Abstract
Key message
Leaf rust resistance gene Lr2a was located to chromosome arm 2DS in three mapping populations, which will facilitate map-based cloning and marker-assisted selection of Lr2a in wheat breeding programs.
Abstract
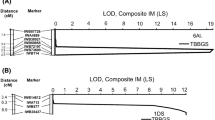
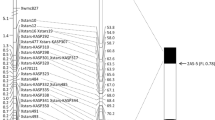
Incorporating effective leaf rust resistance (Lr) genes into high-yielding wheat cultivars has been an efficient method of disease control. One of the most widely used genes in Canada is the multi-allelic resistance gene Lr2, with alleles Lr2a, Lr2b, Lr2c, and Lr2d. The Lr2a allele confers complete resistance to a large portion of the Puccinia triticina (Pt) population in Canada. In this study, Lr2a was genetically mapped in two doubled haploid populations developed from the crosses Superb/BW278 and Superb/86ISMN 2137, and an F2 population developed from the cross Chinese Spring/RL6016. Seedlings were tested with the Lr2a avirulent Pt races 74-2 MGBJ (Superb/BW278) and 12-3 MBDS (Superb/86ISMN 2137 and Chinese Spring/RL6016) in greenhouse assays and were genotyped with 90K wheat Infinium SNP and kompetitive allele-specific PCR (KASP) markers. Lr2a was mapped to a collinear position on chromosome arm 2DS in all three populations, within a 1.00 cM genetic interval between KASP markers kwm1620 and kwm1623. This corresponded to a 305 kb genomic region of chromosome 2D in Chinese Spring RefSeq v2.1. The KASP marker kwh740 was predictive of Lr2a in all mapping populations. A panel of 260 wheats were tested with three Pt isolates, which revealed that Lr2a is common in Canadian wheats. The KASP markers kwh740 and kwm1584 were highly associated with resistance at the Lr2 locus, while kwm1622 was slightly less correlated. Genetic mapping of the leaf rust resistance gene Lr2a and DNA markers developed here will facilitate its use in wheat breeding programs.





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ausemus ER, Harrington JB, Reitz LP, Worzella WW (1946) A summary of genetic studies in hexaploid and tetraploid wheats. Agron J 38:1082–1099. https://doi.org/10.2134/agronj1946.00021962003800120006x
Bokore FE, Knox RE, Cuthbert RD, Pozniak CJ, McCallum BD, N’Diaye A, DePauw RM, Campbell HL, Munro C, Singh A et al (2020) Mapping quantitative trait loci associated with leaf rust resistance in five spring wheat populations using single nucleotide polymorphism markers. PLoS ONE 15(4):e0230855
Bokore FE, Knox RE, Hiebert CW, Cuthbert RD, DePauw RM, Meyer B, N’Diaye A, Pozniak CJ, McCallum BD (2022) A combination of leaf rust resistance genes, including Lr34 and Lr46, is the key to the durable resistance of the Canadian wheat cultivar, Carberry. Front Plant Sci 12:775383. https://doi.org/10.3389/fpls.2021.775383
Cavanagh CR, Chao S, Wang S, Huang BE, Stephen S, Kiani S, Forrest K, Saintenac C, Brown-Guedira GL, Akhunova A, See D, Bai G, Pumphrey M, Tomar L, Wong D, Kong S, Reynolds M, da Silva ML, Bockelman H, Talbert L, Anderson JA, Dreisigacker S, Baenziger S, Carter A, Korzun V, Morrell PL, Dubcovsky J, Morell MK, Sorrells ME, Hayden MJ, Akhunov E (2013) Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proc Nat Academy Sci 110:8057–8062. https://doi.org/10.1073/pnas.1217133110
Chai Y, Pardey PG, Hurley TM, Senay SD, Beddow JM (2020) A probabilistic bio-economic assessment of the global consequences of wheat leaf rust. Phytopathol 110:1886–1896. https://doi.org/10.1094/PHYTO-02-20-0032-R
Cuthbert JL, Somers DJ, Brule-Babel AL, Douglas Brown P, Crow GH (2008) Molecular mapping of quantitative trait loci for yield and yield components in spring wheat (Triticum aestivum L.). Theor Appl Genet 117:595–608
Dyck PL (1979) Identification of the gene for adult plant leaf rust resistance in Thatcher. Can J Plant Sci 59:499–502
Dyck PL, Samborski DJ (1968) Genetics of resistance to leaf rust in the common wheat varieties Webster, Loros, Brevit, Carina, Malakof and Centenario. Can J Genet Cytol 10:7–17. https://doi.org/10.1139/g68-002
Dyck PL, Samborski DJ (1974) Inheritance of virulence in Puccinia recondita on alleles at the Lr2 locus for resistance in wheat. Can J Genet Cytol 16:323–332. https://doi.org/10.1139/g74-036
German SE, Kolmer JA (1992) Effect of gene Lr34 in the enhancement of resistance to leaf rust of wheat. Theor Appl Genet 84:97–105. https://doi.org/10.1007/bf00223987
He F, Pasam R, Shi F, Kant S, Keeble-Gagnere G, Kay P, Forrest K, Fritz A, Hucl P, Wiebe K, Knox R, Cuthbert R, Pozniak C, Akhunova A, Morrell PL, Davies JP, Webb SR, Spangenberg G, Hayes B, Daetwyler H, Tibbits J, Hayden M, Akhunov E (2019) Exome sequencing highlights the role of wild-relative introgression in shaping the adaptive landscape of the wheat genome. Nat Genet 51:896–904
Herrera-Foessel SA, Lagudah ES, Huerta-Espino J, Hayden MJ, Bariana HS, Singh D, Singh RP (2011) New slow-rusting leaf rust and stripe rust resistance genes Lr67 and Yr46 in wheat are pleiotropic or closely linked. Theor Appl Genet 122:239–249
Hiebert CW, Thomas JB, McCallum BD, Humphreys DG, DePauw RM, Hayden MJ, Mago R, Schnippenkoetter W, Spielmeyer W (2010) An introgression on wheat chromosome 4DL in RL6077 (Thatcher*6/PI 250413) confers adult plant resistance to stripe rust and leaf rust (Lr67). Theor Appl Genet 121:1083–1091
Kassa MT et al (2016) Genetic mapping of SrCad and SNP marker development for marker-assisted selection of Ug99 stem rust resistance in wheat. Theor Appl Genet 129:1373–1382
Kolmer JA (1996) Genetics of resistance to wheat leaf rust. Annu Rev Phytopathol 34:435–455. https://doi.org/10.1146/annurev.phyto.34.1.435
Kolmer JA (1999) Virulence dynamics, phenotypic diversity, and virulence complexity in two populations of Puccinia triticina in Canada from 1987 to 1997. Can J Bot 77:333–338. https://doi.org/10.1139/b98-221
Kosambi DD (1943) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Liu JQ, Kolmer JA (1997) Inheritance of leaf rust resistance in wheat cultivars Grandin and CDC Teal. Plant Dis 81:505–508
Long DL, Kolmer JA (1989) A North American system of nomenclature for Puccinia recondita f. sp. tritici. Phytopathology 79:525–529
Lorieux M (2012) MapDisto: fast and efficient computation of genetic linkage maps. Mol Breed 30:1231–1235
Luig NA, McIntosh RA (1968) Location and linkage of genes on wheat chromosome 2D. Can J Genet Cytol 10:99–105
Maccaferri M, Cane MA, Sanguineti MC, Salvi S, Colalongo MC, Massi A, Clarke F, Knox R, Pozniak CJ, Clarke JM, Fahima T, Dubcovsky J, Xu S, Ammar K, Karsai I, Vida G, Tuberosa R (2014) A consensus framework map of durum wheat (Triticum durum Desf.) suitable for linkage disequilibrium analysis and genome-wide association mapping. BMC Genomics 15:873. https://doi.org/10.1186/1471-2164/15/873
Mains EB, Leighty CE, Johnston CO (1926) Inheritance of resistance to leaf rust Puccinia triticina Erikss., in crosses of common wheat. Triticum Vulgare Vill J Agric Res 32:931–972
McCallum BD, DePauw RM (2008) A review of wheat cultivars grown in the Canadian prairies. Can J Plant Sci 88:649–677
McCallum BD, Seto-Goh P (2003) Physiologic specialization of wheat leaf rust (Puccinia triticina) in Canada in 2000. Can J Plant Pathol 25:91–97
McCallum BD, Seto-Goh P (2010) The inheritance of leaf rust resistance in the wheat cultivars ‘Superb’, ‘McKenzie’ and ‘HY644.’ Can J Plant Pathol 32:387–395
McCallum BD, Fetch T, Chong J (2007) Cereal rust control in Canada. Aust J Agr Res 58:639–647
McCallum BD, Seto-Goh P, Xue A (2010) Physiological specialization of Puccinia triticina in Canada in 2007. Can J Plant Pathol 32:229–236
McCallum BD, Hiebert CW, Cloutier S, Bakkeren G, Rosa SB, Humphreys DG, Marais GF, McCartney CA, Panwar V, Rampitsch C, Saville BJ, Wang X (2016) A review of wheat leaf rust research and the development of resistant cultivars in Canada. Can J Plant Pathol 38:1–18. https://doi.org/10.1080/07060661.2016.1145598
McCallum BD, Reimer E, McNabb W, Foster A, Rosa S, Xue A (2021) Physiologic specialization of Puccinia triticina, the causal agent of wheat leaf rust, in Canada in 2015–2019. Can J Plant Pathol 43(sup2):S333–S346
McCartney CA, Somers DJ, McCallum BD, Thomas J, Humphreys DG, Menzies JG, Brown PD (2005) Microsatellite tagging of the leaf rust resistance gene Lr16 on wheat chromosome 2BS. Mol Breed 15:329–337
McIntosh RA, Wellings CR, Park RF (1995) Wheat rust: an atlas of resistance genes. CSIRO, Australia
McIntosh RA, Baker EP (1968) A linkage map for chromosome 2D. In: Finlay KW, Sheperd KW (eds) Proceedings of the Third International Wheat Genetics Symposium. Australian Academy of Science, Canberra, pp 305–309
McMullen M, Markell SG, Rasmussen JB (2008) Rust diseases of wheat in North Dakota. North Dakota State University Extension Bulletin 1361
Meng L, Li H, Zhang L, Wang J (2015) QTL IciMapping: integrated software for genetic linkage map construction and qualitative trait locus mapping in biparental populations. Crop J 3:269–283
Oelke LM, Kolmer JA (2004) Characterization of leaf rust resistance in hard red spring wheat cultivars. Plant Dis 88:1127–1133. https://doi.org/10.1094/PDIS.2004.88.10.1127
Pallotta MA, Warner P, Fox RL, Kuchel H, Jefferies SJ, Langridge P (2003) Marker assisted wheat breeding in the southern region of Australia. In: Pogna NE, Romano M, Pogna EA, Galerio G (eds) Proceedings of the Tenth International Wheat Genetics Symposium, Institutio Sperimentale per la Cerealcoltura, Rome, pp 789–791
Raupp WJ, Sukhwinder-Singh B-G, Gill BS (2001) Cytogenetic and molecular mapping of the leaf rust resistance gene Lr39 in wheat. Theor Appl Genet 102:347–352
Roelfs AP, Singh RP, Saari EE (1992) Rust diseases of wheat. In: Hettel GP (ed) Concepts and Methods of Disease Management. CIMMYT, Mexico
Rowland GG, Kerber ER (1974) Telocentric mapping hexaploid wheat of genes for resistance and other characters derived from Aegilops squarrosa. Can J Genet Cytol 16:137–144
Sharma JS, McCallum BD, Hiebert CW (2022) Development of single nucleotide polymorphism-based functional molecular markers from the Lr22a gene sequence in wheat (Triticum aestivum). Plant Breed 141:204–211. https://doi.org/10.1111/pbr.13007
Shiferaw B, Smale M, Braun HJ, Duveiller E, Reynolds M, Muricho G (2013) Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur 5:291–317
Soliman AS, Heyne EG, Johnston CO (1964) Genetic analysis for leaf rust resistance in the eight differential varieties of wheat. Crop Sci 4:246–248
Sun XC, Bai GH, Carver BF (2009) Molecular markers for leaf rust resistance gene Lr41. Mol Breed 23:311–321
Thind AK, Wicker T, Simkova H, Fossati D, Moullet O, Brabant C, Vrana J, Dolezel J, Krattinger SG (2017) Rapid cloning of genes in hexaploid wheat using cultivar-specific long-range chromosome assembly. Nat Biotechnol 35:793–796. https://doi.org/10.1038/nbt.3877
Thomas J, Chen Q, Howes N (1997) Chromosome doubling of haploids of common wheat with caffeine. Genome 40:552–558
Townley-Smith TF, Humphreys DG, Czarnecki E, Lukow OM, McCallum BM, Fetch TG, Gilbert JA, Menzies JG, Brown PD (2010) Superb hard red spring wheat. Can J Plant Sci 90:347–352
Voorrips RE (2002) MapChart: Software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78
Wang S et al (2014) Characterization of polyploid wheat genomic diversity using a high-density 90 000 single nucleotide polymorphism array. Plant Biotechnol J 12:787–796
Wu Y, Bhat PR, Close TJ, Lonardi S (2008) Efficient and accurate construction of genetic linkage maps form the minimum spanning tree of a graph. PLoS Genet 4:e1000212
Yao Z, You FM, N’Diaye A, Knox RE, McCartney C, Hiebert CW, Pozniak C, Xu W (2020) Evaluation of variant calling tools for large plant genome re-sequencing. BMC Bioinf 21:360
Zhu T, Wang L, Rimbert H, Rodriguez JC, Deal KR, De Oliveira R, Choulet F, Keeble-Gagnère G, Tibbits J, Rogers J, Eversole K, Appels R, Gu YQ, Mascher M, Dvorak J, Luo M-C (2021) Optical maps refine the bread wheat Triticum aestivum cv. Chinese Spring genome assembly. Plant J 107:303–314. https://doi.org/10.1111/tpj.15289
Acknowledgements
The authors thank Mira Popovic, Ghassan Mardli, Leslie Bezte, Alain Ngantcha, and Elsa Reimer at the Morden Research and Development Centre for their contributions to this research. Funding was provided by Western Grains Research Foundation, CAP Ag Action Manitoba, Manitoba Crop Alliance, and Alberta Wheat Commission as part of the Genome Canada projects CTAG2 and 4DWheat.
Funding
Funding was provided by Agriculture and Agri-Food Canada, Western Grains Research Foundation, CAP Ag Action Manitoba, Manitoba Crop Alliance, and Alberta Wheat Commission as part of the Genome Canada projects CTAG2 and 4DWheat.
Author information
Authors and Affiliations
Contributions
DT phenotyped the Chinese Spring/RL6016 population, developed and tested kwm KASP markers, and co-wrote the first draft of the manuscript. ML phenotyped the Superb/BW278 and Superb/86ISMN 2137 DH populations, developed and tested the kwh KASP markers, and co-wrote the first draft of the manuscript. BDM phenotyped all wheat populations. CWH designed many of the kwh KASP markers. CAM designed the kwm KASP markers. BDM, AB, CWH, and CAM conceived, implemented, and supervised various aspects of the study. All authors read, edited, and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Albrecht E. Melchinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Table S1
Kompetitive allele-specific PCR (KASP) markers developed from the Lr2a region of chromosome 2D, SNP name, its location in Chinese Spring RefSeq v1.0 and v2.1, and primer information. Supplementary Table S2 Seedling response to Puccinia triticina Eriks. (Pt) races and validation analysis of Lr2a linked kompetitive allele-specific PCR (KASP) markers Excalibur_c1944_1017_kwh740, kwm1584, kwm1618, kwm1620, kwm1621, kwm1622, and kwm1623 on a wheat panel consisting of 260 wheat lines. Supplementary Table S3 The chromosome 2D genetic map developed for the Superb/BW278 DH population. Summary of data for 71 SNP markers with chromosome assignment and map position and newly developed kompetitive allele-specific PCR (KASP) markers. Supplementary Table S4 The chromosome 2D genetic map developed for the Superb/86ISMN 2137 DH population. Summary of data for 165 SNP markers with chromosome assignment and map position and newly developed kompetitive allele-specific PCR (KASP) markers. Supplementary Table S5 The chromosome 2D genetic map developed for the Chinese Spring/RL6016 F2 population. Summary of data for 45 SNP markers with chromosome assignment and map position and newly developed kompetitive allele-specific PCR (KASP) markers. Supplementary Fig. S1 Pedigree diagram indicating the presence or absence of Lr2a in older Canada Western Red Spring (CWRS) wheat cultivars. Cultivars in red carry Lr2a, cultivars in blue have the susceptible allele at the Lr2 locus, and cultivars in black have not been tested (XLSX 285 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thambugala, D., Lewarne, M.K., McCallum, B.D. et al. Genetic mapping of the wheat leaf rust resistance gene Lr2a and its importance in Canadian wheat cultivars. Theor Appl Genet 136, 198 (2023). https://doi.org/10.1007/s00122-023-04440-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00122-023-04440-9