Abstract
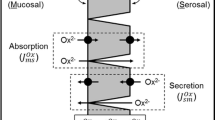
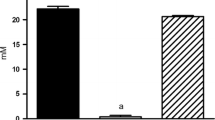
Oxalobacter sp. promotion of enteric oxalate excretion, correlating with reductions in urinary oxalate excretion, was previously reported in rats and mice, but the mechanistic basis for this affect has not been described. The main objective of the present study was to determine whether the apical oxalate transport proteins, PAT1 (slc26a6) and DRA (slc26a3), are involved in mediating the Oxalobacter-induced net secretory flux across colonized mouse cecum and distal colon. We measured unidirectional and net fluxes of oxalate across tissues removed from colonized PAT1 and DRA knockout (KO) mice and also across two double knockout (dKO) mouse models with primary hyperoxaluria, type 1 (i.e., deficient in alanine-glyoxylate aminotransferase; AGT KO), including PAT1/AGT dKO and DRA/AGT dKO mice compared to non-colonized mice. In addition, urinary oxalate excretion was measured before and after the colonization procedure. The results demonstrate that Oxalobacter can induce enteric oxalate excretion in the absence of either apical oxalate transporter and urinary oxalate excretion was reduced in all colonized genotypes fed a 1.5% oxalate-supplemented diet. We conclude that there are other, as yet unidentified, oxalate transporters involved in mediating the directional changes in oxalate transport across the Oxalobacter-colonized mouse large intestine.




Similar content being viewed by others
References
Alper SL, Sharma AK (2013) The SLC26 gene family of anion transporters and channels. Mol Aspects Med 34:494–515
Arvans D, Jung YC, Antonopoulos D, Koval J, Granja I, Bashir M, Karrar E, Roy-Chowdhury J, Musch M, Asplin J, Chang E, Hassan H (2017) Oxalobacter formigenes-derived bioactive factors stimulate oxalate transport by intestinal epithelial cells. J Am Soc Nephrol 28:876–887
Arvans D, Musch M, Chang E, Hassan H, Cheng M (2012) Oxalobacter formigenes conditioned medium stimulates oxalate transport by human intestinal cells. J Investig Med 60:738
Campieri C, Campieri M, Bertuzzi V, Swennen E, Matteuzzi D, Stefoni S, Pirovano F, Centi C, Ulisse S, Famularo G, De Simone C (2001) Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int 60:1097–1105
Dawson PA, Russell CS, Lee S, McLeay SC, van Dongen JM, Cowley DM, Clarke LA, Markovich D (2010) Urolithiasis and hepatotoxicity are linked to the anion transporter Sat1 in mice. J Clin Investig 120:706–712
Freel RW, Hatch M, Green M, Soleimani M (2006) Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol 290:G719–G728
Freel RW, Whittamore JM, Hatch M (2013) Transcellular oxalate and Cl- absorption in mouse intestine is mediated by the DRA anion exchanger Slc26a3, and DRA deletion decreases urinary oxalate. Am J Physiol Gastrointest Liver Physiol 305:G520–G527
Green ML, Hatch M, Freel RW (2005) Ethylene glycol induces hyperoxaluria without metabolic acidosis in rats. Am J Physiol Renal Physiol 289:F536–F543
Hassan H, Arvans D, Cheng M, Musch M, Chang E (2011) Oxalobacter formigenes conditioned medium stimulates oxalate transport by human intestinal cells. J Am Soc Nephrol 22:383A
Hatch M (2017) Gut microbiota and oxalate homeostasis. Ann Transl Med 5:36
Hatch M, Cornelius J, Allison M, Sidhu H, Peck A, Freel RW (2006) Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int 69:691–698
Hatch M, Freel RW (2013) A human strain of Oxalobacter (HC-1) promotes enteric oxalate secretion in the small intestine of mice and reduces urinary oxalate excretion. Urolithiasis 41:379–384
Hatch M, Gjymishka A, Salido EC, Allison MJ, Freel RW (2011) Enteric oxalate elimination is induced and oxalate is normalized in a mouse model of primary hyperoxaluria following intestinal colonization with Oxalobacter. Am J Physiol Gastrointest Liver Physiol 300:G461–G469
Heneghan JF, Akhavein A, Salas MJ, Shmukler BE, Karniski LP, Vandorpe DH, Alper SL (2010) Regulated transport of sulfate and oxalate by SLC26A2/DTDST. Am J Physiol Cell Physiol 298:C1363–C1375
Heneghan JF, Alper SL (2011) This, too, shall pass–like a kidney stone: a possible path to prophylaxis of nephrolithiasis? Focus on “Cholinergic signaling inhibits oxalate transport by human intestinal T84 cells”. Am J Physiol Cell Physiol 302:C18–C20
Hoppe B, Beck B, Gatter N, von Unruh G, Tischer A, Hesse A, Laube N, Kaul P, Sidhu H (2006) Oxalobacter formigenes: a potential tool for the treatment of primary hyperoxaluria type 1. Kidney Int 70:1305–1311
Hoppe B, Groothoff JW, Hulton SA, Cochat P, Niaudet P, Kemper MJ, Deschenes G, Unwin R, Milliner D (2011) Efficacy and safety of Oxalobacter formigenes to reduce urinary oxalate in primary hyperoxaluria. Nephrol Dial Transplant 26:3609–3615
Hoppe B, von Unruh G, Laube N, Hesse A, Sidhu H (2005) Oxalate degrading bacteria: new treatment option for patients with primary and secondary hyperoxaluria? Urol Res 33:372–375
Jiang J, Knight J, Easter LH, Neiberg R, Holmes RP, Assimos DG (2011) Impact of dietary calcium and oxalate, and Oxalobacter formigenes colonization on urinary oxalate excretion. J Urol 186:135–139
Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS (2006) Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38:474–478
Klimesova K, Whittamore JM, Hatch M (2015) Bifidobacterium animalis subsp. lactis decreases urinary oxalate excretion in a mouse model of primary hyperoxaluria. Urolithiasis 43:107–117
Ko N, Knauf F, Jiang Z, Markovich D, Aronson PS (2012) Sat1 is dispensable for active oxalate secretion in mouse duodenum. Am J Physiol Cell Physiol 303:C52–C57
Kwak C, Kim HK, Kim EC, Choi MS, Kim HH (2003) Urinary oxalate levels and the enteric bacterium Oxalobacter formigenes in patients with calcium oxalate urolithiasis. Eur Urol 44:475–481
Liebman M, Al-Wahsh IA (2011) Probiotics and other key determinants of dietary oxalate absorption. Adv Nutr 2:254–260
Lieske JC, Goldfarb DS, De Simone C, Regnier C (2005) Use of a probiotic to decrease enteric hyperoxaluria. Kidney Int 68:1244–1249
Lieske JC, Tremaine WJ, De Simone C, O’Connor HM, Li X, Bergstralh EJ, Goldfarb DS (2010) Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int 78:1178–1185
Markovich D (2012) Slc13a1 and Slc26a1 KO models reveal physiological roles of anion transporters. Physiology (Bethesda) 27:7–14
Mikami K, Akakura K, Takei K, Ueda T, Mizoguchi K, Noda M, Miyake M, Ito H (2003) Association of absence of intestinal oxalate degrading bacteria with urinary calcium oxalate stone formation. Int J Urol 10:293–296
Milliner D, Hoppe B, Groothoff J (2018) A randomised Phase II/III study to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Urolithiasis 46:313–323
Mittal RD, Kumar R, Mittal B, Prasad R, Bhandari M (2003) Stone composition, metabolic profile and the presence of the gut-inhabiting bacterium Oxalobacter formigenes as risk factors for renal stone formation. Med Princ Pract 12:208–213
Neuhaus TJ, Belzer T, Blau N, Hoppe B, Sidhu H, Leumann E (2000) Urinary oxalate excretion in urolithiasis and nephrocalcinosis. Arch Dis Child 82:322–326
Ohana E, Shcheynikov N, Park M, Muallem S (2012) Solute carrier family 26 member a2 (Slc26a2) protein functions as an electroneutral SOFormula/OH–/Cl– exchanger regulated by extracellular Cl–. J Biol Chem 287:5122–5132
Robijn S, Hoppe B, Vervaet BA, D’Haese PC, Verhulst A (2011) Hyperoxaluria: a gut-kidney axis? Kidney Int 80:1146–1158
Satoh H, Susaki M, Shukunami C, Iyama K, Negoro T, Hiraki Y (1998) Functional analysis of diastrophic dysplasia sulfate transporter. Its involvement in growth regulation of chondrocytes mediated by sulfated proteoglycans. J Biol Chem 273:12307–12315
Sidhu H, Schmidt ME, Cornelius JG, Thamilselvan S, Khan SR, Hesse A, Peck AB (1999) Direct correlation between hyperoxaluria/oxalate stone disease and the absence of the gastrointestinal tract-dwelling bacterium Oxalobacter formigenes: possible prevention by gut recolonization or enzyme replacement therapy. J Am Soc Nephrol 10(Suppl 14):S334–S340
Siener R, Bade DJ, Hesse A, Hoppe B (2013) Dietary hyperoxaluria is not reduced by treatment with lactic acid bacteria. J Transl Med 11:306
Siener R, Bangen U, Sidhu H, Honow R, von Unruh G, Hesse A (2013) The role of Oxalobacter formigenes colonization in calcium oxalate stone disease. Kidney Int 83:1144–1149
Troxel SA, Sidhu H, Kaul P, Low RK (2003) Intestinal Oxalobacter formigenes colonization in calcium oxalate stone formers and its relation to urinary oxalate. J Endourol 17:173–176
Whittamore JM, Hatch M (2017) The role of intestinal oxalate transport in hyperoxaluria and the formation of kidney stones in animals and man. Urolithiasis 45:89–108
Whittamore JM, Stephens CE, Hatch M (2018) Absence of the sulfate transporter SAT-1 (Slc26a1) has no impact on oxalate handling by mouse intestine and does not cause hyperoxaluria or hyperoxalemia. Am J Physiol Gastrointest Liver Physiol 316:G82–G94 (E-Pub ahead of print)
Acknowledgements
The authors thank Shreya Mishra, Heran Getachew, and Tisha Van Pelt for excellent technical assistance and animal husbandry.
Funding
This work was supported by NIH Grant Nos. DK088892 and DK081624 in addition to a Grant from the Oxalosis and Hyperoxaluria Foundation.
Author information
Authors and Affiliations
Contributions
MH is responsible for conception and design of the research and MH performed the flux experiments. MH analyzed the data and interpreted the results and MH drafted the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted. This article does not contain any studies with human participants performed by the author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hatch, M. Induction of enteric oxalate secretion by Oxalobacter formigenes in mice does not require the presence of either apical oxalate transport proteins Slc26A3 or Slc26A6. Urolithiasis 48, 1–8 (2020). https://doi.org/10.1007/s00240-019-01144-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-019-01144-y