Abstract
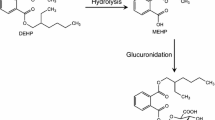
Mono(2-ethylhexyl) phthalate (MEHP) is an active metabolite of di(2-ethylhexyl) phthalate (DEHP), which is an endocrine-disrupting chemical. In the present study, MEHP glucuronidation in humans was studied using recombinant UDP-glucuronosyltransferases (UGTs) and microsomes of the liver and intestine. Among the recombinant UGTs examined, UGT1A3, UGT1A7, UGT1A8, UGT1A9, UGT1A10, UGT2B4, and UGT2B7 glucuronidated MEHP. The kinetics of MEHP glucuronidation by UGT1A3, UGT1A7, UGT1A8, UGT1A10, UGT2B4, and UGT2B7 followed the Michaelis–Menten model, whereas that by UGT1A9 fit the negative allosteric model. CLint values were in the order of UGT1A9 > UGT2B7 > UGT1A7 > UGT1A8 ≥ UGT1A10 > UGT1A3 > UGT2B4. The kinetics of MEHP glucuronidation by liver microsomes followed the Michaelis–Menten model. Diclofenac (20 µM) and raloxifene (20 µM) decreased CLint values to 43 and 36 % that of native microsomes, respectively. The kinetics of MEHP glucuronidation by intestine microsomes fit the biphasic model. Diclofenac (150 and 450 µM) decreased CLint values to 32 and 13 % that of native microsomes for the high-affinity phase, and to 28 and 21 % for the low-affinity phase, respectively. Raloxifene (2.5 and 7.0 µM) decreased CLint values to 35 and 4.1 % that of native microsomes for the high-affinity phase, and to 48 and 53 % for the low-affinity phase, respectively. These results suggest that MEHP glucuronidation in humans is catalyzed by UGT1A3, UGT1A9, UGT2B4, and/or UGT2B7 in the liver, and by UGT1A7, UGT1A8, UGT1A9, UGT1A10, and/or UGT2B7 in the intestine, and also that these UGT isoforms play important and characteristic roles in the detoxification of DEHP.






Similar content being viewed by others
Abbreviations
- DEHP:
-
Di(2-ethylhexyl) phthalate
- MEHP:
-
Mono(2-ethylhexyl) phthalate
- UGT:
-
UDP-glucuronosyltransferase
References
Albro PW (1986) Absorption, metabolism, and excretion of di(2-ethylhexyl) phthalate by rats and mice. Environ Health Perspect 65:293–298
Bernard O, Guillemette C (2004) The main role of UGT1A9 in the hepatic metabolism of mycophenolic acid and the effects of naturally occurring variants. Drug Metab Dispos 32:775–778
Chen Y, Chen S, Li X, Wang X, Zeng S (2006) Genetic variants of human UGT1A3: functional characterization and frequency distribution in a Chinese Han population. Drug Metab Dispos 34:1462–1467
Choi K, Joo H, Campbell JL Jr, Andersen ME, Clewell HJ 3rd (2013) In vitro intestinal and hepatic metabolism of di(2-ethylhexyl) phthalate (DEHP) in human and rat. Toxicol In Vitro 27:1451–1457
Court MH, Krishnaswamy S, Hao Q, Duan SX, Patten CJ, Von Moltke LL, Greenblatt DJ (2003) Evaluation of 3′-azido-3′-deoxythymidine, morphine, and codeine as probe substrates for UDP-glucuronosyltransferase 2B7 (UGT2B7) in human liver microsomes: specificity and influence of the UGT2B7*2 polymorphism. Drug Metab Dispos 31:1125–1133
Fallon JK, Neubert H, Goosen TC, Smith PC (2013) Targeted precise quantification of 12 human recombinant uridine-diphosphate glucuronosyl transferase 1A and 2B isoforms using nano-ultra-high-performance liquid chromatography/tandem mass spectrometry with selected reaction monitoring. Drug Metab Dispos 41:2076–2080
Fan J, Traore K, Li W, Amri H, Huang H, Wu C, Chen H, Zirkin B, Papadopoulos V (2010) Molecular mechanisms mediating the effect of mono-(2-ethylhexyl) phthalate on hormone-stimulated steroidogenesis in MA-10 mouse tumor Leydig cells. Endocrinology 151:3348–3362
Frederiksen H, Skakkebæk NE, Andersson AM (2007) Metabolism of phthalates in humans. Mol Nutr Food Res 51:899–911
Gagné JF, Montminy V, Belanger P, Journault K, Gaucher G, Guillemette C (2002) Common human UGT1A polymorphisms and the altered metabolism of irinotecan active metabolite 7-ethyl-10-hydroxycamptothecin (SN-38). Mol Pharmacol 62:608–617
Guillemette C, Lévesque E, Harvey M, Bellemare J, Menard V (2010) UGT genomic diversity: beyond gene duplication. Drug Metab Rev 42:24–44
Gupta RK, Singh JM, Leslie TC, Meachum S, Flaws JA, Yao HH (2010) Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol Appl Pharmacol 242:224–230
Halden RU (2010) Plastics and health risks. Annu Rev Public Health 31:179–194
Hanioka N, Isobe T, Kinashi Y, Tanaka-Kagawa T, Jinno H (2016) Hepatic and intestinal glucuronidation of mono(2-ethylhexyl) phthalate, an active metabolite of di(2-ethylhexyl) phthalate, in humans, dogs, rats, and mice: an in vitro analysis using microsomal fractions. Arch Toxicol. doi:10.1007/s00204-015-1619-1
Harbourt DE, Fallon JK, Ito S, Baba T, Ritter JK, Glish GL, Smith PC (2012) Quantification of human uridine-diphosphate glucuronosyl transferase 1A isoforms in liver, intestine, and kidney using nanobore liquid chromatography-tandem mass spectrometry. Anal Chem 84:98–105
Heudorf U, Mersch-Sundermann V, Angerer J (2007) Phthalates: toxicology and exposure. Int J Hyg Environ Health 210:623–634
Huang YH, Galijatovic A, Nguyen N, Geske D, Beaton D, Green J, Green M, Peters WH, Tukey RH (2002) Identification and functional characterization of UDP-glucuronosyltransferases UGT1A8*1, UGT1A8*2 and UGT1A8*3. Pharmacogenetics 12:287–297
Ito Y, Yokota H, Wang R, Yamanoshita O, Ichihara G, Wang H, Kurata Y, Takagi K, Nakajima T (2005) Species differences in the metabolism of di(2-ethylhexyl) phthalate (DEHP) in several organs of mice, rats, and marmosets. Arch Toxicol 79:147–154
Izukawa T, Nakajima M, Fujiwara R, Yamanaka H, Fukami T, Takamiya M, Aoki Y, Ikushiro S, Sakaki T, Yokoi T (2009) Quantitative analysis of UDP-glucuronosyltransferase (UGT) 1A and UGT2B expression levels in human livers. Drug Metab Dispos 37:1759–1768
Jinno H, Saeki M, Saito Y, Tanaka-Kagawa T, Hanioka N, Sai K, Kaniwa N, Ando M, Shirao K, Minami H, Ohtsu A, Yoshida T, Saijo N, Ozawa S, Sawada J (2003a) Functional characterization of human UDP-glucuronosyltransferase 1A9 variant, D256N, found in Japanese cancer patients. J Pharmacol Exp Ther 306:688–693
Jinno H, Saeki M, Tanaka-Kagawa T, Hanioka N, Saito Y, Ozawa S, Ando M, Shirao K, Minami H, Ohtsu A, Yoshida T, Saijo N, Sawada J (2003b) Functional characterization of wild-type and variant (T202I and M59I) human UDP-glucuronosyltransferase 1A10. Drug Metab Dispos 31:528–532
Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, Tabacova S, Tyl R, Williams P, Zacharewski T (2002) NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol 16:529–653
Kemp DC, Fan PW, Stevens JC (2002) Characterization of raloxifene glucuronidation in vitro: contribution of intestinal metabolism to presystemic clearance. Drug Metab Dispos 30:694–700
King C, Tang W, Ngui J, Tephly T, Braun M (2001) Characterization of rat and human UDP-glucuronosyltransferases responsible for the in vitro glucuronidation of diclofenac. Toxicol Sci 61:49–53
Koch HM, Bolt HM, Angerer J (2004) Di(2-ethylhexyl) phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch Toxicol 78:123–130
Koch HM, Bolt HM, Preuss R, Angerer J (2005) New metabolites of di(2-ethylhexyl) phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol 79:367–376
Koch HM, Preuss R, Angerer J (2006) Di(2-ethylhexyl) phthalate (DEHP): human metabolism and internal exposure: an update and latest results. Int J Androl 29:155–165
Kokawa Y, Kishi N, Jinno H, Tanaka-Kagawa T, Narimatsu S, Hanioka N (2013) Effect of UDP-glucuronosyltransferase 1A8 polymorphism on raloxifene glucuronidation. Eur J Pharm Sci 49:199–205
Lévesque E, Beaulieu M, Hum DW, Bélanger A (1999) Characterization and substrate specificity of UGT2B4 (E458): a UDP-glucuronosyltransferase encoded by a polymorphic gene. Pharmacogenetics 9:207–216
Mackenzie PI, Owens IS, Burchell B, Bock KW, Bairoch A, Bélanger A, Fournel-Gigleux S, Green M, Hum DW, Iyanagi T, Lancet D, Louisot P, Magdalou J, Chowdhury JR, Ritter JK, Schachter H, Tephly TR, Tipton KF, Nebert DW (1997) The UDP glycosyltransferase gene superfamily: recommended nomenclature update based on evolutionary divergence. Pharmacogenetics 7:255–269
Mackenzie PI, Gregory PA, Gardner-Stephen DA, Lewinsky RH, Jorgensen BR, Nishiyama T, Xie W, Radominska-Pandya A (2003) Regulation of UDP glucuronosyltransferase genes. Curr Drug Metab 4:249–257
Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners JO, Owens IS, Nebert DW (2005) Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics 15:677–685
Martino-Andrade AJ, Chahoud I (2010) Reproductive toxicity of phthalate esters. Mol Nutr Food Res 54:148–157
Muczynski V, Cravedi JP, Lehraiki A, Levacher C, Moison D, Lecureuil C, Messiaen S, Perdu E, Frydman R, Habert R, Rouiller-Fabre V (2012) Effect of mono-(2-ethylhexyl) phthalate on human and mouse fetal testis: in vitro and in vivo approaches. Toxicol Appl Pharmacol 261:97–104
Ohno S, Nakajin S (2009) Determination of mRNA expression of human UDP-glucuronosyltransferases and application for localization in various human tissues by real-time reverse transcriptase-polymerase chain reaction. Drug Metab Dispos 37:32–40
Ritter JK (2000) Roles of glucuronidation and UDP-glucuronosyltransferases in xenobiotic bioactivation reactions. Chem Biol Interact 129:171–193
Sakaguchi K, Green M, Stock N, Reger TS, Zunic J, King C (2004) Glucuronidation of carboxylic acid containing compounds by UDP-glucuronosyltransferase isoforms. Arch Biochem Biophys 424:219–225
Silva MJ, Barr DB, Reidy JA, Kato K, Malek NA, Hodge CC, Hurtz D 3rd, Calafat AM, Needham LL, Brock JW (2003) Glucuronidation patterns of common urinary and serum monoester phthalate metabolites. Arch Toxicol 77:561–567
Silva MJ, Samandar E, Preau JL Jr, Needham LL, Calafat AM (2006) Urinary oxidative metabolites of di(2-ethylhexyl) phthalate in humans. Toxicology 219:22–32
Wang W, Craig ZR, Basavarajappa MS, Gupta RK, Flaws JA (2012) Di(2-ethylhexyl) phthalate inhibits growth of mouse ovarian antral follicles through an oxidative stress pathway. Toxicol Appl Pharmacol 258:288–295
Weuve J, Hauser R, Calafat AM, Missmer SA, Wise LA (2010) Association of exposure to phthalates with endometriosis and uterine leiomyomata: findings from NHANES, 1999–2004. Environ Health Perspect 118:825–832
Yu L, Luan L, Shao Q, Zeng S (2004) Direct determination of S-(−)- and R-(+)-propranolol glucuronide in rat hepatic microsomes by RP-HPLC. Biomed Chromatogr 18:833–837
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (26281028) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no duality of interest to declare.
Rights and permissions
About this article
Cite this article
Hanioka, N., Kinashi, Y., Tanaka-Kagawa, T. et al. Glucuronidation of mono(2-ethylhexyl) phthalate in humans: roles of hepatic and intestinal UDP-glucuronosyltransferases. Arch Toxicol 91, 689–698 (2017). https://doi.org/10.1007/s00204-016-1708-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1708-9