Abstract
The main target of extracorporeal support is to achieve viable gas exchange, while minimizing the risk of ventilator-induced lung injury, achieved through a decreased mechanical ventilation load on the natural lung. However, during veno-venous extracorporeal membrane oxygenation (ECMO), mechanical ventilation is still necessary in order to prevent lung collapse and/or if extracorporeal blood flow is not sufficient to guarantee adequate gas exchange. In this review, we will summarize the physiology of extracorporeal support and the rationale for continuing mechanical ventilation in this context. Furthermore, we will review the current clinical practice among ECMO centers and their suggestions regarding mechanical ventilator settings. While optimal ventilatory settings are still a matter of debate, the use of a strategy combining low tidal volume and limited inspiratory pressures is accepted worldwide. On the contrary, the choice of applied positive end-expiratory pressure (PEEP) varies between the total rest strategy and open lung strategy. Finally, the use of assisted or spontaneous ventilation will be discussed.
Zusammenfassung
Hauptziel der extrakorporalen Lungenunterstützung (ECLS) ist es, einen ausreichenden Gasaustausch sicherzustellen und gleichzeitig das Risiko für beatmungsinduzierte Schädigungen an der Lunge zu minimieren. Dies wird durch eine Verringerung der mechanischen Belastung für die Lunge des Patienten erreicht. Während der venovenösen extrakorporalen Membranoxygenierung (ECMO) erfolgt zusätzlich eine mechanische Beatmung, um einerseits einen pulmonalen Kollaps zu vermeiden und/oder andererseits, wenn der extrakorporale Blutfluss keinen adäquaten Gasaustausch ermöglicht, diesen mit sicherzustellen. In dieser Übersichtsarbeit werden zusammenfassend die Physiologie der extrakorporalen Unterstützung und die Rationale für die Fortführung der mechanischen Beatmung unter diesen Bedingungen dargestellt. Darüber hinaus werden die gegenwärtig von ECMO-Zentren praktizierten klinischen Regime ebenso beschrieben wie die unterschiedlichen Empfehlungen für die Einstellungen an den Beatmungsgeräten. Die optimalen Einstellungen sind nach wie vor Gegenstand kontroverser Diskussionen, doch die Strategie, ein geringes Tidalvolumen und begrenzte inspiratorische Drücke miteinander zu kombinieren, findet weltweit Akzeptanz. Die Wahl des eingesetzten positiven endexpiratorischen Drucks (PEEP) dagegen variiert zwischen der „total rest strategy“ und der „open lung strategy“. Schließlich wird der Einsatz assistierter bzw. spontaner Beatmungsformen diskutiert.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Veno-venous extracorporeal membrane oxygenation (ECMO) is a technique providing temporary respiratory support in patients with respiratory failure refractory to conventional treatment.
During veno-venous ECMO, blood is drained from a patient’s vein and pumped through an artificial, membrane lung, where it is oxygenated and carbon dioxide (CO2) is removed. Thereafter, the blood is delivered back into the patient’s venous system.
Historical background
Following the enthusiasm of the first successful ECMO applications, five decades ago the National Institutes of Health (NIH, Bethesda MD, USA) sponsored the first randomized trial comparing ECMO to conventional therapy in acute respiratory distress syndrome (ARDS) patients [30]. The trial was interrupted for futility after the enrollment of the first 90 patients as mortality reached 90% in both arms. It is worth underlining that the chosen settings of mechanical ventilation likely contributed to the study failure. At that time, the main concern of mechanical ventilation was the high fraction of inspired oxygen, while the detrimental effects of positive pressure ventilation had not been fully appreciated. Indeed, the inspiratory oxygen fraction (FiO2) was reduced in the ECMO group, while all the other ventilatory parameters were mostly unchanged. Of note, tidal volumes were as high as 10–15 ml/kg, leading to airway plateau pressures in the range between 40 and 50 cmH2O [22]. The discouraging results of the National Institute of Health (NIH) study led to the abandonment of the ECMO technique worldwide.
Some years later, Kolobow et al. pointed out that “severely diseased lungs have a chance to heal only if the environment remains conducive to their healing. This environment does not consist of high airway pressures, high tidal volumes, high PEEP, high FiO2, or a severe pulmonary hypoperfusion with severe and lethal lung tissue alkalosis” [12]. The goal of ECMO was shifted from “buying time for the lung to heal” to “rest the lung” and to protect it from further damage, a concept later known as ventilator-induced lung injury (VILI). In the same years, Gattinoni and Kolobow proposed to exploit ECMO to decrease respiratory rate, tidal volume, and airway pressure favoring lung healing (low frequency positive pressure ventilation, LFPPV; [7]). In 1994, however, the negative results of a second randomized clinical trial on the topic where published [21]. As a consequence, only few centers continued to provide veno-venous ECMO as a last resource in selected patients with respiratory failure. However, a renewed interest in ECMO rose after the publication of the CESAR trial, a prospective randomized trial conducted in the United Kingdom [23]. In addition, the 2009 H1N1 influenza pandemic led to the widespread use of ECMO as a successful rescue therapy, first in Australia and New Zealand and then worldwide [3].
Pathophysiology of extracorporeal gas exchange
During extracorporeal support, blood oxygenation and CO2 removal are controlled by different physiologic mechanisms.
The oxygen transfer to the patient depends on the blood flow through the artificial lung, on inlet venous hemoglobin concentration and oxygen saturation. Hemoglobin concentration and mixed venous oxygen saturation (normally around 70%) limit the ability to provide oxygen to the patient. Therefore, high extracorporeal blood flows (4–7 l/min) are needed to provide a normal oxygen supply (i. e., 250 ml/min).
At variance, the CO2 content of blood is high, (normally around 0.5 l/l of blood). Therefore, total CO2 removal (that is the removal of the minute CO2 production) could be achieved at a blood flow in the range of 0.5 l/min [29]. Kolobow and Gattinoni showed that when CO2 was removed by the artificial lung, awake healthy lambs reduced their spontaneous ventilation. By removing CO2 at incremental rates, it was possible to decrease ventilation down to complete apnea when total CO2 production was removed extracorporeally ([11]; apneic oxygenation).
Mechanical ventilation—why?
The main target of extracorporeal support is to minimize the risk of VILI while achieving viable blood gases. The CO2 removal by the artificial lung allows proportional decreases of natural lung ventilation, thus, implementing a protective ventilator strategy. However, a progressive decay of respiratory system compliance, likely caused by lung collapse, can be observed during very low tidal volume ventilation with low PEEP levels. Indeed, it was shown that a PEEP greater than 20 cmH2O was necessary to prevent lung collapse in apneic lambs with healthy lungs, while at lower PEEP levels, a respiratory rate of 2 bpm with plateau pressures of 25–30 cmH2O was required [6].
Additional risks associated with hypoventilation are reabsorption atelectasis, favoring life-threatening hypoxemia during low-flow extracorporeal carbon dioxide removal (ECCO2R) [6] and right ventricular failure as a result of lung collapse and pulmonary hypertension.
Mechanical ventilation—how?
The optimal management of the natural lung during ECMO is still debated, with great variability of reported ventilatory settings and less than 30% of ECMO centers explicitly sharing their mechanical ventilation protocol [16]. From 1986–2006 only minor changes in mechanical ventilation settings have been observed, with reported PEEP values around 10 cmH2O, respiratory rate of 10 bpm, and a mean airway pressure around 16 cmH2O [2].
Subsequently several studies [17, 27, 28] have documented the changes in ventilatory settings occurring after initiation of extracorporeal respiratory support. In all cases, mechanical ventilatory support was reduced: tidal volume on average by 2 ml/kg (from 6 to 4 ml/kg), plateau pressure on average by 5 cmH2O (from 31 to 26 cmH2O), driving pressure by 5 cmH2O (from 19 to 14 cmH2O), respiratory rate, when reported, by 4 bpm (from 22 to 18 bpm), FiO2 on average by 0.3, PEEP by 1 cmH2O (from 14 to 13 cmH2O). Of note, Serpa Neto et al. point out that, in their analysis, driving pressure was the only ECMO ventilatory parameter showing an independent association with in-hospital mortality [28].
Interestingly, Marhong et al. reported mechanical ventilation settings also pre and post ECCO2R initiation and described an increase in PEEP (from 13 to 17) and no change in FiO2 [17].
It might be interesting to underline that some authors suggest reducing tidal volumes to values even lower than 4 ml/kg predicted body weight (PBW). In this regard, Marhong et al. report that 31% of centers used tidal volumes lower that 4 ml/kg PBW and 45% of the centers used tidal volumes between 4 and 6 ml/kg [16]. Favorable outcome has been reported using tidal volumes as low as 1.9 ml/kg [18].
The importance of inspiratory pressure limitation to a maximum of 25–30 cmH2O is recognized by all ECMO centers. Extracorporeal Life Support Organization (ELSO) guidelines recommend a limit at 25 cmH2O [4]; in the CESAR trial peak inspiratory pressures of 20–25 cmH2O were reported as beneficial [23]. Pham et al. suggested that reduced plateau pressure (26 vs 32 cmH2O) during the first day under ECMO in H1N1 ARDS was significantly associated with survival while increasing values of plateau pressure under ECMO were associated with death [24].
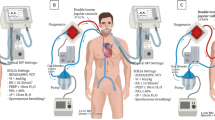
No common guidelines exist about PEEP settings during ECMO. The two extreme approaches are the total lung rest strategy with the application of very low PEEP and tidal volume and possible lung collapse and the open lung strategy with low tidal volume and higher PEEP levels (15–20 cmH2O), possibly coupled with recruitment maneuvers (Fig. 1). It is worth noting that lung collapse might lead to the complete lack of the oxygenation function of the native lung, thus, often requiring higher extracorporeal blood flows. Moreover, diffuse alveolar collapse might lead to increased pulmonary vascular resistance and acute failure of the right heart. In this case, conversion from veno-venous to veno-arterial ECMO might become necessary to unload the right ventricle [9].
ELSO guidelines propose the application of a moderate PEEP level (10 cmH2O; [4]). In the CESAR trial, PEEP was quickly reduced to 10–15 cmH2O [23]; by contrast, other experts suggest keeping PEEP unchanged or even to increase it [27]. The Karolinska group reported an original approach in a group of 13 patients treated with ECMO because of influenza A/H1N1-related severe respiratory failure. Patients were ventilated with pressure control or pressure-support ventilation with settings reduced to resting levels as soon as possible. Peak inspiratory pressures were adjusted to 20–25 cmH2O, PEEP at 5–10 cmH2O, and FiO2 at 0.4. Rather low levels of arterial oxygenation were tolerated (median nearly 85%), as a consequence of a very modest contribution to arterial oxygenation of the collapsed, rested natural lung. Acute right heart failure was treated in 4 patients switching veno-venous ECMO to veno-arterial ECMO [9]. Arterial oxygenation values were on average even lower than that achieved before connection to ECMO.
Schmidt et al., on the other hand, reported that during the first 3 ECMO days higher PEEP levels were independently associated with improved survival [27]. Marhong et al., analyzing the ELSO registry database, reported that in 77% of ECMO centers, a lung rest strategy was the major goal of mechanical ventilation. The application of periodic recruitment maneuvers can be an alternative to avoid lung collapse [16].
Prone position has been shown effective in decreasing mortality in severe ARDS patients [8] but no systematic data on its safety and efficacy in ECMO patients are available. Some centers use prone position during ECMO: it may be however associated with complications such as compression or inadvertent removal of the vascular cannulas which may lead to reduction or interruption of the extracorporeal support [5].
Ventilator FiO2 is reduced during ECMO to limit oxygen toxicity and the risk of resorption atelectasis. In the CESAR trial FiO2 was reduced to 30% [23]. A marked FiO2 reduction implies that, if the native lung still contributes to arterial oxygenation, high extracorporeal blood flows are necessary.
The setting of respiratory rate is another controversial issue. Expert opinions provide a wide range of frequency, from 4 to 30 breaths per minute. Recent literature on mechanical ventilation settings during ECMO reports moderate reduction of respiratory rate after ECMO onset in order to keep pH and CO2 in normal ranges [26].
We strongly advocate a low (<6 bpm) respiratory rate (and an associated decrease of the time spent at high pressure by the native lung) in order to minimize the risk of VILI, given the fact that hypercapnia is rarely present in ARDS patients during ECMO.
Assisted ventilation during ECLS
After sufficient improvement of a patient’s condition, mechanical ventilation can be switched from controlled to assisted mode. Protective assisted mechanical ventilation may improve the muscle function and gas exchange, decreasing the risk of diaphragm dysfunction and the need of sedation and help weaning from the ventilator [25]. Karagiannidis et al. reported in 6 patients treated with ECMO and ventilated with neurally adjusted ventilator assist (NAVA) that ventilatory response to decreased sweep gas flow was rapid, and patients immediately regulated PaCO2 tightly towards a physiological pH value [10]. Mauri et al. reported the successful use of NAVA in the recovery phase of patients with severely impaired lung function. Moreover, manipulation of CO2 elimination, at least during the recovery phase of ARDS, acts as a modulator of the drive to breathe [19].
While most groups delay assisted ventilation until the native lung performance improves substantially, others almost immediately switch the ventilator to pressure support mode. The Karolinska group reported a very low mortality rate (24%) during their experience in 17 severe ARDS patients treated with extracorporeal support coupled with minimal sedation and pressure support ventilation (PSV) with low tidal volumes [15].
In ARDS patients, this approach may require particular attention in the selection of candidates. In fact, the potential benefits of spontaneous ventilation need to be evaluated together with its potential detrimental effects [14]. Indeed, high inspiratory efforts and very high respiratory drive, often independent from the blood gas and pH value have been described in the acute phase of ARDS [13, 20]. Very high inspiratory efforts may worsen lung injury by generating elevated transpulmonary pressures [1]. While the pathophysiology of spontaneous breathing in ARDS patients has been, so far, only partially understood, it is conceivable that pulmonary receptors activated by stimuli such as edema, inflammation, and microembolism might play a key role. In these cases, extracorporeal CO2 removal through full ECMO support is frequently not sufficient to control the inspiratory efforts and keep transpulmonary pressure in a safe range [10] and an approach including deep sedation and controlled mechanical ventilation is indicated.
Conclusions
The management of ventilation during ECMO is still evolving. Lung protection while warranting viable blood gases is the target of the procedure. The early times, aiming at optimal oxygenation achieved by high tidal volumes and airway pressures, have definitely faded away, but the debate about the optimal compromise between lung recruitment and lung rest is not settled yet.
References
Brochard L, Slutsky A, Pesenti A (2017) Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med 195:438–442
Brogan TV, Thiagarajan RR, Rycus PT et al (2009) Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database. Intensive Care Med 35:2105–2114
Davies A, Jones D, Bailey M et al (2009) Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA 302:1888–1895
Extracorporeal Life Support Organization (ELSO) (2013) General guidelines for all ECLS cases. ELSO Guidelines Version 1.3 November 2013. http://www.elso.org/Portals/0/IGD/Archive/FileManager/929122ae88cusersshyerdocumentselsoguidelinesgeneralalleclsversion1.3.pdf. Accessed: 17 Nov 2017
Fan E, Gattinoni L, Combes A et al (2016) Venovenous extracorporeal membrane oxygenation for acute respiratory failure : a clinical review from an international group of experts. Intensive Care Med 42:712–724
Gattinoni L (2016) Ultra-protective ventilation and hypoxemia. Crit Care 20:130
Gattinoni L, Agostoni A, Pesenti A et al (1980) Treatment of acute respiratory failure with low-frequency positive-pressure ventilation and extracorporeal removal of CO2. Lancet 2:292–294
Guerin C, Reignier J, Richard JC et al (2013) Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 368:2159–2168
Holzgraefe B, Broome M, Kalzen H et al (2010) Extracorporeal membrane oxygenation for pandemic H1N1 2009 respiratory failure. Minerva Anestesiol 76:1043–1051
Karagiannidis C, Lubnow M, Philipp A et al (2010) Autoregulation of ventilation with neurally adjusted ventilatory assist on extracorporeal lung support. Intensive Care Med 36:2038–2044
Kolobow T, Gattinoni L, Tomlinson TA et al (1977) Control of breathing using an extracorporeal membrane lung. Anesthesiology 46:138–141
Kolobow T, Solca M, Gattinoni L et al (1981) Adult respiratory distress syndrome (ARDS): why did ECMO fail? Int J Artif Organs 4:58–59
Langer T, Vecchi V, Belenkiy SM et al (2014) Extracorporeal gas exchange and spontaneous breathing for the treatment of acute respiratory distress syndrome: an alternative to mechanical ventilation? Crit Care Med 42:e211–220
Langer T, Santini A, Bottino N et al (2016) “Awake” extracorporeal membrane oxygenation (ECMO): pathophysiology, technical considerations, and clinical pioneering. Crit Care 20:150
Linden V, Palmer K, Reinhard J et al (2000) High survival in adult patients with acute respiratory distress syndrome treated by extracorporeal membrane oxygenation, minimal sedation, and pressure supported ventilation. Intensive Care Med 26:1630–1637
Marhong JD, Telesnicki T, Munshi L et al (2014) Mechanical ventilation during extracorporeal membrane oxygenation. An international survey. Ann Am Thorac Soc 11:956–961
Marhong JD, Munshi L, Detsky M et al (2015) Mechanical ventilation during extracorporeal life support (ECLS): a systematic review. Intensive Care Med 41:994–1003
Mauri T, Bellani G, Foti G et al (2011) Successful use of neurally adjusted ventilatory assist in a patient with extremely low respiratory system compliance undergoing ECMO. Intensive Care Med 37:166–167
Mauri T, Grasselli G, Suriano G et al (2016) Control of respiratory drive and effort in extracorporeal membrane oxygenation patients recovering from severe acute respiratory distress syndrome. Anesthesiology 125:159–167
Mauri T, Langer T, Zanella A et al (2016) Extremely high transpulmonary pressure in a spontaneously breathing patient with early severe ARDS on ECMO. Intensive Care Med 42:2101–2103
Morris AH, Wallace CJ, Menlove RL et al (1994) Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 149:295–305
National Heart, Lung and Blood Institute: Division of Lung Disease (1979) Section four: hemodynamics, gas exchange and mechanics. In: US Department of Health (ed) Extracorporeal support for respiratory insufficiency. A collaborative study in response to RFP-NHLI-73-20. US Department of Health, Washington DC
Peek GJ, Mugford M, Tiruvoipati R et al (2009) Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 374:1351–1363
Pham T, Combes A, Roze H et al (2013) Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 187:276–285
Putensen C, Zech S, Wrigge H et al (2001) Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med 164:43–49
Schmidt M, Pellegrino V, Combes A et al (2014) Mechanical ventilation during extracorporeal membrane oxygenation. Crit Care 18:203
Schmidt M, Stewart C, Bailey M et al (2015) Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome: a retrospective international multicenter study. Crit Care Med 43:654–664
Serpa Neto A, Schmidt M, Azevedo LC et al (2016) Associations between ventilator settings during extracorporeal membrane oxygenation for refractory hypoxemia and outcome in patients with acute respiratory distress syndrome: a pooled individual patient data analysis: mechanical ventilation during ECMO. Intensive Care Med 42:1672–1684
Zanella A, Castagna L, Salerno D et al (2015) Respiratory electrodialysis. A novel, highly efficient extracorporeal CO2 removal technique. Am J Respir Crit Care Med 192:719–726
Zapol WM, Snider MT, Hill JD et al (1979) Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 242:2193–2196
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E. Carlesso, T. Langer and T. Mauri declare that they have no competing interests. A. Pesenti reports personal fees from Maquet, Novalung/Xenios, Baxter and Boehringer Ingelheim, outside the submitted work; in addition, A. Pesenti has patents IT2005MI02020, US201414251924, WO2013IB00432, US201313950892, US201313950717, TO2014A000623, TO2014A000655, TO2014A001096 licensed.
This article does not contain any studies with human participants or animals performed by any of the authors.
The supplement containing this article is not sponsored by industry.
Additional information
Redaktion
M. Quintel, Göttingen
L. Gattinoni, Milan
Rights and permissions
About this article
Cite this article
Pesenti, A., Carlesso, E., Langer, T. et al. Ventilation during extracorporeal support. Med Klin Intensivmed Notfmed 113 (Suppl 1), 26–30 (2018). https://doi.org/10.1007/s00063-017-0384-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00063-017-0384-8
Keywords
- Mechanical ventilation
- Ventilator-induced lung injury
- Respiratory failure
- Positive end expiratory pressure
- Extracorporeal membrane oxygenation