Abstract
Key message
Alloplasmic male sterile breeding lines of Eruca sativa were developed by intergeneric hybridization with CMS- Brassica oleracea, followed by recurrent backcrosses and determination of the breeding value.
Abstract
Male sterile breeding lines of rocket salad (Eruca sativa) were developed by intergeneric hybridization with cytoplasmic male sterile (CMS) cauliflower (Brassica oleracea) followed by recurrent backcrosses. Five amphidiploid F1 plants (2n = 2x = 20, CE), achieved by manual crosses and embryo rescue, showed an intermediate habit. The plants were completely male sterile and lacked seed set after pollination with the Eruca parent. Allotetraploid F1-hybrid plants (4n = 4x = 40, CCEE) obtained after colchicine treatment were backcrossed six times with pollen of the Eruca parent to select alloplasmic diploid E. sativa lines. The hybrid status and the nucleo-cytoplasmic constellation were continuously controlled by RAPD and Southern analysis during subsequent backcrosses. The ploidy level was investigated by flow cytometry and chromosome analysis. Premeiotic (sporophytic) and postmeiotic (pollen abortive) defects during the anther development were observed in the alloplasmic E. sativus plants in comparison to the CMS-cauliflower donor. No further incompatibilities were noticed between the CMS-inducing cybrid cytoplasm and the E. sativa nuclear genome. The final alloplasmic E. sativa lines were diploid with 2n = 2x = 22 chromosomes and revealed complete male sterility and restored female fertility. Plant vigor and yield potential of the CMS-E. sativa BC5 lines were comparable to the parental E. sativus line. In conclusion, the employed cybrid—cytoplasm has been proven as a vital source of CMS for E. sativa. The developed lines are directly applicable for hybrid breeding of rocket salad.
Similar content being viewed by others
Introduction
The trend of Mediterranean cuisine promoted the popularity of rocket salad (Eruca sativa Mill.) during the last two decades. Hence, research and breeding activities were intensified especially for the evaluation of E. sativa regarding genetic resources (Bozokalfa et al. 2011; Egea-Gilabert et al. 2009; Shinwari et al. 2013; Yaniv et al. 1998), resistance to pathogens (Bhargava et al. 1980; Larran et al. 2006; Paz Lima et al. 2004; Sharma 1985; Srinivasan et al. 2012) and tolerance to abiotic stress conditions (Ashraf 1994; de Vos et al. 2013; Singh et al.1992). Hybrid breeding is still limited by the lack of a practicable reproductive system such as cytoplasmic male sterility (CMS) or self-incompatibility (SI) to avoid self-pollination of one of the hybrid cross parents. A sporophytic SI system for E. sativa has been described by Verma and Lewis (1977). However, application in hybrid breeding was not reported yet.
The CMS trait is widely used for commercial hybrid breeding in Brassica napus (Dieterich et al. 2003; Renard et al. 1992), B. oleracea (Branca 2008), B. campestris (Verma et al. 2000), B. rapa (Dong et al. 2013) and B. juncea (Chamola et al. 2013; Wan et al. 2008). For E. sativa a suitable CMS source is not yet available. The transmission of the E. sativa nuclear genome into a well established CMS cytoplasm of the commercially used species of Brassicaceae is a potential option to establish CMS-E. sativa for hybrid breeding.
The Ogura-CMS system based on a cytoplasm from Raphanus sativus (Ogura 1968) offers a promising source. The history of research on Ogura-CMS was recently reviewed by Yamagishi and Bhat (2014). The cytoplasm has been used to generate alloplasmic CMS sources for B. napus, B. juncea, B. oleracea, B. rapa, and R. sativus. It is currently applied worldwide in F1 breeding of the mentioned species (Bonhomme et al. 1992; Dong et al. 2013; González-Melendi et al. 2008; Kirti et al. 1995; Makaroff et al. 1990; Yamagishi and Terachi 1996).
The original Ogura-CMS cytoplasm has been introgressed at first into B. napus and B. oleracea by sexual crosses, but these alloplasmic lines showed, besides the suitable male sterility, undesirable traits such as a disturbed development of nectaries and a chlorotic leaf habit of plants growing at temperatures lower than 15 °C (Bannerot et al. 1977). A replacement of the Raphanus chloroplasts by protoplast fusion has been done to overcome these problems. The developed CMS system was marked as a milestone for hybrid breeding of Brassica crops (Renard et al. 1992). The application of the Ogu-INRA® system in rapeseed led to complete abortion of pollen development in nearly normally developed anthers (Gourret et al. 1992; Pelletier et al. 1983). Histological studies revealed a premature breakdown of the tapetal cell layer as it occurs in the Ogura-radish plants, which impairs pollen development at microspore stage in the absence of functional mitochondria (González-Melendi et al. 2008).
Interspecific crosses within the genus Brassica have been described (for review: Branca 2008; Kaneko et al. 2011; Nishiyama et al. 1991; Ordas and Cartea 2008), however, data on successful intergeneric or intertribal crosses within the Brassicaceae family are rare. Some crosses between Eruca and Brassica have been forced to introduce Eruca traits into Brassica crops. Sexual hybrids produced by the cross E. sativa x B. campestris (Agnihotri et al. 1990) showed an intermediate phenotype, a completely restored fertility and an improved tolerance against Alternaria brassicae and white rust. For B. rapa x E. sativa hybrids induction of male sterility has been mentioned by Matsuzawa et al. (1999). The F1 and F2 plants were partially male fertile and seeds were harvested after self-pollination, up to the F6 generation. Six different types of male sterility were selected, two promising for further breeding application in B. rapa. Somatic hybrids between B. napus and E. sativa showed partial fertility after self-pollination or backcrosses with B. napus (Fahleson et al. 1988). Somatic hybrids generated between E. sativa and B. juncea have shown a variable pollen viability (0–82 %) and a moderate seed set after self-pollination or backcrossing with the B. juncea parent. So far backcrossing with E. sativa was not successful (Sikdar et al. 1990).
The present study reports the development of alloplasmic male sterile breeding lines of E. sativa derived from a sexual intergeneric hybridization with CMS-B. oleracea and recurrent backcrosses until the BC6 generation. A brief agronomical, cytogenetic and molecular characterization, including histological investigation of flower development will be presented.
Materials and methods
Plant material and cultivation
Seeds of E. sativa (2n = 2x = 22, EE) were obtained from a commercial seed trader (International Seeds Processing GmbH, Quedlinburg, Germany). The hermaphrodite cauliflower B. oleracea cv. ‘Korso’ and the CMS counterpart BAZOG 4 (B. oleracea L., 2n = 2x = 18, CC) originated from the Julius-Kühn-Institut´s germplasm collection. The BAZOG 4 CMS line was developed by an independent protoplast fusion approach similar to that described by Pelletier et al. (1983). Whereas the nuclear and the plastid genome of BAZOG 4 descended from the cv. ‘Korso’, a unique mtDNA rearrangement has been identified in comparison to both, the original Ogura mtDNA of Raphanus sativus and the Ogu-INRA® CMS system (Pelletier et al. 1983). Analyses of ptDNA and mtDNA by Southern analyses have been described earlier (Nothnagel and Budahn 1994) and were summarized in Table S1. The BAZOG 4 flowers were characterized by a normal morphology of anthers, but revealed a premature tapetum degeneration followed by a post-meiotic breakdown of microspores.
Parental plants and hybrid plants of the crossing experiments were pre-cultivated in 5 cm plastic pots with a sand-humus soil mixture (v/v 3:1). In the three-leaf stage the seedlings were replanted into 16 cm plastic pots and cultivated under glasshouse conditions. Plant material for the field experiments and seed production was pre-cultivated in 5 cm plastic pots in the same manner as described before and was then replanted in the ground.
Crossing experiments and embryo rescue
The initial crosses of CMS-B. oleracea and E. sativa were carried out manually under insect protected glasshouse conditions. Immature seeds were harvested four to five weeks after pollination for an embryo rescue approach. Embryo sacs were isolated after surface sterilization of pods (3 % NaClO for 15 min, de-ionized water for 1 min) and cultured in Petri dishes on MS medium (Murashige and Skoog 1962) containing MS micro and macro elements including vitamins 4.4 g/L (Duchefa, Haarlem, The Netherlands); 8.0 g/L Bacto-Agar (Becton–Dickinson, Heidelberg, Germany); 30 g/L sucrose; 0.2 mg/L 1-naphtalene acetic acid, pH 5.9–6.1. Petri dishes were placed in a culture chamber with a photoperiod of 16 h and 25/20 °C D/N. For subculture as well as for shoot and root induction, the same medium was used. In vitro plants were transferred to plastic pots with sandy-humus soil (v/v 3:1) in a climatic chamber. Well established plants were cloned by shoot or leaf cuts directly in soil.
Polyploidization, cell flow cytometry and GISH/FISH analysis
Cloned allodiploid F1 plants were treated with colchicine (0.2 %) by drop inoculation on the secondary shoot meristem and incubated for 3 days. The DNA content of the developed shoots was analyzed by cell flow cytometry. Putative tetraploid shoots were cut and rooted in soil.
For the cell flow cytometry using a ‘FACS Calibur’ (Becton Dickenson, BD Biosciences, San Jose, CA) 1 g leaf material was chopped with a razor blade in 500 µl of the nuclei extraction buffer CyStain PI absolute P (Partec, Görlitz, Germany) and stained in 1 ml of the corresponding staining buffer (Partec) with 5 % PVP 25 (SERVA, Heidelberg, Germany) and 0.6 % propidium iodide (SERVA). The nuclei suspension was filtered in a 5 ml Polystyrene Round-Button Tube with Cell-Strainer Cap (BD) immediately after staining.
Metaphase chromosomes of root tips were prepared after tissue maceration in a mixture of 1 % pectolyase and 4 % cellulase (Schrader et al. 2000). One microgram of high molecular unshared genomic DNA of E. sativa was used as a probe in a 20 µl reaction of DIG-Nick Translation Mix (Roche Diagnostics, Mannheim, Germany) for genomic in situ hybridization (GISH). For blocking 30 times excess of B. oleracea DNA was used. The 50 µl hybridization mix for one sample contained 144 ng DIG-labeled Eruca DNA, 4320 ng blocking DNA, 180 ng Biotin labeled 5S rDNA and 5000 ng salmon sperm DNA. A 117 bp fragment of 5S rDNA from soybean (Gottlob-McHugh et al. 1990) was amplified and labeled by PCR. The indirect FISH followed a protocol according to Schrader et al. (2000).
Molecular characterization
Total genomic DNA was extracted from 0.5 g of young leaf tissue using the method of Porebski et al. (1997). The RAPD analyses were performed according to Williams et al. (1990). For the amplification, InviTaq DNA polymerase (Invitek, Berlin, Germany) was used in combination with NH4 reaction buffer. Fragment sizes were estimated using a 100 bp ladder (Life technologies, Carlsbad, USA). PCR amplification of the Ogura-specific orf138 region was carried out with primers described by Yamagishi and Terachi (1996) to detect a specific 278 bp DNA fragment.
For Southern analyses about 8 µg DNA were digested with the restriction enzymes EcoRI and HindIII. After electrophoresis on a 1 % agarose gel the DNA fragments were immobilized on a nylon membrane by 0.5 J/cm2 UV irradiation from both sides in a crosslinker (Biometra, Göttingen, Germany). Hybridization with DIG-labeled probes was performed according to manufacturer instructions (Roche Diagnostics, Mannheim, Germany). The atp1 probe of Arabidopsis thaliana was kindly provided by A. Brennicke (Germany). A PCR fragment from the psbE operon of B. oleracea amplified with P1 and P3 primer described by Bock et al. (1993) was used as cpDNA probe. All probes were labeled via PCR with Digoxigenin-11-dUTP. The membrane was incubated with anti-digoxigenin Fab fragments conjugated to alkaline phosphatase and finally with CDP-Star. Chemiluminescent detection was performed on a Chemiluminescent Detection Film (Roche Diagnostics, Mannheim, Germany).
Flower morphology and histological analysis
Flower morphology, male and female fertility of the hybrid plants were compared to the parental lines during different developmental stages. Flower traits such as petal color and shape, stamen development, expression of nectaries and shape of the stylus were determined using a stereo-microscope.
For detailed comparisons of anther development and microsporogenesis serial sections of flower buds were characterized as described previously (Linke et al. 1999). Flower buds (3–6 mm long) were fixed in Zinc Formal-Fixx (Life Science Int., Frankfurt, Germany), diluted 1:4 with water, dehydrated in a graded ethanol series and embedded in Historesin (Leica, Nussloch, Germany). Semi-thin sections (4 µm) were cut from the polymerized blocks with a microtome RM2155 (Leica, Wetzlar, Germany), stained with 0.01 % toluidine blue, and analyzed using a Nikon 90i microscope equipped with a DS-5Mc digital camera and SIS software (Nikon, Düsseldorf, Germany).
Determination of seed set ability
Plants of the BC2 to BC5 were evaluated for their seed set ability. Harvested pods were measured and the seed number per pod was calculated as index for the female fertility. Seed color, hundred seed weight (HSW) and germination capacity were calculated as indices for the seed quality. Two alloplasmic male sterile E. sativa BC5 lines (8 plants each) and the original E. sativa parent (12 plants) were replanted together in a plastic tunnel for estimating the seed set ability. During the flowering time honey bees were applied for pollination of the CMS-E. sativa lines.
Estimation of growth parameters
Two BC4 lines, four BC5 lines and the Eruca sativa parent were compared for agronomical traits within a parallel trial in the field and in a plastic tunnel. For both trials, plant material was pre-cultivated 30 days under glasshouse conditions and subsequently replanted in the field or tunnel in a randomized block design with five plants per plot each and four plot replications, in a distance of 40 × 40 cm. The number of leaves per plant was counted 40 days post sowing (DPS), the diameter of the plant rosette and the plant leaf biomass were measured at DPS 40 + 58 and DPS 58, respectively.
Statistical analyses
The software package SYSTAT 13® (Chicago, IL: Systat Software, Inc., 2009) was used for the descriptive statistics and ANOVA. Where ANOVA yielded significant F values, means were compared using Tukey’s procedure on a significance level of α = 0.05 marked with small letters in the tables.
Results
Intergeneric hybridizations, backcrosses and plant morphology
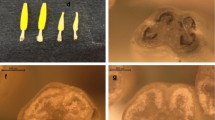
In total, 700 initial crosses have been carried out, but most of the juvenile pods were aborted 10 days after pollination. Approximately 2 % of the pollinated flowers developed pods, 3–5 single ovaries apparently developed into seeds. Since we observed a severe collapse of the juvenile seeds, embryos of the immature seeds were prepared and rescued in vitro. From a total of 30 prepared embryos five F1 plants from independent crosses were obtained. After in vitro cultivation and transfer into soil, the hybrid character was verified by chromosome analysis. The hybrid plants revealed the expected number of 2n = 2x = 20 chromosomes. A discrimination between both haploid chromosome sets (n = 9 C and n = 11 E) was possible by genomic and by gene specific in situ hybridization (GISH, FISH) (Fig. 1).
GISH/FISH staining of mitotic chromosomes of the B. oleracea × E. sativa hybrids within the F1 and BC3 generation. a, b DAPI and corresponding GISH/FISH stained root tip mitosis chromosomes in F1 (9 + 11 = 20), c, d DAPI and corresponding GISH/FISH stained mitosis chromosomes of the root tip of a BC3 plant (2n = 22). Green GISH labeled E. sativa chromosomes. Red 5S rDNA FISH probe
The F1 plants showed an intermediate growth habit. While the CMS-B. oleracea expressed dark-yellow petals and the E. sativa creamy-white petals with purple veins, flowers of the intergeneric hybrid showed light-yellow colored petals with light-purple veins. They developed normal nectaries and carpels and expressed male sterility. No seed set was observed after pollination with the Eruca parent (Fig. 2; Table 1). Therefore, rooted shoot cuttings of the F1 plants were treated successfully with colchicine to obtain tetraploid plants. A total of six allotetraploid hybrid plants from two different crossing events were selected via flow cytometry (Fig. S1) and were backcrossed with the E. sativa parent. Embryo rescue was necessary to generate BC1 plants, because of disturbed seed development in the pods of the allotetraploid F1 plants. The BC1 plants had an intermediate phenotype similar to the F1 plants and showed the same flower morphology as the F1 including male sterility (Fig. 2). Pollinated flowers of the male sterile BC1 plants indicated a moderate seed set and seed germination. The generated BC2 plants corresponded mainly to the phenotype of the E. sativa parent. The flower morphology of the BC2 plants was comparable to the E. sativa parent and the seed set after pollination with pollen of E. sativa was to a large extent normal. In the BC3, the petal color was creamy-white with purple veins indicating a high similarity to the Eruca parent. Filament length and stamen shape were comparable to the Eruca parent (Fig. 2; Table 1). In BC3 and higher backcross generations the seed set estimated as seeds per silique did not differ from that of Eruca parent (Table 2).
Whereas for the BC1 plants significant parts of the Brassica genome were detectable, in all investigated BC3 plants the Brassica chromosomes were eliminated. GISH analysis showed exclusively 2n = 2x = 22 green labeled E. sativa chromosomes (Fig. 1). However, small introgressions of B. oleracea DNA are not detectable by GISH. To select offspring plants with highly reduced B. oleracea introgressions for further backcrosses RAPD analyses were applied. Figure 3 shows a RAPD profile of crossing parents and individual offspring plants of BC1 and BC3. Two BC3 plants exhibit still clearly B. oleracea fragments.
Molecular analysis of the cytoplasms
A stable maternal inheritance of the cytoplasm was verified. The 278 bp mtDNA fragment from orf138 associated with Ogura-CMS was detected by PCR as described by Yamagishi and Terachi (1996) (Fig. 4). Maternal inheritance of the cytoplasm was confirmed by EcoRI and HindIII digestion of total DNA of BC5 and BC6 plants and Southern hybridization with mtDNA or with cpDNA probes. The hybridization patterns of the BC5 and BC6 offspring plants corresponded to those of the CMS-B. oleracea parent (Fig. 5).
Southern hybridization with the mitochondrial probe atp1 (up) and the chloroplast probe P1/P3 from the psbE operon (down). M: DNA Marker VII, DIG-labeled (Roche), Bo: CMS-B. oleracea, 1–5: BC5 plants, 6–11: BC6 plants. The Black and white arrows mark unique fragment bands for E. sativa and B. oleracea, respectively
Anther morphology
An impaired development of both anther morphology and microsporogenesis was observed in the F1 and in all of the five backcross generations BC1 to BC5. The cauliflower CMS type used as female parent in this study showed a proper development of the anthers corresponding to the CMS type described by Ogura (1968) in R. sativus. In the alloplasmic Eruca plants, the anther morphology has been changed dramatically. The development of wild type anthers of B. oleracea and E. sativa was comparable to earlier descriptions (Geddy et al. 2005) (Fig. 6). In the alloplasmic Eruca plants we observed a disturbed formation of the anther locules already during early developmental stages and assigned them as ‘early defect (I)’. The locule growth remained incomplete or occasionally locules were completely undeveloped only showing slight extrusions on the position where normally growth progression occurs (Fig. 6). In rare cases, locules grew out in a correct position, but lacked a structured layer composition. In these cases, sporogenous cells were never observed and the extruded locule-like structure was filled with parenchymal cells. Within individual anthers, the development from one up to four locules could be impaired. Thereby, either single locules or locule pairs of one anther lobe were affected. Regarding the symmetry of the anther structure, locules that belong to the same theca are more likely to be impaired than those of the adaxial or abaxial locule pairs. Within one single flower, approximately three to five of a total of six anthers indicated a disturbed development. Hence, an impaired locule formation during early stages revealed already premeiotic defects by disturbing the sporophyte structure and contributed to male sterility in alloplasmic Eruca plants.
Transverse sections of flowers of the cross parents during post-meiotic microsporogenesis. The cutting plane was optimized to characterize the anther architecture in all of the four locules. a Male fertile cauliflower Brassica oleracea cv. Korso; b Eruca sativa wild type; c male sterile cauliflower CMS-B. oleracea (BAZOG4); d CMS-B. oleracea (BAZOG 4) with collapsed anthers immediately before opening of flower. e–h Transverse sections of flower buds of the alloplasmic male sterile B. oleracea × E. sativa hybrids during post-meiotic microsporogenesis. e F1; f BC3; g BC4; h BC5. A striking number of anthers showed an incomplete or disturbed development of the pollen sacs (locules). It was observed that frequently locule pairs belonging to one theca were affected. Several locules remained undeveloped and did not form sporophytic tissues; instead, slight parenchymal protrusions were observed (g, h). Several thecae revealed a premature fusion of locules (e). Examples of defective locules are indicated by white arrowheads
In those cases where locule formation was properly initiated, development was affected, too, but during advanced stages (Fig. 6). In those locules, the structured layer composition corresponded to those of fertile plants and the initial development of parietal and sporogenous cells was not affected. The breakdown of microspore development started during the meiosis I (Fig. 7). These observations were analogous to the plants of CMS-B. oleracea (not shown here). A first sign for developmental deviation was a premature degeneration of the tapetal layer during the anaphase I. A premature vacuolization of the tapetum occurred and was observed up to the tetrad stage. During the same stages the development of microspores became substantially affected. Degeneration and shrinkage were observed before any differentiation process was initiated. Subsequent stages indicated a complete cessation of microspore development. During the stages, where normally pollen maturation occurs, the (persisting) microspores clumped together and the locules collapsed before bud opening (Fig. 8). At no time, pollen grain spilling was observed. Thus, advanced development was impaired, too, and affected the early post-meiotic stage of microspore development. Advanced disturbances during microsporogenesis were assigned as ‘mid-stage defect (II)’.
a Transverse sections illustrated the typical butterfly symmetry with the four pollen chambers or locules. The locules were arranged as pairs, each pair belonged to a separate unit, the theca. The symmetry axis separating the thecae was drawn by a line. b Distribution example 1: Each theca consisted of only one large pollen chamber; sporophytic tissues and microspores were observed, however, the enlarged size and the presence of only one pollen chamber pointed to a premature fusion of the inter-thecal locule pair. Remnant borders of the original locule within the merged pollen chamber are indicated (arrows). c Distribution example 2: the left theca indicated locule pairs with sporophytic tissue, whereas in the right theca the locule development was incomplete. Formation of the locule was initiated at the correct position, but growth ceased after small protrusions were developed. Sporophytic tissue was not observed, instead, the bulges consisted of parenchymal cells. Bar 100 µm
Tetrad stage and pollen development of fertile Eruca sativa (top line) and alloplasmic male sterile E. sativa plants of the BC4 generation (bottom line) were shown. (a, a’) Tetrad stage of microspores. Degeneration of the tapetum in BC4 is visible. First differences were observed during the tetrad stage. The tapetum cells were dark stained and indicated a strong vacuolization (a’). Although the structure of the tetrads in a’ seemed to be yet unaffected, the size of the tetrads was remarkably smaller than in a. (b, b’) Pre-dehiscent stage: pollen grains were surrounded by the exine wall (b); the tapetum cells revealed an irregular structure by shrinkage and degeneration (b’); tetrads and pre-dehiscent pollen were strongly shrunken and collapsed (b’). (c, c') Single pollen stage, in c'; the remaining pollen collapsed and clumped together, the residual tapetum cells were dispersed. Bar 0.1 mm; M microspores, T tapetum, Te tetrads
Seed production experiment
The bolting behavior of both BC5 CMS lines was similar to the E. sativa parent. All plants bolted without vernalization on average 30 days after replantation. However, the flowering started approximately one week later in both CMS lines than in the E. sativa parental line. A continuous visiting of honey bees on flowers was observed for the both CMS lines and the original E. sativa pollinator plants. Dry mature siliques were harvested by hand continuously over two months. Whereas for the hermaphrodite E. sativa line (pollinator) on average 35 g seeds per plant were harvested, two CMS lines reached approximately 14 g/plant or 40 % compared with the pollinator. The seed set per silique was the same as the fertile Eruca parent while the approximately seed weight was 75 % higher. The seed quality and germination capacity were approximately the same for the three harvested seed lots (Table 2).
Estimation of growth parameters of BC4 and BC5 lines
Two BC4 and four BC5 lines were compared for agronomical traits to the parental Eruca line in a parallel trial in field and plastic tunnel. Under plastic tunnel conditions all BC4 and BC5 lines developed significantly more leaves than the E. sativa parent. The differences were estimated to 1.3–3.4 leaves per plant. Similar effects were observed under field conditions. The BC4 and BC5 lines expressed approximately 1.9–2.0 leaves more than the Eruca control. The mean diameter of the leaf rosette of the BC5 lines, tested twice at DPS 40 and 58, showed for both experiments no differences in comparison to the Eruca parent, but a significant increase in comparison to the BC4 lines (Table 3).
Discussion
Male sterile E. sativa lines have been established by transferring the nucleus of the rocket salad into the cybrid cytoplasm of a CMS-cauliflower via intergeneric sexual hybridization followed by polyploidization enabling recurrent backcrosses. The subsequent elimination of the B. oleracea genome has been documented by RAPD-PCR and GISH analyses. The currently obtained alloplasmic E. sativa lines (BC5) are diploid with 2n = 2x = 22 E. sativa chromosomes, and contain the mtDNA and the cpDNA from CMS-B. oleracea (BAZOG 4).
The CMS-E. sativa lines have an excellent vigor. The growth habit as well as the yield potential corresponded to the Eruca parent, as shown by cultivation experiments in field and plastic tunnel. We did not observe chlorophyll deficiencies, leaf abnormalities or dwarfism due to incompatibilities in interspecific and somatic hybridizations as it was previously described for the Brassicaceae family (Bannerot et al. 1977; De Melo and De Giordano 1994a, b; Navrátilová et al. 1997; Prakash et al. 1995; Tu et al. 2008).
Further developmental disturbances during flowering such as curled petals, crooked style, underdeveloped nectaries, feminization of stamen, contorted pods, decreased seed set or seed abortion reported in relation to interspecific or intergeneric hybridization (Gourret et al. 1992; Kirti et al. 1995; Liu Clarke et al.1999; Sikdar et al. 1990) have not been observed.
With the exception of the stamen formation, the whole reproductive phase of the CMS-E. sativa lines corresponded to those of the Eruca parent. We did not observe any influence by the tri-genomic (cybrid) constellation, consisting by the nuclear DNA from E. sativa, the mt genome from R. sativus and the cp genome from B. oleracea. On the other hand, the development of the male flower organs was strongly impaired leading to abortive pollen development. Already Ogura (1968) described a premature breakdown of the tapetal cell layer and a complete abortion of pollen development in anthers with normal morphology of male sterile radish plants.
In addition, in other CMS systems of the Brassicaceae family an abnormal tapetum development was mentioned. The timeliness of tapetum breakdown was substantial for pollen viability, since both, a premature degeneracy or its delay can contribute to pollen abortion (Gonzáles-Melendi et al. 2008; Kawanabe et al. 2006; Li et al. 2006; Vizcay-Barrena and Wilson 2006).
The CMS-cauliflower parent expressed a premature breakdown of the tapetal cell layer, progressive vacuolization and a complete abortion of pollen development in anthers of normal morphology, similar as described for the Ogura-CMS in R. sativus (Ogura 1968). In most of the CMS-flower phenotypes described here, the principal structure of anthers was not affected and developmental defects were not obvious before postmeitoic stages.
In contrast, the broader usage of the unmodified Ogura cytoplasm in alloplasmic Brassicaceae led, beside chlorophyll deficiency and low nectar production, to various defects of the male organs beginning from abortion or disorganization of the microsporangia and anthers (Polowick and Sawhney 1991). In addition, the development of petaloid or carpelloid structures and also defects during the tapetal and microspore development have been reported (Bannerot et al. 1974; Gourret et al. 1992; Heyn 1976). Yang et al. (2008) described petaloid anthers within Ogu-plasmic B. juncea and Dong et al. (2013) showed that stamen had shorter filaments and undeveloped anthers, silique growth retarded within Ogu-plasmic B. rapa. Hence, the range of flower malformations can vary by a different extent. Beyond postmeiotic pollen abortion, also homeotic conversions of male organs were observed in alloplasmic CMS-lines of Brassicaceae (reviewed by Yamagishi and Bhat 2014).
The CMS-E. sativa plants revealed two distinct developmental phases where male reproduction was disturbed. A first defect was addressed to the sporophytic part of the plant during early stages of anther formation (defect (I)—early stage). In these cases, the ‘envelope’ enclosing the germline, especially the locule-structure was affected. A second defect was manifested during the early generative stage when meiosis has been already initiated. In this case, the development of the tapetum was disturbed and in parallel, also the development of the germline was impaired (defect (II), assigned to the ‘mid stage’ of development). Both defects were largely specific and can be precisely assigned in a distinct temporal and spatial manner by morphological characterization. Hence, flower morphology of the alloplasmic CMS-E. sativa lines revealed more severe distortions than the original CMS-B. oleracea line.
Regarding the fact, that chlorotic effects have not been observed in the CMS-E. sativa lines it can be concluded, that the final CMS-E. sativa line of this study contains solely the plastids of B. oleracea. Concerning the tri-genomic state of organelles in the alloplasmic Eruca lines, it can be concluded, that anther defects were due to an impaired interaction between the nuclear and the mitochondrial genomes. Regarding the wide range of male flower defects observed in the different nuclear backgrounds of alloplasmic Brassicaceae, it can be expected that different nuclear-mitochondrial interactions contribute to the broad range of male defects. Previous data had shown a substantial input of mitochondrial genomes on male flower development by analyses of cybrids obtained from Nicotiana or Brassica only differing in their mitochondrial genome (Belliard et al. 1979; Kofer al. 1991).
Hence, the developed intergeneric Eruca hybrids represent a novel example for mitochondrial influences on certain periods of male flower development in alloplasmic and cybrid Brassica using the Ogura cytoplasm. Although CMS has been attributed to specific mitochondrial genes, diverse floral phenotypes induced by the same cytoplasm in different species have not been fully investigated (Yamagishi and Bhat 2014). Polymorphic phenotypes are assumed to result from differences in the energy status of cells due to expression of CMS-inducing mitochondrial genes in different nuclear backgrounds (Yamagishi and Bhat 2014). Nevertheless, how mitochondrial genes like orf138 finally contribute to the diversity of flower defects remained yet unsolved.
Hence, the utilized cybrid cytoplasm was proven to be a vital source of CMS for E. sativa. In accordance with predominant reports of Ogura cytoplasm-based CMS-B. oleracea (De Melo and De Giordano 1994a, b), B. napus (Delourme et al. 1998), B. rapa (Dong et al. 2013) or B. juncea (Kirti et al. 1995), we did not observe a restoration of the male fertility.
The excellent seed quantity and quality of both BC5 lines in the seed production experiment suggest that neither the premeiotic (sporophytic) nor the postmeiotic (pollen abortive) disturbances influenced the development of the gynoecium and the final seed set. The lower seed yield (~40 %) of the BC5 lines compared with the pollinator line harvested in our experimental approach may have different reasons. While we can exclude a spontaneous flower abortion, we assume that a number of factors were responsible as the ratio between the male sterile and the male fertile pollinator plants, synchronization of flowering and in consequence a time-delayed mature of siliques of the CMS-lines. Our current research is aimed at optimizing the pollination efficiency by bees and conditions of seed production for possible application in hybrid breeding.
The plant material offers a promising tool to promote further studies of cytoplasmic-nuclear interaction and potential cause of CMS manifestation in Brassicaceae. The complete male sterility and the restored female fertility of the alloplasmic E. sativus lines as well as the high yield potential and viability open the possibility for a direct application in hybrid breeding approaches.
Author contribution statement
Nothnagel T: Plant development, intraspecific hybridization, histological analysis. Klocke E: Southern analysis. Schrader O: Flow cytometry, GISH, FISH. Bettina Linke: Histology and flower biology. Budahn H: RAPD- and orf138- analysis.
References
Agnihotri A, Gupta V, Lakshmikumaran MS, Shivanna KR, Prakash S, Jagannathan V (1990) Production of Eruca-Brassica hybrids by embryo rescue. Plant Breed 104:281–289. doi:10.1111/j.1439-0523.1990.tb00437.x
Ashraf M (1994) Organic substances responsible for salt tolerance in Eruca sativa. Biol Plantarum 36:255–259. doi:10.1007/BF02921095
Bannerot H, Boulidard L, Cauderon Y, Tempe J (1974) Transfer of cytoplasmic male sterility from Raphanus sativus to Brassica oleracea. In: Eucarpia Meeting Cruciferae, Dundee, Scotland, pp 52–54
Bannerot H, Boulidard L, Chupeau Y (1977) Unexpected difficulties met with the radish cytoplasm. Eucarpia Crucif Newsl 2:16
Belliard G, Vedel F, Pelletier G (1979) Mitochondrial recombination in cytoplasmic hybrids of Nicotiana tabacum by protoplast fusion. Nature 218:401–402. doi:10.1038/281401a0
Bhargava SN, Shukla DN, Singh N (1980) Root- and foot-rot of Eruca sativa caused by Alternaria alternata (Fr) Keissler. Curr Sci India 49:452
Bock R, Hagemann R, Kössel H, Kudla J (1993) Tissue- and stage-specific modulation of RNA editing of the psbF and psbL transcript from spinach plastids—a new regulatory mechanism? Mol Gen Genet 240:238–244. doi:10.1007/BF00277062
Bonhomme S, Budar F, Lancelin D, Small I, Defrance MC, Pelletier G (1992) Sequence and transcript analysis of the Nco2.5 Ogura-specific fragment correlated with cytoplasmic male sterility in Brassica hybrids. Mol Gen Genet 235:340–348. doi:10.1007/BF00279379
Bozokalfa MK, Esiyok D, Ilbi H, Kavak S, Asçiogul TK (2011) Evaluation of phenotypic diversity and geographical variation of cultivated (Eruca sativa L.) and wild (Diplotaxis tenuifolia L.) rocket plant. Plant Genetic Resour 9:454–463. doi:10.1017/S1479262111000657
Branca F (2008) Cauliflower and broccoli. In: Prohens J, Nuez F (eds) Handbook of plant breeding—Vegetables, vol 1. Springer, Berlin, pp 151–186
Chamola R, Balyan HS, Bhat SR (2013) Effect of alien cytoplasm and fertility restorer genes on agronomic and physiological traits of Brassica juncea (L.) Czern. Plant Breed 132:681–687. doi:10.1111/pbr.12080
De Melo P, De Giordano L (1994a) Effect of Ogura male-sterile cytoplasm on the performance of cabbage hybrid variety. I. Vegetative characteristics. Euphytica 76:117–123. doi:10.1007/BF00024028
De Melo P, De Giordano L (1994b) Effect of Ogura male-sterile cytoplasm on the performance of cabbage hybrid variety. II. Commercial characteristics. Euphytica 78:149–154. doi:10.007/BF00021411
De Vos AC, Broekman R, de Almeida Guerra CC, van Rijsselberghe M, Rozema J (2013) Developing and testing new halophyte crops: a case study of salt tolerance of two species of the Brassicaceae, Diplotaxis tenuifolia and Cochlearia officinalis. Environ Exp Bot 92:154–164. doi:10.1016/j.envexpbot.2012.08.003
Delourme R, Foisset N, Horvais R, Barret P, Champagne G, Cheung WY, Landry BS, Renard M (1998) Characterisation of the radish introgression carrying the Rfo restorer gene for the Ogu-INRA cytoplasmic male sterility in rapeseed (Brassica napus L.). Theor Appl Genet 97:129–134. doi:10.1007/s001220050876
Dieterich JH, Braun HP, Schmitz UK (2003) Alloplasmic male sterility in Brassica napus (CMS ‘Tournefortii-Stiewe’) is associated with a special gene arrangement around a novel atp9 gene. Mol Gen Genom 269:723–731. doi:10.1007/s00438-003-0886-3
Dong X, Kim WK, Lim YP, Kim YK, Hur Y (2013) Ogura-CMS in Chinese cabbage (Brassica rapa ssp. pekinensis) causes delayed expression of many nuclear genes. Plant Sci 199–200:7–17. doi:10.1016/j.plantsci.2012.11.001
Egea-Gilabert C, Fernández JA, Migliaro D, Martínez-Sánchez JJ, Vicente MJ (2009) Genetic variability in wild vs. cultivated Eruca vesicaria populations as assessed by morphological, agronomical and molecular analyses. Sci Hortic 121:260–266. doi:10.1016/j.scienta.2009.02.020
Fahleson J, Råhlén L, Glimelius K (1988) Analysis of plants regenerated from protoplast fusions between Brassica napus and Eruca sativa. Theor Appl Genet 76:507–512. doi:10.1007/BF00260900
Geddy R, Mahé L, Brown GG (2005) Cell-specific regulation of a Brassica napus CMS-associated gene by a nuclear restorer with related effects on a floral homeotic gene promoter. Plant J 41:333–345. doi:10.1111/j.1365-323.2004.02305.x
González-Melendi P, Uyttewaal M, Morcillo CN, Mora JRH, Fajardo S, Budar F, Lucas MM (2008) A light and electron microscopy analysis of the events leading to male sterility in Ogu-INRA CMS of rapeseed (Brassica napus). J Exp Bot 59:827–838. doi:10.1093/jxb/erm365
Gottlob-McHugh SG, Levesque M, MacKenzie K, Olson M, Yarosh O, Johnson DA (1990) Organization of the 5S rRNA genes in the soybean Glycine max (L.) Merrill and conservation of the 5S rDNA repeat structure in higher plants. Genome 33:94–100. doi:10.1139/g30-072
Gourret JP, Delourme R, Renard M (1992) Expression of ogu cytoplasmic male sterility in cybrids of Brassica napus. Theor Appl Genet 83:549–556. doi:10.1007/BF00226898
Heyn FW (1976) Transfer of restorer genes from Raphanus to cytoplasmic male-sterile Brassica napus. Crucif Newsl 1:15–16
Kaneko Y, Bang SW, Matsuzawa Y (2011) Raphanus. In: Kole C (ed) Wild crop relatives: genomic and breeding resources, vegetables. Springer, Berlin, pp 247–258
Kawanabe T, Ariizumi T, Kawai-Yamada M, Uchimiya H, Toriyama K (2006) Abolition of the tapetum suicide program ruins microsporogenesis. Plant Cell Physiol 47:784–787. doi:10.1093/pcp/pcj039
Kirti PB, Banga SS, Prakash S, Chopra VL (1995) Transfer of Ogu cytoplasmic male sterility to Brassica juncea and improvement of the male sterile line through somatic cell fusion. Theor Appl Genet 91:517–521. doi:10.1007/BF00222982
Kofer W, Glimelius K, Bonnett HT (1991) Modifications of mitochondrial DNA cause changes in floral development in homeotic-like mutants of tobacco. Plant Cell 3:759–769. doi:10.2307/3869270
Larran S, Ronco L, Mónaco C, Andreau RH (2006) First report of Peronospora parasitica on rocket (Eruca sativa) in Argentina. Australas Plant Path 35:377–378. doi:10.1071/AP06024
Li N, Zhang DS, Liu HS, Yin CS, Li XX, Liang WQ, Yuan Z, Xu B, Chu HW, Wang J, Wen TQ, Huang H, Luo D, Ma H, Zhang DB (2006) The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell 18:2999–3014. doi:10.1105/tpc.106.044107
Linke B, Nothnagel T, Börner T (1999) Morphological characterization of modified flower morphology of three novel male sterile carrot sources. Plant Breed 118:543–548. doi:10.1046/1439-0523.1999.004202.x
Liu Clarke JH, Chèvre AM, Landgren M, Glimelius K (1999) Characterization of sexual progenies of male-sterile somatic cybrids between Brassica napus and Brassica tournefortii. Theor Appl Genet 99:605–610. doi:10.1007/s001220051275
Makaroff CA, Apel IJ, Palmer JD (1990) Characterization of radish mitochondrial atpA; influence of nuclear background on transcription of atpA-associated sequences and relationship with male sterility. Plant Mol Biol 15:735–746. doi:10.1007/BF00016123
Matsuzawa Y, Mekiyanon S, Kaneko Y, Bang SW, Wakui K, Takahata Y (1999) Male sterility in alloplasmic Brassica rapa L. carrying Eruca sativa cytoplasm. Plant Breed 118:82–84. doi:10.1046/j.1439-0539-0523.1999.118001082.x
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Navrátilová B, Bůžek J, Široký J, Havránek P (1997) Construction of intergeneric somatic hybrids between Brassica oleracea and Armoracia rusticana. Biol Plant 39:531–541. doi:10.1023/A:1000970529643
Nishiyama I, Sarashima M, Matsuzawa Y (1991) Critical discussion on abortive interspecific crosses in Brassica. Plant Breed 107:288–302. doi:10.1111/j.1439-0523.1991.tb00552.x
Nothnagel T, Budahn H (1994) Ein CMS—assoziiertes OGURA—mtDNA Fragment in zwei selektierten Linien von Brassica oleracea var. botrytis. Vortr Pflanzenzücht 28:134–136 (in German)
Ogura H (1968) Studies on the new male sterility in Japanese radish, with special references to the utilization of this sterility towards the practical raising of hybrid seeds. Mem Fac Agr Kogoshima Univ 6:39–78
Ordas A, Cartea ME (2008) Cabbage and Kale. In: Prohens J, Nuez F (eds) Handbook of plant breeding—vegetables, vol 1. Springer, Berlin, pp 119–149
Paz Lima MLP, Café-Filho AC, Nogueira NL, Rossi ML, Schuta LR (2004) First report of clubroot of Eruca sativa caused by Plasmodiophora brassicae in Brazil. Plant Dis 88:573. doi:10.1094/PDIS.2004.88.5.573B
Pelletier G, Primard C, Vedel F, Chetrit P, Remy R, Rousselle Renard M (1983) Intergeneric cytoplasmic hybridization in Cruciferae by protoplast fusion. Mol Gen Genet 191:244–250. doi:10.1007/BF00334821
Polowick PL, Sawhney VK (1991) Microsporogenesis in a normal line and in the ogu cytoplasmic male-sterile line of Brassica napus. Sex Plant Reprod 4:22–27. doi:10.1007/BF00202884
Porebski S, Bailey LG, Baum BR (1997) Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol Biol Rep 15:8–15. doi:10.1007/BF02772108
Prakash S, Kirti PB, Chopra VL (1995) Cytoplasmic male sterility (CMS) systems other than Ogu and Polima in Brassica—Current status. In: Proceedings of 9th International Rapeseed Congress. Cambridge, UK, pp 44–48
Renard M, Delourme R, Mesquida J, Pelletier G, Primard C, Boulidard L, Dore C, Ruffio V, Hervé Y, Morice J (1992) Male sterilities and F1 hybrids in Brassica. In: Dattée Y, Dumas C, Gallais A (eds) Reproductive biology and plant breeding. Springer, Heidelberg, pp 107–109
Schrader O, Budahn H, Ahne R (2000) Detection of 5S and 25S rRNA genes in Sinapis alba, Raphanus sativus and Brassica napus by double fluorescence in situ hybridization. Theor Appl Genet 100:665–669. doi:10.1007/s001220051337
Sharma AK (1985) An unrecorded leaf-blight disease of taramira (Eruca sativa Mill.) from India caused by Alternaria brassicicola (Schew.) wiltshire. Curr Sci 54:942–943 (Acc. Number: WOS:A1985ARH7200017 ISSN: 0011-3891)
Shinwari S, Akbar F, Rabbani MA, Mumtaz AS, Shinwari ZK (2013) Evaluation of genetic diversity in different genotypes of Eruca sativa from Pakistan by SDS-PAGE analysis. Pak J Bot 45:1235–1240
Sikdar SR, Chatterjee G, Das S, Sen SK (1990) “Erussica” the intergeneric fertile somatic hybrid developed through protoplast fusion between Eruca sativa LAM. and Brassica juncea (L.) Czern. Theor Appl Genet 79:561–567. doi:10.1007/BF00226168
Singh R, Verma B, Prakash C, Prasad SN (1992) Effect of irrigation on yield and water-use of Indian mustard (Brassica juncea), rocket salad (Eruca sativa), safflower (Carthamus tinctorius) and pigeonpea (Cajanus cajan). Indian J Agr Sci 62:254–257
Srinivasan K, Spadaro D, Poli A, Gilardi G, Gullino ML, Garibaldi A (2012) Genetic diversity and pathogenicity of Fusarium oxysporum isolated from wilted rocket plants in Italy. Phytoparasitica 40:157–170. doi:10.1007/s12600-011-0207-z
Tu YQ, Sun J, Liu Y, Ge XH, Zhao ZG, Yao XC, Li ZY (2008) Production and characterization of intertribal somatic hybrids of Raphanus sativus and Brassica rapa with dye and medicinal plant Isatis indigotica. Plant Cell Rep 27:873–883. doi:10.1007/s00299-008-0513-1
Verma SC, Lewis D (1977) Three gene system of sporophytic incompatibility in Eruca sativa (Cruciferae). Heredity 39:188
Verma JK, Sodhi YS, Mukhopadhyay A, Arumugam N, Gupta V, Pental D, Pradhan AK (2000) Identification of stable maintainer and fertility restorer lines for ‘Polima’ CMS in Brassica campestris. Plant Breed 119:90–92. doi:10.1046/j.1439-0523.2000.00430.x
Vizcay-Barrena G, Wilson ZA (2006) Altered tapetal PCD and pollen wall development in the Arabidopsis ms1 mutant. J Exp Bot 57:2709–2717. doi:10.1093/jxb/erl032
Wan ZJ, Jing B, Tu JX, Ma CZ, Shen JX, Yi B, Wen J, Huang T, Wang XJ, Fu TD (2008) Genetic characterization of a new cytoplasmic male sterility system (hau) in Brassica juncea and its transfer to B. napus. Theor Appl Genet 116:355–362. doi:10.1007/s00122-007-0673-3
Williams JGK, Kubelik AP, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucl Acids Res 18:6531–6535. doi:10.1093/nar/18.22.6531
Yamagishi H, Bhat SR (2014) Cytoplasmic male sterility in Brassicaceae crops. Breed Sci 64:38–47. doi:10.1270/jsbbs.64.38
Yamagishi H, Terachi T (1996) Molecular and biological studies on the male-sterile cytoplasm in Cruciferae. III. Distribution of Ogura-type cytoplasm among Japanese wild radishes and Asian radish cultivars. Theor Appl Genet 93:325–332. doi:10.1007/BF00223172
Yang JH, Qi XH, Zang MF, Yu JQ (2008) MADS-box genes are associated with cytoplasmic homeosis in cytoplasmic male-sterile stem mustard as partially mimicked by specifically inhibiting mtETC. Plant Growth Regul 56:191–201. doi:10.1007/s10725-008-9300-9
Yaniv Z, Schafferman D, Amar Z (1998) Tradition, uses and biodiversity of rocket (Eruca sativa, Brassicaceae) in Israel. Econ Bot 52:394–400. doi:10.1007/BF02862069
Acknowledgments
The authors thank Rosemarie Dippe, Ilona Kison, Susanne Kozber, Karla Müller, Martina Fuss, Kerstin Maier and Simone Abel for the excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Furthermore we declare that the experiments comply with the current laws of Germany.
Additional information
Communicated by I. Rajcan.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nothnagel, T., Klocke, E., Schrader, O. et al. Development of male sterile Eruca sativa carrying a Raphanus sativus/Brassica oleracea cybrid cytoplasm. Theor Appl Genet 129, 331–344 (2016). https://doi.org/10.1007/s00122-015-2630-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-015-2630-x