Abstract
Partial extracorporeal CO2 removal allows a decreasing tidal volume without respiratory acidosis in patients with acute respiratory distress syndrome. This, however, may be associated with worsening hypoxemia, due to several mechanisms, such as gravitational and reabsorption atelectasis, due to a decrease in mean airway pressure and a critically low ventilation-perfusion ratio, respectively. In addition, an imbalance between alveolar and artificial lung partial pressures of nitrogen may accelerate the process. Finally, the decrease in the respiratory quotient, leading to unrecognized alveolar hypoxia and monotonous low plateau pressures preventing critical opening, may contribute to hypoxemia.
Similar content being viewed by others
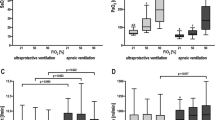
The capability to remove some of the metabolically produced CO2 with artificial lungs using a low extracorporeal blood flow (<1 l/min) and a high extracorporeal gas flow provides a key to significantly reduce mechanical ventilation (the ultra-protective strategy) [1]. This, and the easily established vascular access, make the method attractive for acute respiratory distress syndrome (ARDS) patients, in whom even the standard 6 ml/kg tidal volume (TV) may still produce unacceptable stress and strain [2]. In a recent multicenter study, Fanelli et al. [1] tested the feasibility of the ultra-protective strategy in combination with extracorporeal carbon dioxide removal (ECCO2R) in 15 patients with moderate ARDS. The authors concluded that one could use ultra-protective ventilation with a TV of 4 ml/kg without resulting relevant respiratory acidosis. The price of attaining this goal, however, appears quite high. Six of the 15 patients (40 %) with initially moderate ARDS experienced life-threatening hypoxemia and required either extracorporeal membrane oxygenation (ECMO) or the prone position. The authors concluded that the impact of the ultra-protective strategy, ECCO2R, on hypoxemia is not clear. We believe, however, that the observed hypoxemia is due to well-defined mechanisms acting simultaneously, which may be summarized as follows:
-
1)
Gravitational atelectasis: Whenever ventilation decreases (reduction of TV and/or respiratory rate) the mean airway pressure decreases, and the lungs tend to collapse. The greater the decrease in ventilation and the greater the lung weight, the greater will be the collapse. This phenomenon, albeit to a lesser extent, occurs in normal lungs on induction of anesthesia [3]. This has been observed experimentally during ECCO2R and mechanical ventilation [4]. Positive end-expiratory pressure (PEEP) must be increased by an amount sufficient to maintain the same mean airway pressure in order to preserve the open lung volume [5] otherwise some amount of gravitational collapse is unavoidable. In the study of Fanelli et al., the PEEP increase was so low as to be non-significant.
-
2)
Absorption atelectasis: At least two conditions favor the appearance of absorption atelectasis. Firstly, when ventilation is very low the amount of oxygen provided to some pulmonary units may be lower than the amount removed by the blood perfusing those units. This produces a collapse of the pulmonary unit when its ventilation (VA)/perfusion (Q) ratio reaches a critical level [6]. Secondly, if the artificial lung is ventilated with a fraction of inspired oxygen (FiO2) greater than that used for the natural lung, the partial pressure of nitrogen in the blood perfusing the natural lung is lower than that in the alveoli [7]. Under these conditions the critical VA/Q is reached more rapidly, since not only oxygen but also nitrogen leaves the alveoli. In the study by Fanelli et al., the artificial lungs were ventilated with an FiO2 of 1.0 in some of the centers, while the natural lungs were ventilated with an FiO2 of 0.5.
-
3)
Opening pressures: Sufficient pressure must be applied to reopen the atelectatic areas. Studies show that at least one normal TV every 2 min was mandatory in order to correct or prevent atelectasis, otherwise a consistent reduction in lung volume was observed [4]. This low frequency has an effect similar to sighing [8]. In ARDS patients with a plateau pressure of 25 cmH2O, as employed in the study by Fanelli et al., we may estimate that 30 to 40 % of the recruitable lung always remains closed [9, 10]. This implies that oxygenation cannot take place even during inspiration [11], and that newly formed atelectasis cannot be reopened.
-
4)
Alveolar PaO 2 and respiratory quotient: During ECCO2R, the respiratory quotient (R) decreases when the CO2 eliminated by the natural lungs decreases. To maintain the same alveolar partial pressure of oxygen (PaO2), the FiO2 must be increased. This has been proved experimentally [12] and can be easily derived from the alveolar gas equation [13]. The lower the FiO2, the greater the impact of R on alveolar PaO2. For example, with FiO2 = 0.3, partial pressure of CO2 = 50 mmHg, and R = 1.0, PaO2 is approximately 164 mmHg. With FiO2 kept constant at 0.3, alveolar PaO2 decreases to 113 mmHg when R is reduced to 0.5 and to as low as 48 mmHg when R is decreased to 0.3. The risk of unrecognized alveolar hypoxia as the cause of hypoxemia must be kept in mind during ECCO2R, particularly if a FiO2 below 0.4 is being used.
All discussed causes of hypoxemia may be prevented or corrected with the proper use of PEEP, FiO2 and recruitment maneuvers, e.g., intermittent sighs.
Abbreviations
- ARDS:
-
acute respiratory distress syndrome
- ECCO2R:
-
extracorporeal carbon dioxide removal
- FiO2 :
-
fraction of inspired oxygen
- PaO2 :
-
alveolar partial pressure of oxygen
- PEEP:
-
positive end-expiratory pressure
- Q:
-
perfusion
- R:
-
respiratory quotient
- TV:
-
tidal volume
- VA:
-
ventilation
References
Fanelli V et al. Feasibility and safety of low-flow extracorporeal carbon dioxide removal to facilitate ultra-protective ventilation in patients with moderate acute respiratory distress syndrome. Crit Care. 2016;20(1):36.
Terragni PP et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;175(2):160–6.
Brismar B et al. Pulmonary densities during anesthesia with muscular relaxation—a proposal of atelectasis. Anesthesiology. 1985;62(4):422–8.
Gattinoni L et al. Low-frequency positive pressure ventilation with extracorporeal carbon dioxide removal (LFPPV-ECCO2R): an experimental study. Anesth Analg. 1978;57(4):470–7.
Gattinoni L, Iapichino G, Kolobow T. Hemodynamic, mechanical and renal effects during “apneic oxygenation” with extracorporeal carbon dioxide removal, at different levels of intrapulmonary pressure in lambs. Int J Artif Organs. 1979;2(5):249–53.
Dantzker DR, Wagner PD, West JB. Proceedings: Instability of poorly ventilated lung units during oxygen breathing. J Physiol. 1974;242(2):72P.
Kolobow T et al. An alternative to breathing. J Thorac Cardiovasc Surg. 1978;75(2):261–6.
Pelosi P et al. Sigh in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;159(3):872–80.
Borges JB et al. Reversibility of lung collapse and hypoxemia in early acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174(3):268–78.
Crotti S et al. Recruitment and derecruitment during acute respiratory failure: a clinical study. Am J Respir Crit Care Med. 2001;164(1):131–40.
Cressoni M et al. Lung inhomogeneities and time course of ventilator-induced mechanical injuries. Anesthesiology. 2015;123(3):618–27.
Gattinoni L et al. Control of intermittent positive pressure breathing (IPPB) by extracorporeal removal of carbon dioxide. Br J Anaesth. 1978;50(8):753–8.
Riley RL, Lilienthal Jr JL, et al. On the determination of the physiologically effective pressures of oxygen and carbon dioxide in alveolar air. Am J Physiol. 1946;147:191–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author declares that he has no competing interests.
See related research by Fanelli et al., http://ccforum.biomedcentral.com/articles/10.1186/s13054-016-1211-y
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gattinoni, L. Ultra-protective ventilation and hypoxemia. Crit Care 20, 130 (2016). https://doi.org/10.1186/s13054-016-1310-9
Published:
DOI: https://doi.org/10.1186/s13054-016-1310-9