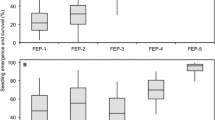
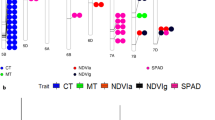
Abstract
Key message
Promising genome regions for improving cold tolerance of sorghum were identified on chromosomes SBI-01, SBI-03, SBI-07, and SBI-10. Chlorophyll fluorescence had no major effect on growth rates at low temperatures.
Abstract
Developing fast growing sorghum seedlings is an important breeding goal for temperate climates since low springtime temperatures are resulting in a prolonged juvenile development. The adaptation of sorghum to tropical and subtropical highlands gives hint for certain genetic variation. The goals of the present study were to detect marker-trait associations for leaf and dry matter growth rate and for chlorophyll fluorescence and content (SPAD) in relation to temperature. A diversity set comprising 194 genotypes was tested in eight controlled environments with temperatures ranging from 9.4 to 20.8 °C. Significant marker-trait associations (p < 0.05) were identified for each individual temperature regime and on the parameters of regression analyses describing the responses of growth or chlorophyll related traits to temperatures. The diversity set was fingerprinted with 171 diversity array technology (DArT) and 31 simple-sequence repeat (SSR) markers. SSRs were used to analyze the population structure while association studies were performed on DArT markers. Promising marker-trait associations for growth rates in relation to temperature were detected on chromosomes SBI-01, SBI-03, SBI-07, and SBI-10. Many promising loci were also significantly associated to the results obtained in individual low-temperature environments. Marker-trait associations for chlorophyll content and fluorescence did occasionally co-locate to those for growth during juvenile development but there was no evidence supporting our hypothesis that seedling growth at low temperatures is largely influenced by SPAD or fluorescence.





Similar content being viewed by others
References
Bekele WA, Fiedler K, Shiringani A, Schnaubelt D, Windpassinger S, Uptmoor R, Friedt W, Snowdon RJ (2014) Unravelling the genetic complexity of sorghum seedling development under low temperature conditions. Plant Cell Environ 37:707–723
Bouchet S, Pot D, Deu M, Rami JF, Billot C, Perrier X, Rivallan R, Gardes L, Xia L, Wenzl P, Kilian A, Glaszmann JC (2012) Genetic structure, linkage disequilibrium, and signature of selection in sorghum: lessons from physically anchored DArT markers. PLoS One 7:e33470
Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23:2633–2635
Burow G, Burke J, Xin ZG, Franks C (2011) Genetic dissection of early-season cold tolerance in sorghum (Sorghum bicolor (L.) Moench). Mol Breeding 28:391–402
Collins NC, Tardieu F, Tuberosa R (2008) Quantitative trait loci and crop performance under abiotic stress: where do we stand? Plant Physiol 147:469–486
El-Lithy ME, Clerkx EJM, Ruys GJ, Koornneef M, Vreugdenhil D (2004) Quantitative trait locus analysis of growth-related traits in a new Arabidopsis recombinant. Plant Physiol 135:444–458
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Fiedler K, Bekele W, Friedt W, Snowdon R, Stützel H, Zacharias UR (2012) Genetic dissection of the temperature dependent emergence processes in sorghum using a cumulative emergence model and stability parameters. Theor Appl Genet 125:1647–1661
Foyer CH, Vanacker H, Gomez LD, Harbinson J (2002) Regulation of photosynthesis and antioxidant metabolism in maize leaves at optimal and chilling temperatures: review. Plant Physiol Biochem 40:659–668
Fracheboud Y, Haldimann P, Leipner J, Stamp P (1999) Chlorophyll fluorescence as a selection tool for cold tolerance of photosynthesis in maize (Zea mays L.). J Exp Bot 50:1533–1540
Fracheboud Y, Jompuk C, Ribaut JM, Stamp P, Leipner J (2004) Genetic analysis of cold-tolerance of photosynthesis in maize. Plant Mol Biol 56:241–253
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Hill J, Becker HC, Tigerstedt P (1998) Quantitative and ecological aspects of plant breeding. Chapman Hall, London
Hund A, Fracheboud Y, Soldati A, Stamp P (2008) Cold tolerance of maize seedlings as determined by root morphology and photosynthetic traits. Eur J Agron 28:178–185
Kebede H, Subudhi PK, Rosenow DT, Nguyen HT (2001) Quantitative trait loci influencing drought tolerance in grain sorghum (Sorghum bicolor L. Moench). Theor Appl Genet 103:266–276
Knoll J, Gunaratna N, Ejeta G (2008) QTL analysis of early-season cold tolerance in sorghum. Theor Appl Genet 116:577–587
Kocova M, Hola D, Wilhelmova N, Rothova O (2009) The influence of low-temperature on the photochemical activity of chloroplasts and activity of antioxidant enzymes in maize leaves. Biol Plant 53:475–483
Krause GH (1988) Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiol Plant 74:566–574
Kumar SR, Hammer GL, Broad I, Harland P, McLean G (2009) Modelling environmental effects on phenology and canopy development of diverse sorghum genotypes. Field Crops Res 111:157–165
Lafarge T, de Raissac M, Tardieu F (1998) Elongation rate of sorghum leaves has a common response to meristem temperature in diverse African and European environmental conditions. Field Crops Res 58:69–79
Ma CX, Casella G, Wu R (2002) Functional mapping of quantitative trait loci underlying the character process: a theoretical framework. Genetics 161:1751–1762
Maccaferri M, Sanguineti MC, Demontis A, El-Ahmed A, del Moral LG, Maalouf F, Nachit M, Nserallah N, Ouabbou H, Rhouma S, Royo C, Villegas D, Tuberosa R (2011) Association mapping in durum wheat grown across a broad range of water regimes. J Exp Bot 62:409–438
Mace ES, Jordan DR (2010) Integrating sorghum whole genome sequence information with a compendium of sorghum QTL studies reveals uneven distribution of QTL and of gene-rich regions with significant implications for crop improvement. Theor Appl Genet 123:169–191
Mace ES, Xia L, Jordan DR, Halloran K, Parh DK, Huttner E, Wenzl P, Kilian A (2008) DArT markers: diversity analyses and mapping in Sorghum bicolor. BMC Genom 9:26
Malosetti M, Visser RGF, Celis-Gamboa C, van Eeuwijk FA (2006) QTL methodology for response curves on the basis of non-linear mixed models, with an illustration to senescence in potato. Theor Appl Genet 113:288–300
Padilla JM, Otegui ME (2005) Co-ordination between leaf initiation and leaf appearance in field-grown maize (Zea mays): genotypic differences in response of rates to temperature. Ann Bot 96:997–1007
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Rami JF, Dufour P, Trouche G, Fliedel G, Mestres C, Davrieux F, Blanchard P, Hamon P (1998) Quantitative trait loci for grain quality, productivity, morphological and agronomical traits in sorghum (Sorghum bicolor L. Moench). Theor Appl Genet 97:605–616
Reymond M, Muller B, Leonardi A, Charcosset A, Tardieu F (2003) Combining quantitative trait loci analysis and an ecophysiological model to analyze the genetic variability of the responses of maize leaf growth to temperature and water deficit. Plant Physiol 131:664–675
Richards RA (2000) Selectable traits to increase crop photosynthesis and yield of grain crops. J Exp Bot 51:447–458
Ritter KB, Jordan DR, Chapman SC, Godwin ID, Mace ES, McIntyre CL (2008) Identification of QTL for sugar-related traits in a sweet × grain sorghum (Sorghum bicolor L. Moench) recombinant inbred population. Mol Breeding 22:367–384
Sadok W, Naudin P, Boussuge B, Muller B, Welcker C, Tardieu F (2007) Leaf growth rate per unit thermal time follows QTL-dependent daily patterns in hundreds of maize lines under naturally fluctuating conditions. Plant Cell Environ 30:135–146
Savitch LV, Ivanov AG, Gudynaite-Savitch L, Huner NPA, Simmonds J (2011) Cold stress effects on PSI photochemistry in Zea mays: differential increase of FQR-dependent cyclic electron flow and functional implications. Plant Cell Physiol 52:1042–1054
Shiringani AL, Frisch M, Friedt W (2010) Genetic mapping of QTLs for sugar-related traits in a RIL population of Sorghum bicolor L. Moench. Theor Appl Genet 121:323–336
Soldati A, Stehli A, Stamp P (1999) Temperature adaptation of tropical highland maize (Zea mays L.) during early growth and in controlled conditions. Eur J Agron 10:111–117
Steponkus PL (1984) Role of the plasma-membrane in freezing-injury and cold-acclimation. Annu Rev Plant Physiol Plant Mol Biol 35:543–584
Stitt M, Hurry V (2002) A plant for all seasons: alterations in photosynthetic carbon metabolism during cold acclimation in Arabidopsis. Curr Opin Plant Biol 5:199–206
Subudhi PK, Rosenow DT, Nguyen HT (2000) Quantitative trait loci for the stay green trait in sorghum (Sorghum bicolor L. Moench): consistency across genetic backgrounds and environments. Theor Appl Genet 101:733–741
Tao YZ, Henzell RG, Jordan DR, Butler DG, Kelly AM, McIntyre CL (2000) Identification of genomic regions associated with stay green in sorghum by testing RILs in multiple environments. Theor Appl Genet 100:1225–1232
Thomas H, Howarth CJ (2000) Five ways to stay green. J Exp Bot 51:329–337
Thornley JHM, Johnson IR (1990) Plant and crop modeling: a mathematical approach to plant and crop physiology. Oxford Science, USA
Trachsel S, Messmer R, Stamp P, Ruta N, Hund A (2010) QTLs for early vigor of tropical maize. Mol Breeding 25:91–103
Uptmoor R, Osei-Kwarteng M, Gürtler S, Stützel H (2009) Modelling the effects of drought stress on leaf development in a Brassica oleracea doubled haploid population using two-phase linear functions. J Am Soc Hortic Sci 134:543–552
van Eeuwijk FA, Bink M, Chenu K, Chapman SC (2010) Detection and use of QTL for complex traits in multiple environments. Curr Opin Plant Biol 13:193–205
Via S, Gomulkiewicz R, De Jong G, Scheiner SM, Schlichting CD, Van Tienderen PH (1995) Adaptive phenotypic plasticity: consensus and controversy. Trends Ecol Evol 10:212–217
Werck-Reichert D, Hehn A, Didierjean L (2000) Cytochromes P450 for engineering herbicide tolerance. Trends Plant Sci 5:116–123
Yin XY, Struik PC, van Eeuwijk FA, Stam P, Tang JJ (2005) QTL analysis and QTL-based prediction of flowering phenology in recombinant inbred lines of barley. J Exp Bot 56:967–976
Yu JM, Tuinstra MR (2001) Genetic analysis of seedling growth under cold temperature stress in grain sorghum. Crop Sci 41:1438–1443
Zhang ZW, Ersoz E, Lai CQ, Todhunter RJ, Tiwari HK, Gore MA, Bradbury PJ, Yu JM, Arnett DK, Ordovas JM, Buckler ES (2010) Mixed linear model approach adapted for genome-wide association studies. Nat Genet 42:355–360
Acknowledgments
We thank Katharina Meyer for excellent technical assistance and gratefully acknowledge the German Federal Ministry of Education and Research (BMBF) for funding the project (BioEnergie 2021, Project No. 03154211).
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical standards
The experiments comply with the current German laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hai-Chun Jing.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fiedler, K., Bekele, W.A., Duensing, R. et al. Genetic dissection of temperature-dependent sorghum growth during juvenile development. Theor Appl Genet 127, 1935–1948 (2014). https://doi.org/10.1007/s00122-014-2350-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-014-2350-7