen. nov., sp. nov.: oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Arch Microbiol 141(1):1–7
Freel RW, Hatch M, Green M, Soleimani M (2006) Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol 290(4):G719–G728. doi:10.1152/ajpgi.00481.2005
Freel RW, Whittamore JM, Hatch M (2013) Transcellular oxalate and Cl− absorption in mouse intestine is mediated by the DRA anion exchanger Slc26a3, and DRA deletion decreases urinary oxalate. Am J Physiol Gastrointest Liver Physiol 305(7):G520–G527. doi:10.1152/ajpgi.00167.2013
Jiang ZR, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS (2006) Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38(4):474–478. doi:10.1038/ng1762
Dawson PA, Russell CS, Lee S, McLeay SC, van Dongen JM, Cowley DM, Clarke LA, Markovich D (2010) Urolithiasis and hepatotoxicity are linked to the anion transporter Sat1 in mice. J Clin Investig 120(3):706–712. doi:10.1172/jci31474
Hatch M, Freel RW (2005) Intestinal transport of an obdurate anion: oxalate. Urol Res 33(1):1–16. doi:10.1007/s00240-004-0445-3
Hatch M, Freel RW (2008) The roles and mechanisms of intestinal oxalate transport in oxalate homeostasis. Semin Nephrol 28(2):143–151. doi:10.1016/j.semnephrol.2008.01.007
Powell DW (1981) Barrier function of epithelia. Am J Physiol Gastrointest Liver Physiol 241(4):G275–G288
Knauf F, Ko N, Jiang ZR, Robertson WG, Van Itallie CM, Anderson JM, Aronson PS (2011) Net intestinal transport of oxalate reflects passive absorption and SLC26A6-mediated secretion. J Am Soc Nephrol 22(12):2247–2255. doi:10.1681/asn.2011040433
Pappenheimer JR (1990) Paracellular intestinal-absorption of glucose, creatinine, and mannitol in normal animals—relation to body size. Am J Physiol Gastrointest Liver Physiol 259(2):G290–G299
Walker NM, Simpson JE, Brazill JM, Gill RK, Dudeja PK, Schweinfest CW, Clarke LL (2009) Role of down-regulated in adenoma anion exchanger in HCO3 − secretion across murine duodenum. Gastroenterology 136(3):893–901. doi:10.1053/j.gastro.2008.11.016
Singh AK, Riederer B, Chen MM, Xiao F, Krabbenhoft A, Engelhardt R, Nylander O, Soleimani M, Seidler U (2010) The switch of intestinal Slc26 exchangers from anion absorptive to HCO3 − secretory mode is dependent on CFTR anion channel function. Am J Physiol Cell Physiol 298(5):C1057–C1065. doi:10.1152/ajpcell.00454.2009
Singh AK, Liu YJ, Riederer B, Engelhardt R, Thakur BK, Soleimani M, Seidler U (2013) Molecular transport machinery involved in orchestrating luminal acid-induced duodenal bicarbonate secretion in vivo. J Physiol Lond 591(21):5377–5391. doi:10.1113/jphysiol.2013.254854
Liu XM, Li TL, Riederer B, Lenzen H, Ludolph L, Yeruva S, Tuo BG, Soleimani M, Seidler U (2015) Loss of Slc26a9 anion transporter alters intestinal electrolyte and HCO3 − transport and reduces survival in CFTR-deficient mice. Pflug Archiv Eur J Physiol 467(6):1261–1275. doi:10.1007/s00424-014-1543-x
Inagaki E, Natori Y, Ohgishi Y, Hayashi H, Suzuki Y (2005) Segmental difference of mucosal damage along the length of a mouse small intestine in an Ussing chamber. J Nutr Sci Vitaminol 51(6):406–412
Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL (2007) PAT-1 (Slc26a6) is the predominant apical membrane Cl−/HCO3 − exchanger in the upper villous epithelium of the murine duodenum. Am J Physiol Gastrointest Liver Physiol 292(4):G1079–G1088. doi:10.1152/ajpgi.00354.2006
Walker NM, Simpson JE, Hoover EE, Brazill JM, Schweinfest CW, Soleimani M, Clarke LL (2011) Functional activity of Pat-1 (Slc26a6) Cl−/HCO3 − exchange in the lower villus epithelium of murine duodenum. Acta Physiol 201(1):21–31. doi:10.1111/j.1748-1716.2010.02210.x
Freel RW, Hatch M, Vaziri ND (1998) Conductive pathways for chloride and oxalate in rabbit ileal brush-border membrane vesicles. Am J Physiol Cell Physiol 275(3):C748–C757
Barrett KE, Keely SJ (2000) Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol 62:535–572. doi:10.1146/annurev.physiol.62.1.535
Freel RW, Hatch M (2008) Enteric oxalate secretion is not directly mediated by the human CFTR chloride channel. Urol Res 36(3–4):127–131. doi:10.1007/s00240-008-0142-8
Gibney EM, Goldfarb DS (2003) The association of nephrolithiasis with cystic fibrosis. Am J Kidney Dis 42(1):1–11. doi:10.1016/s0272-6386(03)00403-7
Hoppe B, von Unruh GE, Blank G, Rietschel E, Sidhu H, Laube N, Hesse A (2005) Absorptive hyperoxaluria leads to an increased risk for urolithiasis or nephrocalcinosis in cystic fibrosis. Am J Kidney Dis 46(3):440–445. doi:10.1053/j.ajkd.2005.06.003
Knauf F, Thomson RB, Heneghan JF, Jiang Z, Adebamiro A, Thomson CL, Barone C, Asplin JR, Egan ME, Alper SL, Aronson PS (2016) Loss of cystic fibrosis transmembrane regulator impairs intestinal oxalate secretion. J Am Soc Nephrol. doi:10.1681/asn.2016030279
Ko SBH, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S (2002) A molecular mechanism for aberrant CFTR-dependent HCO3 − transport in cystic fibrosis. EMBO J 21(21):5662–5672. doi:10.1093/emboj/cdf580
Ko SBH, Zeng WZ, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S (2004) Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol 6(4):343–350. doi:10.1038/ncb1115
Rossmann H, Jacob P, Baisch S, Hassoun R, Meier J, Natour D, Yahya K, Yun C, Biber J, Lackner KJ, Fiehn W, Gregor M, Seidler U, Lamprecht G (2005) The CFTR associated protein CAP70 interacts with the apical Cl−/HCO3 − exchanger DRA in rabbit small intestinal mucosa. Biochemistry 44(11):4477–4487. doi:10.1021/bi048828b
Singh AK, Sjoblom M, Zheng W, Krabbenhoft A, Riederer B, Rausch B, Manns MP, Soleimani M, Seidler U (2008) CFTR and its key role in in vivo resting and luminal acid-induced duodenal HCO3 − secretion. Acta Physiol 193(4):357–365. doi:10.1111/j.1748-1716.2008.01854.x
Singh AK, Riederer B, Krabbenhoft A, Rausch B, Bonhagen J, Lehmann U, de Jonge HR, Donowitz M, Yun C, Weinman EJ, Kocher O, Hogema BM, Seidler U (2009) Differential roles of NHERF1, NHERF2, and PDZK1 in regulating CFTR-mediated intestinal anion secretion in mice. J Clin Investig 119(3):540–550. doi:10.1172/jci35541
Regeer RR, Lee A, Markovich D (2003) Characterization of the human sulfate anion transporter (hsat-1) protein and gene (SAT1; SLC26A1). DNA Cell Biol 22(2):107–117. doi:10.1089/104454903321515913
Ko N, Knauf F, Jiang Z, Markovich D, Aronson PS (2012) Sat1 is dispensable for active oxalate secretion in mouse duodenum. Am J Physiol Cell Physiol 303(1):C52–C57. doi:10.1152/ajpcell.00385.2011
Whittamore JM, Frost SC, Hatch M (2015) Effects of acid-base variables and the role of carbonic anhydrase on oxalate secretion by the mouse intestine in vitro. Physiol Rep 3(2). doi:10.14814/phy2.12282
Morozumi M, Green M, Freel RW, Hatch M (2004) The effect of oxalate loading or acidified media on the expression of mRNA encoding candidate oxalate transporters in Caco-2 monolayers. In: 10th international symposium on urolithiasis, Hong Kong, 2004. pp 178–180
Freel RW, Morozumi M, Hatch M (2009) Parsing apical oxalate exchange in Caco-2BBe1 monolayers: siRNA knockdown of SLC26A6 reveals the role and properties of PAT-1. Am J Physiol Gastrointest Liver Physiol 297(5):G918–G929. doi:10.1152/ajpgi.00251.2009
Mount DB, Romero MF (2004) The SLC26 gene family of multifunctional anion exchangers. Pflug Archiv Eur J Physiol 447(5):710–721. doi:10.1007/s00424-003-1090-3
Dorwart MR, Shcheynikov N, Yang DK, Muallem S (2008) The solute carrier 26 family of proteins in epithelial ion transport. Physiology 23(2):104–114. doi:10.1152/physiol.00037.2007
Alper SL, Sharma AK (2013) The SLC26 gene family of anion transporters and channels. Mol Asp Med 34(2–3):494–515. doi:10.1016/j.mam.2012.07.009
Shcheynikov N, Ohana E, Muallem S (2016) Properties and function of the solute carrier 26 family of anion transporters. In: Hamilton KL, Devor DC (eds) Ion channels and transporters of epithelia in health and disease. physiology in health and disease. Springer, New York, pp 465–490
Karniski LP, Lotscher M, Fucentese M, Hilfiker H, Biber J, Murer H (1998) Immunolocalization of sat-1 sulfate/oxalate/bicarbonate anion exchanger in the rat kidney. Am J Physiol Renal Physiol 275(1):F79–F87
Satoh H, Susaki M, Shukunami C, Iyama K, Negoro T, Hiraki Y (1998) Functional analysis of diastrophic dysplasia sulfate transporter—its involvement in growth regulation of chondrocytes mediated by sulfated proteoglycans. J Biol Chem 273(20):12307–12315. doi:10.1074/jbc.273.20.12307
Xie QH, Welch R, Mercado A, Romero MF, Mount DB (2002) Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am J Physiol Renal Physiol 283(4):F826–F838. doi:10.1152/ajprenal.00079.2002
Lee A, Beck L, Markovich D (2003) The mouse sulfate anion transporter gene Sat1 (Slc26a1): cloning, tissue distribution, gene structure, functional characterization, and transcriptional regulation by thyroid hormone. DNA Cell Biol 22(1):19–31. doi:10.1089/104454903321112460
Krick W, Schnedler N, Burckhardt G, Burckhardt BC (2009) Ability of sat-1 to transport sulfate, bicarbonate, or oxalate under physiological conditions. Am J Physiol Renal Physiol 297(1):F145–F154. doi:10.1152/ajprenal.90401.2008
Quondamatteo F, Krick W, Hagos Y, Kruger MH, Neubauer-Saile K, Herken R, Ramadori G, Burckhardt G, Burckhardt BC (2006) Localization of the sulfate/anion exchanger in the rat liver. Am J Physiol Gastrointest Liver Physiol 290(5):G1075–G1081. doi:10.1152/ajpgi.00492.2005
Brzica H, Breljak D, Krick W, Lovric M, Burckhardt G, Burckhardt BC, Sabolic I (2009) The liver and kidney expression of sulfate anion transporter sat-1 in rats exhibits male-dominant gender differences. Pflug Archiv Eur J Physiol 457(6):1381–1392. doi:10.1007/s00424-008-0611-5
Breljak D, Brzica H, Vrhovac I, Micek V, Karaica D, Ljubojevic M, Sekovanic A, Jurasovic J, Rasic D, Peraica M, Lovric M, Schnedler N, Henjakovic M, Wegner W, Burckhardt G, Burckhardt BC, Sabolic I (2015) In female rats, ethylene glycol treatment elevates protein expression of hepatic and renal oxalate transporter sat-1 (Slc26a1) without inducing hyperoxaluria. Croat Med J 56(5):447–459. doi:10.3325/cmj.2015.56.447
Dawson PA, Sim P, Mudge DW, Cowley D (2013) Human SLC26A1 gene variants: a pilot study. Sci World J. doi:10.1155/2013/541710
Haila S, Hastbacka J, Bohling T, Karjalainen-Lindsberg ML, Kere J, Saarialho-Kere U (2001) SLC26A2 (diastrophic dysplasia sulfate transporter) is expressed in developing and mature cartilage but also in other tissues and cell types. J Histochem Cytochem 49(8):973–982
Park M, Ohana E, Choi SY, Lee M-S, Park JH, Muallem S (2014) Multiple Roles of the SO4 2−/Cl−/OH− exchanger protein Slc26a2 in chondrocyte functions. J Biol Chem 289(4):1993–2001. doi:10.1074/jbc.M113.503466
Hastbacka J, Delachapelle A, Mahtani MM, Clines G, Reevedaly MP, Daly M, Hamilton BA, Kusumi K, Trivedi B, Weaver A, Coloma A, Lovett M, Buckler A, Kaitila I, Lander ES (1994) The diastrophic dysplasia gene encodes a novel sulfate transporter—positional cloning by fine-structure linkage disequilibrium mapping. Cell 78(6):1073–1087. doi:10.1016/0092-8674(94)90281-x
Karniski LP (2001) Mutations in the diastrophic dysplasia sulfate transporter (DTDST) gene: correlation between sulfate transport activity and chondrodysplasia phenotype. Hum Mol Genet 10(14):1485–1490. doi:10.1093/hmg/10.14.1485
Rossi A, Superti-Furga A (2001) Mutations in the diastrophic dysplasia sulfate transporter (DTDST) gene (SLC26A2): 22 Novel mutations, mutation review, associated skeletal phenotypes, and diagnostic relevance. Hum Mutat 17(3):159–171. doi:10.1002/humu.1
Forlino A, Piazza R, Torre SD, Tatangelo L, Bonafe L, Gualeni B, Romano A, Pecora F, Superti-Furga A, Cetta G, Rossi A (2005) A diastrophic dysplasia sulfate transporter (SLC26A2) mutant mouse: morphological and biochemical characterization of the resulting chondrodysplasia phenotype. Hum Mol Genet 14(6):859–871. doi:10.1093/hmg/ddi079
Haila S, Saarialho-Kere U, Karjalainen-Lindsberg ML, Lohi H, Airola K, Holmberg C, Hastbacka J, Kere J, Hoglund P (2000) The congenital chloride diarrhea gene is expressed in seminal vesicle, sweat gland, inflammatory colon epithelium, and in some dysplastic colon cells. Histochem Cell Biol 113(4):279–286
Dawson PA, Rakoczy J, Simmons DG (2012) Placental, renal, and ileal sulfate transporter gene expression in mouse gestation. Biol Reprod 87(2). doi:10.1095/biolreprod.111.098749
Heneghan JF, Akhavein A, Salas MJ, Shmukler BE, Karniski LP, Vandorpe DH, Alper SL (2010) Regulated transport of sulfate and oxalate by SLC26A2/DTDST. Am J Physiol Cell Physiol 298(6):C1363–C1375. doi:10.1152/ajpcell.00004.2010
Ohana E, Shcheynikov N, Park M, Muallem S (2012) Solute carrier family 26 member a2 (Slc26a2) protein functions as an electroneutral SO4 2−/OH−/Cl− exchanger regulated by extracellular Cl−. J Biol Chem 287(7):5122–5132. doi:10.1074/jbc.M111.297192
Talbot C, Lytle C (2010) Segregation of Na+/H+ exchanger-3 and Cl−/HCO3 exchanger SLC26A3 (DRA) in rodent cecum and colon. Am J Physiol Gastrointest Liver Physiol 299(2):G358–G367. doi:10.1152/ajpgi.00151.2010
Silberg DG, Wang W, Moseley RH, Traber PG (1995) The down-regulated in adenoma (dra) gene encodes an intestine-specific membrane sulfate transport protein. J Biol Chem 270(20):11897–11902
Hoglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg ML, Airola K, Holmberg C, de la Chapelle A, Kere J (1996) Mutations of the down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet 14(3):316–319. doi:10.1038/ng1196-316
Turnberg LA (1971) Abnormalities in intestinal electrolyte transport in congenital chloridorrhoea. Gut 12(7):544–551. doi:10.1136/gut.12.7.544
Bieberdorf FA, Fordtran JS, Gorden P (1972) Pathogenesis of congenital alkalosis with diarrhea—implications for physiology of normal ileal electrolyte absorption and secretion. J Clin Investig 51(8):1958–1968. doi:10.1172/jci107002
Holmberg C, Perheentupa J, Launiala K (1975) Colonic electrolyte transport in health and in congenital chloride diarrhea. J Clin Investig 56(2):302–310. doi:10.1172/jci108094
Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang ZH, Soleimani M (2006) slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem 281(49):37962–37971. doi:10.1074/jbc.M607527200
Schweinfest CW, Henderson KW, Suster S, Kondoh N, Papas TS (1993) Identification of a colon mucosa gene that is down-regulated in colon adenomas and adenocarcinomas. Proc Natl Acad Sci USA 90(9):4166–4170. doi:10.1073/pnas.90.9.4166
Byeon MK, Westerman MA, Maroulakou IG, Henderson KW, Suster S, Zhang XK, Papas TS, Vesely J, Willingham MC, Green JE, Schweinfest CW (1996) The down-regulated in adenoma (DRA) gene encodes an intestine-specific membrane glycoprotein. Oncogene 12(2):387–396
Jacob P, Rossmann H, Lamprecht G, Kretz A, Neff C, Lin-Wu E, Gregor M, Groneberg DA, Kere J, Seidler U (2002) Down-regulated in adenoma mediates apical Cl−/HCO3 − exchange in rabbit, rat, and human duodenum. Gastroenterology 122(3):709–724. doi:10.1053/gast.2002.31875
Wang ZH, Wang T, Petrovic S, Tuo BG, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M (2005) Renal and intestinal transport defects in Slc26a6-null mice. Am J Physiol Cell Physiol 288(4):C957–C965. doi:10.1152/ajpcell.00505.2004
Barmeyer C, Ye JHQ, Sidani S, Geibel J, Binder HJ, Rajendran VM (2007) Characteristics of rat downregulated in adenoma (rDRA) expressed in HEK 293 cells. Pflug Archiv Eur J Physiol 454(3):441–450. doi:10.1007/s00424-007-0213-7
Wedenoja S, Ormala T, Berg UB, Halling SFE, Jalanko H, Karikoski R, Kere J, Holmberg C, Hoglund P (2008) The impact of sodium chloride and volume depletion in the chronic kidney disease of congenital chloride diarrhea. Kidney Int 74(8):1085–1093. doi:10.1038/ki.2008.401
Chernova MN, Jiang LW, Shmukler BE, Schweinfest CW, Blanco P, Freedman SD, Stewart AK, Alper SL (2003) Acute regulation of the SLC26A3 congenital chloride diarrhoea anion exchanger (DRA) expressed in Xenopus oocytes. J Physiol Lond 549(1):3–19. doi:10.1113/jphysiol.2003.039818
Clark JS, Vandorpe DH, Chernova MN, Heneghan JF, Stewart AK, Alper SL (2008) Species differences in Cl− affinity and in electrogenicity of SLC26A6-mediated oxalate/Cl− exchange correlate with the distinct human and mouse susceptibilities to nephrolithiasis. J Physiol Lond 586(5):1291–1306. doi:10.1113/jphysiol.2007.143222
Lamprecht G, Baisch S, Schoenleber E, Gregor M (2005) Transport properties of the human intestinal anion exchanger DRA (down-regulated in adenoma) in transfected HEK293 cells. Pflug Archiv Eur J Physiol 449(5):479–490. doi:10.1007/s00424-004-1342-x
Stewart AK, Shmukler BE, Vandorpe DH, Reimold F, Heneghan JF, Nakakuki M, Akhavein A, Ko S, Ishiguro H, Alper SL (2011) SLC26 anion exchangers of guinea pig pancreatic duct: molecular cloning and functional characterization. Am J Physiol Cell Physiol 301(2):C289–C303. doi:10.1152/ajpcell.00089.2011
Whittamore JM, Freel RW, Hatch M (2013) Sulfate secretion and chloride absorption are mediated by the anion exchanger DRA (Slc26a3) in the mouse cecum. Am J Physiol Gastrointest Liver Physiol 305(2):G172–G184. doi:10.1152/ajpgi.00084.2013
Shcheynikov N, Wang Y, Park M, Ko SBH, Dorwart M, Naruse S, Thomas PJ, Muallem S (2006) Coupling modes and stoichiometry of Cl−/HCO3 − exchange by slc26a3 and slc26a6. J Gen Physiol 127(5):511–524. doi:10.1085/jgp.200509392
Ohana E, Shcheynikov N, Yang DK, So I, Muallem S (2011) Determinants of coupled transport and uncoupled current by the electrogenic SLC26 transporters. J Gen Physiol 137(2):239–251. doi:10.1085/jgp.201010531
Song Y, Yamamoto A, Steward MC, Ko SBH, Stewart AK, Soleimani M, Liu BC, Kondo T, Jin CX, Ishiguro H (2012) Deletion of Slc26a6 alters the stoichiometry of apical Cl−/HCO3 − exchange in mouse pancreatic duct. Am J Physiol Cell Physiol 303(8):C815–C824. doi:10.1152/ajpcell.00151.2012
Wang ZH, Petrovic S, Mann E, Soleimani M (2002) Identification of an apical Cl−/HCO3 − exchanger in the small intestine. Am J Physiol Gastrointest Liver Physiol 282(3):G573–G579. doi:10.1152/ajpgi.00338.2001
Walker NM, Simpson JE, Yen PF, Gill RK, Rigsby EV, Brazill JM, Dudeja PK, Schweinfest CW, Clarke LL (2008) Down-regulated in adenoma Cl−/HCO3 − exchanger couples with Na+/H+ exchanger 3 for NaCl absorption in murine small intestine. Gastroenterology 135(5):1645–1653. doi:10.1053/j.gastro.2008.07.083
Xia WL, Yu Q, Riederer B, Singh AK, Engelhardt R, Yeruva S, Song PH, Tian DA, Soleimani M, Seidler U (2014) The distinct roles of anion transporters Slc26a3 (DRA) and Slc26a6 (PAT-1) in fluid and electrolyte absorption in the murine small intestine. Pflug Archiv Eur J Physiol 466(8):1541–1556. doi:10.1007/s00424-013-1381-2
Lohi H, Lamprecht G, Markovich D, Heil A, Kujala M, Seidler U, Kere J (2003) Isoforms of SLC26A6 mediate anion transport and have functional PDZ interaction domains. Am J Physiol Cell Physiol 284(3):C769–C779. doi:10.1152/ajpcell.00270.2002
Anderle P, Sengstag T, Mutch DM, Rumbo M, Praz V, Mansourian R, Delorenzi M, Williamson G, Roberts MA (2005) Changes in the transcriptional profile of transporters in the intestine along the anterior–posterior and crypt-villus axes. BMC Genom 6. doi:10.1186/1471-2164-6-69
Hassan HA, Cheng M, Aronson PS (2012) Cholinergic signaling inhibits oxalate transport by human intestinal T84 cells. Am J Physiol Cell Physiol 302(1):C46–C58. doi:10.1152/ajpcell.00075.2011
Fordtran JS, Locklear TW (1966) Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am J Dig Dis 11(7):503–521. doi:10.1007/bf02233563
Li H, Ye ZQ, He W, Xia D, Aliya AY, Shen JH, Chen ZQ (2012) Screening of differentially expressed genes in the jejunum of rats with idiopathic hyperoxaluria. Chin Med J 125(2):312–315. doi:10.3760/cma.j.issn.0366-6999.2012.02.027
Monico CG, Weinstein A, Jiang ZR, Rohlinger AL, Cogal AG, Bjornson BB, Olson JB, Bergstralh EJ, Milliner DS, Aronson PS (2008) Phenotypic and functional analysis of human SLC26A6 variants in patients with familial hyperoxaluria and calcium oxalate nephrolithiasis. Am J Kidney Dis 52(6):1096–1103. doi:10.1053/j.ajkd.2008.07.041
Corbetta S, Eller-Vainicher C, Frigerio M, Vataperta R, Costa E, Vicentini L, Baccarelli A, Beck-Peccoz P, Spada A (2009) Analysis of the 206M polymorphic variant of the SLC26A6 gene encoding a Cl− oxalate transporter in patients with primary hyperparathyroidism. Eur J Endocrinol 160(2):283–288. doi:10.1530/eje-08-0623
Field M (2003) Intestinal ion transport and the pathophysiology of diarrhea. J Clin Investig 111(7):931–943. doi:10.1172/jci200318326
Kato A, Romero MF (2011) Regulation of electroneutral NaCl absorption by the small intestine. Annu Rev Physiol 73:261–281. doi:10.1146/annurev-physiol-012110-142244
Hassan HA, Mentone S, Karniski LP, Rajendran VM, Aronson PS (2007) Regulation of anion exchanger Slc26a6 by protein kinase C. Am J Physiol Cell Physiol 292(4):C1485–C1492. doi:10.1152/ajpcell.00447.2006
Amin R, Sharma S, Ratakonda S, Hassan HA (2013) Extracellular nucleotides inhibit oxalate transport by human intestinal Caco-2-BBe cells through PKC-delta activation. Am J Physiol Cell Physiol 305(1):C78–C89. doi:10.1152/ajpcell.00339.2012
Reimold FR, Heneghan JF, Stewart AK, Zelikovic I, Vandorpe DH, Shmukler BE, Alper SL (2011) Pendrin function and regulation in Xenopus oocytes. Cell Physiol Biochem 28(3):435–450. doi:10.1159/000335106
Newton AC (1995) Protein-kinase-C - Structure, function, and regulation. J Biol Chem 270(48):28495–28498. doi:10.1074/jbc.270.43.25526
Mellor H, Parker PJ (1998) The extended protein kinase C superfamily. Biochem J 332:281–292
Zeng L, Webster SV, Newton PM (2012) The biology of protein kinase C. Calcium Signal 740:639–661. doi:10.1007/978-94-007-2888-2_28
Di Mari JF, Mifflin RC, Powell DW (2005) The role of protein kinase C in gastrointestinal function and disease. Gastroenterology 128(7):2131–2146. doi:10.1053/j.gastro.2004.09.078
Nishizuka Y (1984) The role of Protein Kinase-C in cell-surface signal transduction and tumor promotion. Nature 308(5961):693–698. doi:10.1038/308693a0
Flores CA, Cid LP, Sepulveda FV (2010) Strain-dependent differences in electrogenic secretion of electrolytes across mouse colon epithelium. Exp Physiol 95(6):686–698. doi:10.1113/expphysiol.2009.051102
Davies SP, Reddy H, Caivano M, Cohen P (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351:95–105. doi:10.1042/0264-6021:3510095
Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JSC, Alessi DR, Cohen P (2007) The selectivity of protein kinase inhibitors: a further update. Biochem J 408:297–315. doi:10.1042/bj20070797
Soltoff SP (2007) Rottlerin: an inappropriate and ineffective inhibitor of PKC delta. Trends Pharmacol Sci 28(9):453–458. doi:10.1016/j.tips.2007.07.003
Wu-Zhang AX, Newton AC (2013) Protein kinase C pharmacology: refining the toolbox. Biochem J 452:195–209. doi:10.1042/bj20130220
Tuo BG, Chow JYC, Barrett KE, Isenberg JI (2004) Protein kinase C potentiates cAMP-stimulated mouse duodenal mucosal bicarbonate secretion in vitro. Am J Physiol Gastrointest Liver Physiol 286(5):G814–G821. doi:10.1152/ajpgi.00251.2003
Tuo BG, Riederer B, Wang ZH, Colledge WH, Soleimani M, Seidler U (2006) Involvement of the anion exchanger SLC26A6 in prostaglandin E2- but not forskolin-stimulated duodenal HCO3 − secretion. Gastroenterology 130(2):349–358. doi:10.1053/j.gastro.2005.10.017
Song JC, Hanson CM, Tsai V, Farokhzad OC, Lotz M, Matthews JB (2001) Regulation of epithelial transport and barrier function by distinct protein kinase C isoforms. Am J Physiol Cell Physiol 281(2):C649–C661
Del Castillo IC, Fedor-Chaiken M, Song JC, Starlinger V, Yoo J, Matlin KS, Matthews JB (2005) Dynamic regulation of Na+−K+−2Cl− cotransporter surface expression by PKC-ε in Cl−secretory epithelia. Am J Physiol Cell Physiol 289(5):C1332–C1342. doi:10.1152/ajpcell.00580.2004
Heneghan JF, Alper SL (2012) This, too, shall pass—like a kidney stone: a possible path to prophylaxis of nephrolithiasis? Focus on “Cholinergic signaling inhibits oxalate transport by human intestinal T84 cells”. Am J Physiol Cell Physiol 302(1):C18–C20. doi:10.1152/ajpcell.00389.2011
Field M, McColl I (1973) Ion-transport in rabbit ileal mucosa 3. Effects of catecholamines. Am J Physiol 225(4):852–857
Hubel KA (1976) Intestinal ion-transport—effect of norepinephrine, pilocarpine, and atropine. Am J Physiol 231(1):252–257
Chang EB, Field M, Miller RJ (1982) Alpha-2-adrenergic receptor regulation of ion-transport in rabbit ileum. Am J Physiol Gastrointest Liver Physiol 242(3):G237–G242
Sellin JH, Desoignie R (1987) Regulation of Na-Cl absorption in rabbit proximal colon in vitro. Am J Physiol Gastroint Liver Physiol 252(1):G45–G51
Hirota CL, McKay DM (2006) Cholinergic regulation of epithelial ion transport in the mammalian intestine. Br J Pharmacol 149(5):463–479. doi:10.1038/sj.bjp.0706889
Novak I (2011) Purinergic signalling in epithelial ion transport: regulation of secretion and absorption. Acta Physiol 202(3):501–522. doi:10.1111/j.1748-1716.2010.02225.x
Burnstock G (2014) Purinergic signalling in the gastrointestinal tract and related organs in health and disease. Purinergic Signal 10(1):3–50. doi:10.1007/s11302-013-9397-9
Abraham C, Scaglione-Sewell B, Skarosi SF, Qin WY, Bissonnette M, Brasitus TA (1998) Protein kinase C alpha modulates growth and differentiation in Caco-2 cells. Gastroenterology 114(3):503–509. doi:10.1016/s0016-5085(98)70533-5
Wald FA, Oriolo AS, Mashukova A, Fregien NL, Langshaw AH, Salas PJI (2008) Atypical protein kinase C (iota) activates ezrin in the apical domain of intestinal epithelial cells. J Cell Sci 121(5):644–654. doi:10.1242/jcs.016246
Suzuki T, Elias BC, Seth A, Shen L, Turner JR, Giorgianni F, Desiderio D, Guntaka R, Rao R (2009) PKC-η regulates occludin phosphorylation and epithelial tight junction integrity. Proc Natl Acad Sci USA 106(1):61–66. doi:10.1073/pnas.0802741106
Alrefai WA, Scaglione-Sewell B, Tyagi S, Wartman L, Brasitus TA, Ramaswamy K, Dudeja PK (2001) Differential regulation of the expression of Na+/H+ exchanger isoform NHE3 by PKC-alpha in Caco-2 cells. Am J Physiol Cell Physiol 281(5):C1551–C1558
Saksena S, Gill RK, Syed IA, Tyagi S, Alrefai WA, Ramaswamy K, Dudeja PK (2002) Inhibition of apical Cl−/OH− exchange activity in Caco-2 cells by phorbol esters is mediated by PKC-ε. Am J Physiol Cell Physiol 283(5):C1492–C1500. doi:10.1152/ajpcell.00473.2001
Stenson WF, Easom RA, Riehl TE, Turk J (1993) Regulation of paracellular permeability in Caco-2 cell monolayers by protein-kinase-C. Am J Physiol Gastrointest Liver Physiol 265(5):G955–G962
Turner JR, Angle JM, Black ED, Joyal JL, Sacks DB, Madara JL (1999) PKC-dependent regulation of transepithelial resistance: roles of MLC and MLC kinase. Am J Physiol Cell Physiol 277(3):C554–C562
Ohana E, Shcheynikov N, Moe OW, Muallem S (2013) SLC26A6 and NaDC-1 transporters interact to regulate oxalate and citrate homeostasis. J Am Soc Nephrol 24(10):1617–1626
Moe OW, Preisig PA (2006) Dual role of citrate in mammalian urine. Curr Opin Nephrol Hypertens 15(4):419–424. doi:10.1097/01.mnh.0000232882.35469.72
Sakhaee K, Maalouf NM, Sinnott B (2012) Kidney Stones 2012: pathogenesis, diagnosis, and management. J Clin Endocrinol Metab 97(6):1847–1860. doi:10.1210/jc.2011-3492
Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS (2001) Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci USA 98(16):9425–9430. doi:10.1073/pnas.141241098
Pajor AM (2014) Sodium-coupled dicarboxylate and citrate transporters from the SLC13 family. Pflug Archiv Eur J Physiol 466(1):119–130. doi:10.1007/s00424-013-1369-y
Garcia-Perez I, Villasenor A, Wijeyesekera A, Posma JM, Jiang ZR, Stamler J, Aronson P, Unwin R, Barbas C, Elliott P, Nicholson J, Holmes E (2012) Urinary metabolic phenotyping the slc26a6 (Chloride-oxalate exchanger) null mouse model. J Proteome Res 11(9):4425–4435. doi:10.1021/pr2012544
Whittamore JM, Hatch M (2015) Chronic metabolic acidosis reduces urinary oxalate excretion and promotes intestinal oxalate secretion in the rat. Urolithiasis 43(6):489–499. doi:10.1007/s00240-015-0801-5
Charney AN, Goldfarb DS, Dagher PC (1995) Metabolic disorders associated with gastrointestinal disease. In: Arieff AI, DeFronzo RA (eds) Fluid, electrolyte, and acid-base disorders, 2nd edn. Churchill Livingstone, New York, pp 813–836
Gennari FJ, Weise WJ (2008) Acid-base disturbances in gastrointestinal disease. Clin J Am Soc Nephrol 3(6):1861–1868. doi:10.2215/cjn.02450508
Charney AN, Feldman GM (1984) Systemic acid-base-disorders and intestinal electrolyte transport. Am J Physiol Gastrointest Liver Physiol 247(1):G1–G12
Charney AN, Dagher PC (1996) Acid-base effects on colonic electrolyte transport revisited. Gastroenterology 111(5):1358–1368. doi:10.1053/gast.1996.v111.agast961111358
Charney AN, Egnor RW, Alexander-Chacko J, Cassai N, Sidhu GS (2002) Acid-base effects on intestinal Na+ absorption and vesicular trafficking. Am J Physiol Cell Physiol 283(3):C971–C979. doi:10.1152/ajpcell.00079.2002
Charney AN, Egnor RW, Henner D, Rashid H, Cassai N, Sidhu GS (2004) Acid-base effects on intestinal Cl− absorption and vesicular trafficking. Am J Physiol Cell Physiol 286(5):C1062–C1070. doi:10.1152/ajpcell.00454.2003
Alvarez BV, Vilas GL, Casey JR (2005) Metabolon disruption: a mechanism that regulates bicarbonate transport. EMBO J 24(14):2499–2511. doi:10.1038/sj.emboj.7600736
Bushinsky DA, Grynpas MD, Asplin JR (2001) Effect of acidosis on urine supersaturation and stone formation in genetic hypercalciuric stone-forming rats. Kidney Int 59(4):1415–1423. doi:10.1046/j.1523-1755.2001.0590041415.x
Hatch M, Freel RW (2003) Renal and intestinal handling of oxalate following oxalate loading in rats. Am J Nephrol 23(1):18–26. doi:10.1159/000066300
Osther PJ, Bollerslev J, Norgard JR, Engel K, Kildeberg P (1994) Effects of acute acid loading on the risk of calcium-phosphate and calcium-oxalate crystallization in urine. Scanning Microsc 8(1):63–69
Ahlstrand C, Tiselius HG (1987) Urine composition and stone formation during treatment with acetazolamide. Scand J Urol Nephrol 21(3):225–228
Higashihara E, Nutahara K, Takeuchi T, Shoji N, Araie M, Aso Y (1991) Calcium-metabolism in acidotic patients induced by carbonic-anhydrase inhibitors—responses to citrate. J Urol 145(5):942–948
Welch BJ, Graybeal D, Moe OW, Maalouf NM, Sakhaee K (2006) Biochemical and stone-risk profiles with topiramate treatment. Am J Kidney Dis 48(4):555–563. doi:10.1053/j.ajkd.2006.07.003
Kaplon DM, Penniston KL, Nakada SY (2011) Patients with and without prior urolithiasis have hypocitraturia and incident kidney stones while on topiramate. Urology 77(2). doi:10.1016/j.urology.2010.06.048
Encinosa WE, Bernard DM, Chen CC, Steiner CA (2006) Healthcare utilization and outcomes after bariatric surgery. Med Care 44(8):706–712. doi:10.1097/01.mlr.0000220833.89050.ed
Canales BK, Hatch M (2014) Kidney stone incidence and metabolic urinary changes after modern bariatric surgery: review of clinical studies, experimental models, and prevention strategies. Surg Obes Relat Dis 10(4):734–742. doi:10.1016/j.soard.2014.03.026
Hatch M, Canales BK (2015) The mechanistic basis of hyperoxaluria following gastric bypass in obese rats. Urolithiasis. doi:10.1007/s00240-015-0836-7
Dawson KA, Allison MJ, Hartman PA (1980) Characteristics of anaerobic oxalate-degrading enrichment cultures from the rumen. Appl Environ Microbiol 40(4):840–846
Knight J, Deora R, Assimos DG, Holmes RP (2013) The genetic composition of Oxalobacter formigenes and its relationship to colonization and calcium oxalate stone disease. Urolithiasis 41(3):187–196. doi:10.1007/s00240-013-0566-7
Asplin JR (2016) The management of patients with enteric hyperoxaluria. Urolithiasis 44(1):33–43. doi:10.1007/s00240-015-0846-5
Liebman M, Al-Wahsh IA (2011) Probiotics and other key determinants of dietary oxalate absorption. Adv Nutr 2(3):254–260. doi:10.3945/an.111.000414
Abratt VR, Reid SJ (2010) Oxalate-degrading bacteria of the human gut as probiotics in the management of kidney stone disease. Adv Appl Microbiol 72:63–87
Hoppe B, Groothoff JW, Hulton SA, Cochat P, Niaudet P, Kemper MJ, Deschenes G, Unwin R, Milliner D (2011) Efficacy and safety of Oxalobacter formigenes to reduce urinary oxalate in primary hyperoxaluria. Nephrol Dial Transplant 26(11):3609–3615. doi:10.1093/ndt/gfr107
Jiang J, Knight J, Easter LH, Neiberg R, Holmes RP, Assimos DG (2011) Impact of dietary calcium and oxalate, and Oxalobacter formigenes colonization on urinary oxalate excretion. J Urol 186(1):135–139. doi:10.1016/j.juro.2011.03.006
Lieske JC, Tremaine WJ, De Simone C, O’Connor HM, Li X, Bergstralh EJ, Goldfarb DS (2010) Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int 78(11):1178–1185. doi:10.1038/ki.2010.310
Siener R, Bade DJ, Hesse A, Hoppe B (2013) Dietary hyperoxaluria is not reduced by treatment with lactic acid bacteria. J Transl Med 11:306. doi:10.1186/1479-5876-11-306
Ellis ML, Shaw KJ, Jackson SB, Daniel SL, Knight J (2015) Analysis of commercial kidney stone probiotic supplements. Urology 85(3):517–521. doi:10.1016/j.urology.2014.11.013
Hassan H, Arvans D, Cheng M, Musch MW, Chang EB (2011) Oxalobacter formigenes conditioned medium stimulates oxalate transport by human intestinal cells. J Am Soc Nephrol 22:383A (Abstract)
Arvans D, Musch M, Chang E, Hassan H, Cheng M (2012) Oxalobacter formigenes conditioned medium stimulates oxalate transport by human intestinal cells. J Investig Med 60(4):738–738 (Abstract)
Raheja G, Singh V, Ma K, Boumendjel R, Borthakur A, Gill RK, Saksena S, Alrefai WA, Ramaswamy K, Dudeja PK (2010) Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am J Physiol Gastrointest Liver Physiol 298(3):G395–G401. doi:10.1152/ajpgi.00465.2009
Borthakur A, Gill RK, Tyagi S, Koutsouris A, Alrefai WA, Hecht GA, Ramaswamy K, Dudeja PK (2008) The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J Nutr 138(7):1355–1359
Singh V, Kumar A, Raheja G, Anbazhagan AN, Priyamvada S, Saksena S, Jhandier MN, Gill RK, Alrefai WA, Borthakur A, Dudeja PK (2014) Lactobacillus acidophilus attenuates downregulation of DRA function and expression in inflammatory models. Am J Physiol Gastrointest Liver Physiol 307(6):G623–G631. doi:10.1152/ajpgi.00104.2014
Klimesova K, Whittamore JM, Hatch M (2015) Bifidobacterium animalis subsp. lactis decreases urinary oxalate excretion in a mouse model of primary hyperoxaluria. Urolithiasis 43(2):107–117. doi:10.1007/s00240-014-0728-2
Kumar A, Hecht C, Priyamvada S, Anbazhagan AN, Alakkam A, Borthakur A, Alrefai WA, Gill RK, Dudeja PK (2014) Probiotic Bifidobacterium species stimulate human SLC26A3 gene function and expression in intestinal epithelial cells. Am J Physiol Cell Physiol 307(12):C1084–C1092. doi:10.1152/ajpcell.00194.2014
Murphy C, Murphy S, O’Brien F, O’Donoghue M, Boileau T, Sunvold G, Reinhart G, Kiely B, Shanahan F, O’Mahony L (2009) Metabolic activity of probiotics-oxalate degradation. Vet Microbiol 136(1–2):100–107. doi:10.1016/j.vetmic.2008.10.005
Sheldon RJ, Malarchik ME, Fox DA, Burks TF, Porreca F (1989) Pharmacological characterization of neural mechanisms regulating mucosal ion-transport in mouse jejunum. J Pharmacol Exp Ther 249(2):572–582
Seidler U, Blumenstein I, Kretz A, Viellard-Baron D, Rossmann H, Colledge WH, Evans M, Ratcliff R, Gregor M (1997) A functional CFTR protein is required for mouse intestinal cAMP-, cGMP- and Ca2+-dependent HCO3 − secretion. J Physiol Lond 505(2):411–423. doi:10.1111/j.1469-7793.1997.411bb.x
Acknowledgements
The authors wish to thank Tara Braun, Kristina Fernandez, Heran Getachew, Shreya Mishra, Candi Morris, Susie Robertson and Tisha Van Pelt who provided valuable technical assistance and animal husbandry over the period spanning the collection of data presented here. We are also grateful to Dr. Robert W. Freel, who performed some of the experiments reported and for many useful discussions on epithelial oxalate transport and the transporters involved. This work, and the data presented herein, has been supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants DK-056245, DK-081624, DK-088892 to M. Hatch, and U54 DK-083908 (sub-award to J. M. Whittamore), from the National Institutes of Health, together with funding from the Oxalosis and Hyperoxaluria Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures involving animals were performed under protocols approved by the University of Florida Institutional Animal Care and Use Committee (IACUC), in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Whittamore, J.M., Hatch, M. The role of intestinal oxalate transport in hyperoxaluria and the formation of kidney stones in animals and man. Urolithiasis 45, 89–108 (2017). https://doi.org/10.1007/s00240-016-0952-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-016-0952-z