Abstract
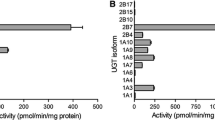
Mono(2-ethylhexyl) phthalate (MEHP) is an active metabolite of di(2-ethylhexyl) phthalate (DEHP) and has endocrine-disrupting effects. MEHP is metabolized into glucuronide by UDP-glucuronosyltransferase (UGT) enzymes in mammals. In the present study, the hepatic and intestinal glucuronidation of MEHP in humans, dogs, rats, and mice was examined in an in vitro system using microsomal fractions. The kinetics of MEHP glucuronidation by liver microsomes followed the Michaelis–Menten model for humans and dogs, and the biphasic model for rats and mice. The K m and V max values of human liver microsomes were 110 µM and 5.8 nmol/min/mg protein, respectively. The kinetics of intestinal microsomes followed the biphasic model for humans, dogs, and mice, and the Michaelis–Menten model for rats. The K m and V max values of human intestinal microsomes were 5.6 µM and 0.40 nmol/min/mg protein, respectively, for the high-affinity phase, and 430 µM and 0.70 nmol/min/mg protein, respectively, for the low-affinity phase. The relative levels of V max estimated by Eadie–Hofstee plots were dogs (2.0) > mice (1.4) > rats (1.0) ≈ humans (1.0) for liver microsomes, and mice (8.5) > dogs (4.1) > rats (3.1) > humans (1.0) for intestinal microsomes. The percentages of the V max values of intestinal microsomes to liver microsomes were mice (120 %) > rats (57 %) > dogs (39 %) > humans (19 %). These results suggest that the metabolic abilities of UGT enzymes expressed in the liver and intestine toward MEHP markedly differed among species, and imply that these species differences are strongly associated with the toxicity of DEHP.




Similar content being viewed by others
Abbreviations
- DEHP:
-
Di(2-ethylhexyl) phthalate
- MEHP:
-
Mono(2-ethylhexyl) phthalate
- UGT:
-
UDP-glucuronosyltransferase
References
Albro PW (1986) Absorption, metabolism, and excretion of di(2-ethylhexyl) phthalate by rats and mice. Environ Health Perspect 65:293–298
Choi K, Joo H, Campbell JL Jr, Andersen ME, Clewell HJ 3rd (2013) In vitro intestinal and hepatic metabolism of Di(2-ethylhexyl) phthalate (DEHP) in human and rat. Toxicol In Vitro 27:1451–1457
Fan J, Traore K, Li W, Amri H, Huang H, Wu C, Chen H, Zirkin B, Papadopoulos V (2010) Molecular mechanisms mediating the effect of mono-(2-ethylhexyl) phthalate on hormone-stimulated steroidogenesis in MA-10 mouse tumor Leydig cells. Endocrinology 151:3348–3362
Frederiksen H, Skakkebæk NE, Andersson AM (2007) Metabolism of phthalates in humans. Mol Nutr Food Res 51:899–911
Gupta RK, Singh JM, Leslie TC, Meachum S, Flaws JA, Yao HH (2010) Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol Appl Pharmacol 242:224–230
Halden RU (2010) Plastics and health risks. Annu Rev Public Health 31:179–194
Heudorf U, Mersch-Sundermann V, Angerer J (2007) Phthalates: toxicology and exposure. Int J Hyg Environ Health 210:623–634
Ito Y, Yokota H, Wang R, Yamanoshita O, Ichihara G, Wang H, Kurata Y, Takagi K, Nakajima T (2005) Species differences in the metabolism of di(2-ethylhexyl) phthalate (DEHP) in several organs of mice, rats, and marmosets. Arch Toxicol 9:147–154
Kamrin MA (2009) Phthalate risks, phthalate regulation, and public health: a review. J Toxicol Environ Health B Crit Rev 12:157–174
Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, Tabacova S, Tyl R, Williams P, Zacharewski T (2002) NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol 16:529–653
Kiang TK, Ensom MH, Chang TK (2005) UDP-glucuronosyltransferases and clinical drug-drug interactions. Pharmacol Ther 106:97–132
Koch HM, Bolt HM, Angerer J (2004) Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch Toxicol 78:123–130
Mackenzie PI, Owens IS, Burchell B, Bock KW, Bairoch A, Bélanger A, Fournel-Gigleux S, Green M, Hum DW, Iyanagi T, Lancet D, Louisot P, Magdalou J, Chowdhury JR, Ritter JK, Schachter H, Tephly TR, Tipton KF, Nebert DW (1997) The UDP glycosyltransferase gene superfamily: recommended nomenclature update based on evolutionary divergence. Pharmacogenetics 7:255–269
Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners JO, Owens IS, Nebert DW (2005) Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomic 15:677–685
Martino-Andrade AJ, Chahoud I (2010) Reproductive toxicity of phthalate esters. Mol Nutr Food Res 54:148–157
Muczynski V, Cravedi JP, Lehraiki A, Levacher C, Moison D, Lecureuil C, Messiaen S, Perdu E, Frydman R, Habert R, Rouiller-Fabre V (2012) Effect of mono-(2-ethylhexyl) phthalate on human and mouse fetal testis: in vitro and in vivo approaches. Toxicol Appl Pharmacol 261:97–104
Peck CC, Albro PW (1982) Toxic potential of the plasticizer Di(2-ethylhexyl) phthalate in the context of its disposition and metabolism in primates and man. Environ Health Perspect 45:11–17
Ritter JK (2000) Roles of glucuronidation and UDP-glucuronosyltransferases in xenobiotic bioactivation reactions. Chem Biol Interact 129:171–193
Silva MJ, Barr DB, Reidy JA, Kato K, Malek NA, Hodge CC, Hurtz D 3rd, Calafat AM, Needham LL, Brock JW (2003) Glucuronidation patterns of common urinary and serum monoester phthalate metabolites. Arch Toxicol 77:561–567
Silva MJ, Samandar E, Preau JL Jr, Needham LL, Calafat AM (2006) Urinary oxidative metabolites of di(2-ethylhexyl) phthalate in humans. Toxicology 219:22–32
Tukey RH, Strassburg CP (2000) Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol 40:581–616
Wang W, Craig ZR, Basavarajappa MS, Gupta RK, Flaws JA (2012) Di(2-ethylhexyl) phthalate inhibits growth of mouse ovarian antral follicles through an oxidative stress pathway. Toxicol Appl Pharmacol 258:288–295
Weuve J, Hauser R, Calafat AM, Missmer SA, Wise LA (2010) Association of exposure to phthalates with endometriosis and uterine leiomyomata: findings from NHANES, 1999–2004. Environ Health Perspect 118:825–832
Yu L, Luan L, Shao Q, Zeng S (2004) Direct determination of S-(-)- and R-(+)-propranolol glucuronide in rat hepatic microsomes by RP-HPLC. Biomed Chromatogr 18:833–837
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (26281028) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no duality of interest to declare.
Rights and permissions
About this article
Cite this article
Hanioka, N., Isobe, T., Kinashi, Y. et al. Hepatic and intestinal glucuronidation of mono(2-ethylhexyl) phthalate, an active metabolite of di(2-ethylhexyl) phthalate, in humans, dogs, rats, and mice: an in vitro analysis using microsomal fractions. Arch Toxicol 90, 1651–1657 (2016). https://doi.org/10.1007/s00204-015-1619-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-015-1619-1