Abstract
Purpose
In stable ventilatory and metabolic conditions, changes in end-tidal carbon dioxide (EtCO2) might reflect changes in cardiac index (CI). We tested whether EtCO2 detects changes in CI induced by volume expansion and whether changes in EtCO2 during passive leg raising (PLR) predict fluid responsiveness. We compared EtCO2 and arterial pulse pressure for this purpose.
Methods
We included 65 patients [Simplified Acute Physiology Score (SAPS) II = 57 ± 19, 37 males, under mechanical ventilation without spontaneous breathing, 15 % with chronic obstructive pulmonary disease, baseline CI = 2.9 ± 1.1 L/min/m2] in whom a fluid challenge was decided due to circulatory failure and who were monitored by an expiratory-CO2 sensor and a PiCCO2 device. In all patients, we measured arterial pressure, EtCO2, and CI before and after a fluid challenge. In 40 patients, PLR was performed before fluid administration. The PLR-induced changes in arterial pressure, EtCO2, and CI were recorded.
Results
Considering the whole population, the fluid-induced changes in EtCO2 and CI were correlated (r 2 = 0.45, p = 0.0001). Considering the 40 patients in whom PLR was performed, volume expansion increased CI ≥15 % in 21 “volume responders.” A PLR-induced increase in EtCO2 ≥5 % predicted a fluid-induced increase in CI ≥15 % with sensitivity of 71 % (95 % confidence interval: 48–89 %) and specificity of 100 (82–100) %. The prediction ability of the PLR-induced changes in CI was not different. The area under the receiver-operating characteristic (ROC) curve for the PLR-induced changes in pulse pressure was not significantly different from 0.5.
Conclusion
The changes in EtCO2 induced by a PLR test predicted fluid responsiveness with reliability, while the changes in arterial pulse pressure did not.
Similar content being viewed by others
Introduction
Volume expansion is often the first-line treatment during acute circulatory failure. Nevertheless, fluid administration results in a significant improvement of cardiac index (CI) in only half of cases if preload responsiveness is not previously assessed [1]. Among the different tests that have been developed for detecting preload responsiveness [2], the passive leg raising (PLR) test consists in moving the patient from the semirecumbent position to a position in which the legs are elevated at 45° [3]. This postural change transfers blood from the leg and abdominal compartments [4] toward cardiac cavities and acts like an endogenous fluid challenge. The main advantage of the PLR test is that it remains valuable for detecting preload responsiveness in case of spontaneous breathing activity [5–11], acute respiratory distress syndrome with low tidal volume and lung compliance [12], and cardiac arrhythmias [5, 13], conditions in which the respiratory variation of stroke volume or surrogates is not valid [5, 12, 14].
Nevertheless, a disadvantage of the PLR test is that it requires a direct measure of CI. Indeed, arterial pressure alone does not allow precise assessment of PLR hemodynamic effects [15]. This is even true for the arterial pulse pressure, i.e., the value of arterial pressure which is best correlated with stroke volume [16]. This is likely due to the fact that changes in arterial pulse pressure are only roughly correlated with changes in stroke volume [17].
In this context, measuring the end-tidal carbon dioxide (EtCO2) might be an attractive method for assessing the effects of the PLR test when no direct monitoring of CI is available. Indeed, the amount of exhaled CO2 is proportional to CI in stable respiratory and metabolic conditions [18], and its changes have been demonstrated to reflect CI changes [19, 20]. Thus, provided that ventilation is unaltered and cell metabolism is stable, which might be true over short periods of time, EtCO2 might allow continuous and noninvasive estimation of CI changes during PLR.
The goals of the present study were to test (1) whether the changes in EtCO2 observed during volume expansion are able to track the simultaneous changes in CI, and (2) whether the changes in EtCO2 during a PLR test could predict fluid responsiveness.
Patients and methods
Patients
This prospective study was approved by the Institutional Review Board of our institution. Patients were included according to an emergency procedure. Deferred informed consent was requested from the patient’s surrogate as soon as possible. As he/she recovered consciousness, deferred informed consent was requested from the patient. If the patient or his/her next of kin refused consent, patient’s data were not entered into analysis.
Sixty-five patients were included in the study. They were included if they were routinely monitored by a PiCCO2 device (Pulsion Medical Systems, Munich, Germany) and by a mainstream CO2 sensor (M2741A; Philips, Böblingen, Germany, connected to the M2772A airway adapter) and if the attending physician planned to administer a volume expansion due to hemodynamic instability. Patients also had to be intubated and ventilated (Evita 4; Dräger Medical, Lübeck, Germany) in the control assisted mode with no inspiratory effort, as assessed by observing the flow curve displayed by the ventilator. Patients were excluded if they were less than 18 years old and if PLR was contraindicated (head trauma, known deep vein thrombosis of the inferior limbs, venous compression stocking).
Study design and measurements
At baseline, a first set of measurements was performed, including heart rate, systemic arterial pressure, CI (measured by transpulmonary thermodilution), and EtCO2. A volume expansion was administered (500 mL saline over 30 min [21]). Immediately after fluid infusion, we again measured heart rate, systemic arterial pressure, CI (measured by transpulmonary thermodilution), and EtCO2.
In 40 out of the 65 patients, a PLR test was performed before volume expansion. For this purpose, immediately after the first set of measurements, patients were transferred from the initial semirecumbent position to the PLR position, in which the legs are elevated at 45° and the trunk is in horizontal position. The postural change was performed by using the automatic motion of the bed and without changing the hip angle [4]. When the PLR had induced its maximal effect on CI, a second set of measurements was recorded, including heart rate, systemic arterial pressure, CI (measured by pulse contour analysis), and EtCO2. The effects of PLR on CI were assessed by pulse contour analysis rather than by transpulmonary thermodilution because pulse contour analysis allows easy assessment of transient changes in CI induced by PLR. The patient was moved back to the semirecumbent position. When the hemodynamic variables reached their baseline values again and before fluid administration, we measured heart rate, systemic arterial pressure, CI (measured by transpulmonary thermodilution), and EtCO2. This second transpulmonary thermodilution was performed after the PLR test and before volume expansion in order to very precisely measure the effects of volume expansion on CI, without taking into account the small changes that may spontaneously occur between before and after the PLR. Transpulmonary thermodilution was performed by averaging the value resulting from three successive bolus injections performed with 15 mL cold saline [22]. To assess the changes in EtCO2, it was carefully checked by observing the EtCO2 curve that the baseline EtCO2 and the EtCO2 upstroke delay did not change during PLR and volume expansion; i.e., the changes in EtCO2 were not related to changes in inspired CO2 or to increased airway resistance at expiration [18] [see Electronic Supplementary Material (ESM) Fig. 1].
Statistical analysis
Patients in whom volume expansion increased CI by more than 15 % were defined as “volume responders” and the remaining ones as “volume nonresponders.” This cutoff was justified by the fact that the least significant change (LSC) of CI measured by transpulmonary thermodilution is 12 % when three cold measurements are averaged [22].
All data except the dose of norepinephrine, and the relative changes in CI, in arterial pulse pressure, and in EtCO2 were normally distributed (Kolmogorov–Smirnov test) and are expressed as mean ± standard deviation (SD) or as median [25–75 % interquartile range, IQR], as appropriate. Pairwise comparisons of values between different study times were performed by paired Student’s t test or Wilcoxon test, as appropriate. Comparisons between volume responders and nonresponders were performed by two-tailed Student t test or Mann–Whitney U test, as appropriate. Correlations were tested by the Pearson method.
In the 40 patients in whom a PLR test was performed, the ability of the PLR-induced changes in arterial pulse pressure, in CI, and in EtCO2 to detect volume responsiveness was tested and compared by constructing receiver-operating characteristic (ROC) curves for each variable. The areas under the ROC curves were compared by the Hanley–McNeil test [23]. Sensitivity and specificity are expressed as mean (95 % confidence interval).
We calculated the coefficient of variation of EtCO2 as being the standard deviation divided by the mean of the measurements performed before the PLR test and before volume expansion, i.e., at times when the hemodynamic status was supposed to be similar. The precision was calculated as being two times the coefficient of variation, and the LSC as precision times √2. The LSC is the minimum change that needs to be measured by a device in order to recognize a real change. p-Value ≤0.05 was considered statistically significant. Statistical analysis was performed using MedCalc 8.1.0.0 (Mariakerke, Belgium).
Results
Patients’ characteristics
Patients’ characteristics are detailed in Table 1. The population did not include any surgical patients. A majority suffered from septic shock (91 %) and acute respiratory distress syndrome (77 %) (Table 1). Atrial fibrillation was observed in 17 % of patients. All other patients were in sinus rhythm. All patients were sedated, but none were paralyzed. All patients were mechanically ventilated without spontaneous breathing. Fifteen percent of patients had previous chronic obstructive pulmonary disease. No patient exhibited right ventricular dilation (defined by ratio of right over left ventricular diameter >0.6) or paradoxical septal motion at echocardiography. No patient exhibited clinical signs of intraabdominal hypertension. The study was conducted over 1 year. Ten patients were excluded because of lack of cardiac index measurement.
Relationship between the changes in CI, EtCO2, and arterial pulse pressure induced by volume expansion
Considering all 65 episodes of volume expansion, CI increased by 12 [3–24] %, EtCO2 by 5 [0–13] %, and arterial pulse pressure by 7 [2–22] %. CI increased by more than 15 % in 34 volume responders. In these patients, CI increased by 27 [23–42] %. Individual changes in EtCO2, CI, and arterial pulse pressure in responders and nonresponders are displayed in ESM Fig. 2.
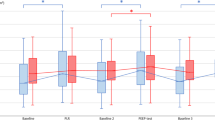
Receiver-operating characteristic (ROC) curves showing the ability of the PLR-induced changes in cardiac index (CI), in end-tidal carbon dioxide (EtCO2), and in arterial pulse pressure to predict an increase in cardiac index ≥15 % during the subsequent fluid administration. n = 40, *p ≤ 0.05 for the comparison between areas under the curves
The correlation between absolute values of EtCO2 and of CI was not significant, neither before nor after volume expansions. The correlation between the changes in EtCO2 and in CI induced by volume expansions was significant (r 2 = 0.45, p ≤ 0.0001).
The correlation between absolute values of arterial pulse pressure and of CI was significant before and after volume expansions (r 2 = 0.20, p ≤ 0.0001 and r 2 = 0.15, p = 0.0009, respectively). The correlation between the changes in arterial pulse pressure and in CI induced by volume expansions was significant (r 2 = 0.31, p < 0.0001).
The precision of EtCO2 was 1.3 ± 2.1 %, and the LSC was 1.8 ± 3.0 %.
Ability of the PLR-induced changes in CI, EtCO2, and arterial pulse pressure to predict fluid responsiveness
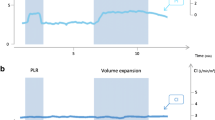
A typical CO2 tracing in a responder is displayed in Fig. 1. In all patients, the maximal effects of PLR on CI and EtCO2 were observed within 1 min. Considering the 40 patients in whom PLR was performed, the correlation between the changes in EtCO2 and in CI induced by PLR was significant (r 2 = 0.42, p < 0.0001). The correlation between the changes in arterial pulse pressure and in CI induced by PLR was significant (r 2 = 0.23, p ≤ 0.0001).
Among these 40 patients, volume expansion increased CI by more than 15 % (29 [22–45] %) in 21 volume responders. The PLR-induced changes in CI and EtCO2 were significantly higher in the 21 volume responders than in the 19 volume nonresponders (Table 2). There was a significant correlation between the changes in CI induced by fluid administration and the changes in EtCO2 induced by the PLR test (r = 0.79, p < 0.001). A PLR-induced increase in CI ≥10 % predicted a fluid-induced increase in CI ≥15 % with sensitivity of 95 [76–100] % and specificity of 95 [74–100] % (Table 3; Fig. 2). A PLR-induced increase in EtCO2 ≥5 % predicted a fluid-induced increase in CI ≥15 % with sensitivity of 71 [48–89] % and specificity of 100 [82–100] % (Table 3; Fig. 2). The areas under the ROC curves constructed for the PLR-induced increase in CI and EtCO2 were not statistically different (Table 3; Fig. 2). The area under the ROC curve for the PLR-induced changes in arterial pulse pressure was not significantly different from 0.5 (Table 3; Fig. 2).
Discussion
This study showed that EtCO2 could be used as a noninvasive tool for assessing the response of CI to volume expansion and that the changes in EtCO2 observed during a PLR test allowed prediction of fluid responsiveness. EtCO2 was better than arterial pulse pressure for this purpose.
Exhaled CO2 is determined by three factors: the production of CO2 by cell metabolism, the pulmonary flow (i.e., cardiac output) that drives CO2 from the periphery to the lungs, and the ability of the lung to clear the venous blood of CO2 [24]. Thus, if two of the three factors are constant, changes in EtCO2 might reflect changes of the third. Based upon this reasoning, previous studies showed a good correlation between EtCO2 and cardiac output during cardiopulmonary resuscitation in animals [25] and humans [26–28]. Some studies also established that the EtCO2 improvement during resuscitation is a prognostic indicator of resuscitated cardiac arrest [29–31] and that the changes in EtCO2 could track changes in cardiac output during cardiopulmonary bypass [32, 33]. The present study investigated EtCO2 as a surrogate of cardiac output for dynamic prediction of fluid responsiveness.
By showing that the changes in EtCO2 and in CI induced by volume expansion were significantly correlated, we suggest that EtCO2 could be used as a noninvasive tool for monitoring CI in response to preload changes, even though the correlation between the changes in EtCO2 and CI was not perfect. Interestingly, the correlation between the absolute values of EtCO2 and CI was not significant. This is explained by the fact that the absolute value of EtCO2 is influenced by many other factors than CI (cell metabolism, minute ventilation, respiratory dead space), but that these factors are unchanged in the meantime of volume expansion. The amplitude of the preload-induced changes in EtCO2 was small, but it was larger than the least significant change we determined from two EtCO2 measurements. In other words, in patients with stable condition, EtCO2 is a very stable variable, so that even small changes are easily detectable.
The PLR maneuver induces passive transfer of blood contained in the venous compartment of the lower limb [34] and of the abdominal compartment [4] to the heart and increases the right and left cardiac preload [35]. A number of original studies [5–11, 13] showed that the PLR test is reliable for predicting fluid responsiveness, and a meta-analysis [15] confirmed this diagnostic accuracy. By showing that PLR-induced increases in CI are able to predict the response of CI to a subsequent fluid challenge, the present study is in accordance with all these previous results. It also confirms previous observations [15] that arterial pulse pressure is less reliable than CI for assessing the hemodynamic effects of PLR. The pulse pressure is physiologically related to stroke volume [36]. Nevertheless, peripheral pulse pressure is also influenced by arterial compliance and by the pulse wave amplification phenomenon [36]. These issues of arterial compliance and pulse wave amplification phenomenon likely explain why the changes in arterial pulse pressure only roughly indicate changes in CI induced by volume expansion [17]. We confirmed in the present study that the correlation between the absolute values and fluid-induced changes in CI and in arterial pulse pressure were actually weak. As a consequence, the PLR-induced changes in arterial pulse pressure were unable to predict fluid responsiveness.
By contrast, the PLR-induced changes in EtCO2 predicted fluid responsiveness with reliability. Our results confirm a recent study conducted in a similar population [37]. Thus, EtCO2 could be regarded as a surrogate of CI for assessing the effects of PLR when no device is available for measuring CI directly. This can be the case in the perioperative setting or at the early phase of shock. Obviously, monitoring EtCO2 alone might be insufficient when the circulatory failure is refractory to the initial therapy and when additional hemodynamic information is mandatory [38, 39]. Additionally, it must be recalled that trends in EtCO2 might no longer reflect trends in CI in nonventilated patients. Also, it must be underlined that EtCO2 might help monitoring CI only over short periods. Indeed, it is plausible that, during acute circulatory failure, if the hemodynamic improvement persists, cell metabolism could shift from anaerobic to aerobic conditions, eventually leading to a change in CO2 production.
We acknowledge some limitations to our study. First, although several devices are commercially available for monitoring EtCO2, we only tested the mainstream technique and we cannot certify that the cutoff value reported in the present study would be identical with other EtCO2 monitoring devices. Second, although it is conceivable that CI also changes EtCO2 in spontaneously breathing patients provided that ventilation is regular, we did not test this hypothesis. Third, since a majority of patients were ventilated with tidal volume lower than 8 mL/kg, a condition where pulse pressure variation might not be valid for predicting fluid responsiveness [40, 41], we could not compare the predictive value of this variable with that of the PLR-induced changes in EtCO2. Finally, our results only apply to the specific population that we studied, i.e., patients under mechanical ventilation without spontaneous breathing activity, with normal or moderately depressed left ventricular function and normal right ventricle. Also, we could not investigate the effects of an increased intraabdominal pressure, since this condition could alter the hemodynamic effects of PLR [42].
Conclusions
The changes in EtCO2 induced by a PLR test predicted fluid responsiveness with reliability, while the changes in arterial pulse pressure did not. EtCO2 monitoring should thus be regarded as a noninvasive surrogate of CI during PLR when no device is available for measuring CI.
References
Michard F, Teboul JL (2002) Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 121:2000–2008
Marik PE, Monnet X, Teboul JL (2011) Hemodynamic parameters to guide fluid therapy. Ann Intensive Care 1:1
Monnet X, Teboul JL (2008) Passive leg raising. Intensive Care Med 34:659–663
Jabot J, Teboul JL, Richard C, Monnet X (2009) Passive leg raising for predicting fluid responsiveness: importance of the postural change. Intensive Care Med 35:85–90
Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, Teboul JL (2006) Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med 34:1402–1407
Biais M, Nouette-Gaulain K, Cottenceau V, Revel P, Sztark F (2008) Uncalibrated pulse contour-derived stroke volume variation predicts fluid responsiveness in mechanically ventilated patients undergoing liver transplantation. Br J Anaesth 101:761–768
Lafanechere A, Pene F, Goulenok C, Delahaye A, Mallet V, Choukroun G, Chiche JD, Mira JP, Cariou A (2006) Changes in aortic blood flow induced by passive leg raising predict fluid responsiveness in critically ill patients. Crit Care 10:R132
Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul JL (2007) Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med 33:1125–1132
Maizel J, Airapetian N, Lorne E, Tribouilloy C, Massy Z, Slama M (2007) Diagnosis of central hypovolemia by using passive leg raising. Intensive Care Med 33:1133–1138
Monnet X, Osman D, Ridel C, Lamia B, Richard C, Teboul JL (2009) Predicting volume responsiveness by using the end-expiratory occlusion in mechanically ventilated intensive care unit patients. Crit Care Med 37:951–956
Preau S, Saulnier F, Dewavrin F, Durocher A, Chagnon JL (2010) Passive leg raising is predictive of fluid responsiveness in spontaneously breathing patients with severe sepsis or acute pancreatitis. Crit Care Med 38:989–990
Monnet X, Bleibtreu A, Ferré A, Dres M, Gharbi R, Richard C, Teboul JL (2012) Passive leg raising and end-expiratory occlusion tests perform better than pulse pressure variation in patients with low respiratory system compliance. Crit Care Med 40:152–157
Thiel SW, Kollef MH, Isakow W (2009) Non-invasive stroke volume measurement and passive leg raising predict volume responsiveness in medical ICU patients: an observational cohort study. Crit Care 13:R111
Soubrier S, Saulnier F, Hubert H, Delour P, Lenci H, Onimus T, Nseir S, Durocher A (2007) Can dynamic indicators help the prediction of fluid responsiveness in spontaneously breathing critically ill patients? Intensive Care Med 33:1117–1124
Cavallaro F, Sandroni C, Marano C, La Torre G, Mannocci A, De Waure C, Bello G, Maviglia R, Antonelli M (2010) Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults: systematic review and meta-analysis of clinical studies. Intensive Care Med 36:1475–1483
Lamia B, Chemla D, Richard C, Teboul JL (2005) Clinical review: interpretation of arterial pressure wave in shock states. Crit Care 9:601–606
Monnet X, Letierce A, Hamzaoui O, Chemla D, Anguel N, Osman D, Richard C, Teboul JL (2011) Arterial pressure allows monitoring the changes in cardiac output induced by volume expansion but not by norepinephrine*. Crit Care Med 39:1394–1399
Tautz TJ, Urwyler A, Antognini JF, Riou B (2010) Case scenario: increased end-tidal carbon dioxide: a diagnostic dilemma. Anesthesiology 112:440–446
Weil MH, Bisera J, Trevino RP, Rackow EC (1985) Cardiac output and end-tidal carbon dioxide. Crit Care Med 13:907–909
Gudipati CV, Weil MH, Bisera J, Deshmukh HG, Rackow EC (1988) Expired carbon dioxide: a noninvasive monitor of cardiopulmonary resuscitation. Circulation 77:234–239
Vincent JL, Weil MH (2006) Fluid challenge revisited. Crit Care Med 34:1333–1337
Monnet X, Persichini R, Ktari M, Jozwiak M, Richard C, Teboul JL (2011) Precision of the transpulmonary thermodilution measurements. Crit Care 15:R204
Hanley JA, McNeil BJ (1983) A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148:839–843
Anderson CT, Breen PH (2000) Carbon dioxide kinetics and capnography during critical care. Crit Care 4:207–215
Ornato JP, Garnett AR, Glauser FL (1990) Relationship between cardiac output and the end-tidal carbon dioxide tension. Ann Emerg Med 19:1104–1106
Garnett AR, Ornato JP, Gonzalez ER, Johnson EB (1987) End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. JAMA 257:512–515
Steedman DJ, Robertson CE (1990) Measurement of end-tidal carbon dioxide concentration during cardiopulmonary resuscitation. Arch Emerg Med 7:129–134
Falk JL, Rackow EC, Weil MH (1988) End-tidal carbon dioxide concentration during cardiopulmonary resuscitation. N Engl J Med 318:607–611
Cantineau JP, Lambert Y, Merckx P, Reynaud P, Porte F, Bertrand C, Duvaldestin P (1996) End-tidal carbon dioxide during cardiopulmonary resuscitation in humans presenting mostly with asystole: a predictor of outcome. Crit Care Med 24:791–796
Grmec S, Klemen P (2001) Does the end-tidal carbon dioxide (EtCO2) concentration have prognostic value during out-of-hospital cardiac arrest? Eur J Emerg Med 8:263–269
Levine RL, Wayne MA, Miller CC (1997) End-tidal carbon dioxide and outcome of out-of-hospital cardiac arrest. N Engl J Med 337:301–306
Baraka AS, Aouad MT, Jalbout MI, Kaddoum RN, Khatib MF, Haroun-Bizri ST (2004) End-tidal CO2 for prediction of cardiac output following weaning from cardiopulmonary bypass. J Extra Corpor Technol 36:255–257
Saleh HZ, Pullan DM (2011) Monitoring cardiac output trends with end-tidal carbon dioxide pressures in off-pump coronary bypass. Ann Thorac Surg 91:e81–e82
Rutlen DL, Wackers FJ, Zaret BL (1981) Radionuclide assessment of peripheral intravascular capacity: A technique to measure intravascular volume changes in the capacitance circulation in man. Circulation 64:146–152
Boulain T, Achard JM, Teboul JL, Richard C, Perrotin D, Ginies G (2002) Changes in BP induced by passive leg raising predict response to fluid loading in critically ill patients. Chest 121:1245–1252
Chemla D, Hebert JL, Coirault C, Zamani K, Suard I, Colin P, Lecarpentier Y (1998) Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol 274:H500–H505
Monge Garcia MI, Gil Cano A, Gracia Romero M, Monterroso Pintado R, Perez Madueno V, Diaz Monrove JC (2012) Non-invasive assessment of fluid responsiveness by changes in partial end-tidal CO2 pressure during a passive leg-raising maneuver. Ann Intensive Care 2:9
Vincent JL, Rhodes A, Perel A, Martin GS, Rocca GD, Vallet B, Pinsky MR, Hofer CK, Teboul JL, de Boode WP, Scolletta S, Vieillard-Baron A, De Backer D, Walley KR, Maggiorini M, Singer M (2011) Clinical review: update on hemodynamic monitoring - a consensus of 16. Crit Care 15:229
Antonelli M, Levy M, Andrews PJ, Chastre J, Hudson LD, Manthous C, Meduri GU, Moreno RP, Putensen C, Stewart T, Torres A (2007) Hemodynamic monitoring in shock and implications for management. International Consensus Conference, Paris, France, 27–28 April 2006. Intensive Care Med 33:575–590
De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL (2005) Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med 31:517–523
Vallee F, Richard JC, Mari A, Gallas T, Arsac E, Verlaan PS, Chousterman B, Samii K, Genestal M, Fourcade O (2009) Pulse pressure variations adjusted by alveolar driving pressure to assess fluid responsiveness. Intensive Care Med 35:1004–1010
Malbrain ML, Reuter DA (2010) Assessing fluid responsiveness with the passive leg raising maneuver in patients with increased intra-abdominal pressure: be aware that not all blood returns! Crit Care Med 38:1912–1915
Conflicts of interest
Profs. Jean-Louis Teboul and Xavier Monnet are members of the Medical Advisory Board of Pulsion Medical Systems.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Monnet, X., Bataille, A., Magalhaes, E. et al. End-tidal carbon dioxide is better than arterial pressure for predicting volume responsiveness by the passive leg raising test. Intensive Care Med 39, 93–100 (2013). https://doi.org/10.1007/s00134-012-2693-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2693-y