Abstract
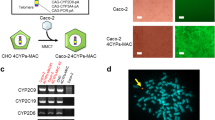
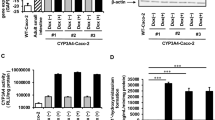
The small intestine plays a critical role in the absorption and metabolism of orally administered drugs. Therefore, a model capable of evaluating drug absorption and metabolism in the small intestine would be useful for drug discovery. Patients with genotype UGT1A1*6 (exon 1, 211G > A) treated with the antineoplastic drug SN-38 have been reported to exhibit decreased glucuronide conjugation and increased incidence of intestinal toxicity and its severe side effects, including severe diarrhea. To ensure the safety of drugs, we must develop a drug metabolism and toxicity evaluation model which considers UGT1A1*6. In this study, we generated CYP3A4·POR·UGT1A1 KI- and CYP3A4·POR·UGT1A1*6 KI-Caco-2 cells for pharmaceutical research using a PITCh system. The CYP3A4·POR·UGT1A1 KI-Caco-2 cells were shown to express functional CYP3A4 and UGT1A1. The CYP3A4·POR·UGT1A1*6 KI-Caco-2 cells were sensitive to SN-38-induced intestinal toxicity. We thus succeeded in generating CYP3A4·POR·UGT1A1 KI- and CYP3A4·POR·UGT1A1*6 KI-Caco-2 cells, which can be used in pharmaceutical research. We also developed an intestinal epithelial cell model of patients with UGT1A1*6 and showed that it was useful as a tool for drug discovery.






Similar content being viewed by others
Data availability
The authors declare that all the data related to this study are available within the paper or can be obtained from the authors upon reasonable request.
References
Balimane PV, Chong S (2005) Cell culture-based models for intestinal permeability: a critique. Drug Discov Today 10:335–343. https://doi.org/10.1016/S1359-6446(04)03354-9
Cheng L, Li M, Hu J et al (2014) UGT1A1*6 polymorphisms are correlated with irinotecan-induced toxicity: a system review and meta-analysis in Asians. Cancer Chemother Pharmacol 73:551–560. https://doi.org/10.1007/s00280-014-2382-3
Crespi CL, Bruce W, Penman MH (1996) Development of Caco-2 cells expressing high levels of cDNA derived CYP3A4.pdf. Pharm Res 13:1635–1641
Cummins CL, Mangravite LM, Benet LZ (2001) Characterizing the expression of CYP3A4 and efflux transporters (P-gp, MRP1, and MRP2) in CYP3A4-transfected Caco-2 cells after induction with sodium butyrate and the phorbol ester 12-O-tetradecanoylphorbol-13-acetate. Pharm Res 18:1102–1109. https://doi.org/10.1023/A:1010914624111
Cummins CL, Jacobsen W, Christians U, Benet LZ (2004) CYP3A4-transfected Caco-2 cells as a tool for understanding biochemical absorption barriers: studies with sirolimus and midazolam. J Pharmacol Exp Ther 308:143–155. https://doi.org/10.1124/jpet.103.058065
Etienne-Grimaldi M-C, Boyer J-C, Thomas F et al (2015) UGT1A1 genotype and irinotecan therapy: general review and implementation in routine practice. Fundam Clin Pharmacol 29:219–237. https://doi.org/10.1111/fcp.12117
Farkas D, Greenblatt DJ (2008) Influence of fruit juices on drug disposition: discrepancies between in vitro and clinical studies. Expert Opin Drug Metab Toxicol 4:381–393. https://doi.org/10.1517/17425255.4.4.381
Fujita K, Kubota Y, Ishida H, Sasaki Y (2015) Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J Gastroenterol 21:12234–12248. https://doi.org/10.3748/wjg.v21.i43.12234
Guo LQ, Fukuda K, Ohta T, Yamazoe Y (2000) Role of furanocoumarin derivatives on grapefruit juice-mediated inhibition of human CYP3A activity. Drug Metab Dispos 28:766–771
Hu M, Li Y, Davitt CM et al (1999) Transport and metabolic characterization of Caco-2 cells expressing CYP3A4 and CYP3A4 plus oxidoreductase. Pharm Res 16:1352–1359. https://doi.org/10.1023/a:1018986605929
Ichikawa M, Negoro R, Kawai K et al (2021) Vinblastine treatment decreases the undifferentiated cell contamination of human iPSC-derived intestinal epithelial-like cells. Mol Ther Methods Clin Dev 20:463–472. https://doi.org/10.1016/j.omtm.2021.01.005
Kabeya T, Qiu S, Hibino M et al (2018) Cyclic AMP signaling promotes the differentiation of human induced pluripotent stem cells into intestinal epithelial cells. Drug Metab Dispos 46:1411–1419. https://doi.org/10.1124/dmd.118.082123
Kaniwa N, Kurose K, Jinno H et al (2005) Racial variability in haplotype frequencies of UGT1A1 and glucuronidation activity of a novel single nucleotide polymorphism 686C> T (P229L) found in an African-American. Drug Metab Dispos 33:458–465. https://doi.org/10.1124/dmd.104.001800
Kawai K, Negoro R, Ichikawa M et al (2020) Establishment of SLC15A1/PEPT1-knockout human-induced pluripotent stem cell line for intestinal drug absorption studies. Mol Ther Methods Clin Dev 17:49–57. https://doi.org/10.1016/j.omtm.2019.11.008
Lim GE, Li T, Buttar HS (2003) Interactions of grapefruit juice and cardiovascular medications: a potential risk of toxicity. Exp Clin Cardiol 8:99–107
Lown KS, Bailey DG, Fontana RJ et al (1997) Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest 99:2545–2553. https://doi.org/10.1172/JCI119439
Nagar S, Blanchard RL (2006) Pharmacogenetics of uridine diphosphoglucuronosyltransferase (UGT) 1A family members and its role in patient response to irinotecan. Drug Metab Rev 38:393–409. https://doi.org/10.1080/03602530600739835
Nakade S, Tsubota T, Sakane Y et al (2014) Microhomology-mediated end-joining-dependent integration of donor DNA in cells and animals using TALENs and CRISPR/Cas9. Nat Commun 5:1–8. https://doi.org/10.1038/ncomms6560
Negoro R, Takayama K, Nagamoto Y et al (2016) Modeling of drug-mediated CYP3A4 induction by using human iPS cell-derived enterocyte-like cells. Biochem Biophys Res Commun 472:631–636. https://doi.org/10.1016/j.bbrc.2016.03.012
Negoro R, Takayama K, Kawai K et al (2018) Efficient generation of small intestinal epithelial-like cells from human iPSCs for drug absorption and metabolism studies. Stem Cell Reports 11:1539–1550. https://doi.org/10.1016/j.stemcr.2018.10.019
Negoro R, Kawai K, Ichikawa M et al (2020) Establishment of MDR1-knockout human induced pluripotent stem cell line. Drug Metab Pharmacokinet 35:288–296. https://doi.org/10.1016/j.dmpk.2020.01.009
Nie Y-L, He H, Li J-F et al (2017) Hepatic expression of transcription factors affecting developmental regulation of UGT1A1 in the Han Chinese population. Eur J Clin Pharmacol 73:29–37. https://doi.org/10.1007/s00228-016-2137-7
Ozawa T, Takayama K, Okamoto R et al (2015) Generation of enterocyte-like cells from human induced pluripotent stem cells for drug absorption and metabolism studies in human small intestine. Sci Rep 5:16479. https://doi.org/10.1038/srep16479
Paine MF, Fisher MB (2000) Immunochemical identification of UGT isoforms in human small bowel and in caco-2 cell monolayers. Biochem Biophys Res Commun 273:1053–1057. https://doi.org/10.1006/bbrc.2000.3064
Pelkonen O, Turpeinen M, Hakkola J et al (2008) Inhibition and induction of human cytochrome P450 enzymes: current status. Arch Toxicol 82:667–715. https://doi.org/10.1007/s00204-008-0332-8
Peters SA, Jones CR, Ungell A-L, Hatley OJD (2016) Predicting drug extraction in the human gut wall: assessing contributions from drug metabolizing enzymes and transporter proteins using preclinical models. Clin Pharmacokinet 55:673–696. https://doi.org/10.1007/s40262-015-0351-6
Prueksaritanont T, Gorham LM, Hochman JH et al (1996) Comparative studies of drug-metabolizing enzymes in dog, monkey, and human small intestines, and in Caco-2 cells. Drug Metab Dispos 24:634–642
Sai K, Saeki M, Saito Y et al (2004) UGT1A1 haplotypes associated with reduced glucuronidation and increased serum bilirubin in irinotecan-administered Japanese patients with cancer. Clin Pharmacol Ther 75:501–515. https://doi.org/10.1016/j.clpt.2004.01.010
Sakuma T, Nishikawa A, Kume S et al (2014) Multiplex genome engineering in human cells using all-in-one CRISPR/Cas9 vector system. Sci Rep 4:4–9. https://doi.org/10.1038/srep05400
Sakuma T, Nakade S, Sakane Y et al (2016) MMEJ-Assisted gene knock-in using TALENs and CRISPR-Cas9 with the PITCh systems. Nat Protoc 11:118–133. https://doi.org/10.1038/nprot.2015.140
Sun H, Chow EC, Liu S et al (2008) The Caco-2 cell monolayer: usefulness and limitations. Expert Opin Drug Metab Toxicol 4:395–411. https://doi.org/10.1517/17425255.4.4.395
Takayama K, Morisaki Y, Kuno S et al (2014) Prediction of interindividual differences in hepatic functions and drug sensitivity by using human iPS-derived hepatocytes. Proc Natl Acad Sci 111:16772–16777. https://doi.org/10.1073/pnas.1413481111
Takayama K, Negoro R, Yamashita T et al (2019) Generation of human iPSC-derived intestinal epithelial cell monolayers by CDX2 transduction. Cell Mol Gastroenterol Hepatol 8:513–526. https://doi.org/10.1016/j.jcmgh.2019.06.004
Takenaka T, Harada N, Kuze J et al (2014) Human small intestinal epithelial cells differentiated from adult intestinal stem cells as a novel system for predicting oral drug absorption in humans. Drug Metab Dispos 42:1947–1954. https://doi.org/10.1124/dmd.114.059493
Takenaka T, Harada N, Kuze J et al (2016) Application of a human intestinal epithelial cell monolayer to the prediction of oral drug absorption in humans as a superior alternative to the Caco-2 cell monolayer. J Pharm Sci 105:915–924. https://doi.org/10.1016/j.xphs.2015.11.035
Takenaka T, Kazuki K, Harada N et al (2017) Development of Caco-2 cells co-expressing CYP3A4 and NADPH-cytochrome P450 reductase using a human artificial chromosome for the prediction of intestinal extraction ratio of CYP3A4 substrates. Drug Metab Pharmacokinet 32:61–68. https://doi.org/10.1016/j.dmpk.2016.08.004
Yamashita S, Konishi K, Yamazaki Y et al (2002) New and better protocols for a short-term Caco-2 cell culture system. J Pharm Sci 91:669–679. https://doi.org/10.1002/jps.10050
Yoshida S, Honjo T, Iino K et al (2021) Generation of human-induced pluripotent stem cell-derived functional enterocyte-like cells for pharmacokinetic studies. Stem Cell Rep 16:295–308. https://doi.org/10.1016/j.stemcr.2020.12.017
Zhou S-F (2009) Polymorphism of human cytochrome P450 2D6 and its clinical significance. Clin Pharmacokinet 48:761–804. https://doi.org/10.2165/11318070-000000000-00000
Acknowledgements
This research was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Number 21K15264), Japan Agency for Medical Research and Development (AMED) (Grant Number 21mk0101214), The Nakatomi Foundation, The Promotion and Mutual Aid Corporation for Private Schools of Japan (PMAC) and the Research Promotion Program for Acquiring Grants-in-Aid-for Scientific Research of the Ritsumeikan University.
Author information
Authors and Affiliations
Contributions
RN conceived and supervised the study. RN and NY designed the experiments. RN, NY and KW performed the experiments. RN, NY, KW, YK and TF analyzed the data. RN wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Negoro, R., Yamada, N., Watanabe, K. et al. Generation of Caco-2 cells stably expressing CYP3A4·POR·UGT1A1 and CYP3A4·POR·UGT1A1*6 using a PITCh system. Arch Toxicol 96, 499–510 (2022). https://doi.org/10.1007/s00204-021-03175-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-021-03175-0