Abstract
The timing of extracorporeal membrane oxygenation (ECMO) initiation and its outcome in the management of respiratory and cardiac failure have received considerable attention, but very little attention has been given to mechanical ventilation during ECMO. Mechanical ventilation settings in non-ECMO studies have been shown to have an effect on survival and may also have contributed to a treatment effect in ECMO trials. Protective lung ventilation strategies established for non-ECMO-supported respiratory failure patients may not be optimal for more severe forms of respiratory failure requiring ECMO support. The influence of positive end-expiratory pressure on the reduction of the left ventricular compliance may be a matter of concern for patients receiving ECMO support for cardiac failure. The objectives of this review were to describe potential mechanisms for lung injury during ECMO for respiratory or cardiac failure, to assess the possible benefits from the use of ultra-protective lung ventilation strategies and to review published guidelines and expert opinions available on mechanical ventilation-specific management of patients requiring ECMO, including mode and ventilator settings. Articles were identified through a detailed search of PubMed, Ovid, Cochrane databases and Google Scholar. Additional references were retrieved from the selected studies. Growing evidence suggests that mechanical ventilation settings are important in ECMO patients to minimize further lung damage and improve outcomes. An ultra-protective ventilation strategy may be optimal for mechanical ventilation during ECMO for respiratory failure. The effects of airway pressure on right and left ventricular afterload should be considered during venoarterial ECMO support of cardiac failure. Future studies are needed to better understand the potential impact of invasive mechanical ventilation modes and settings on outcomes.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Review
Introduction
Over the past decade, the use of two distinct modalities of extracorporeal membrane oxygenation (ECMO) for respiratory and cardiac support in adults has increased. Venovenous (VV)-ECMO may be initiated as a treatment strategy for patients with severe acute respiratory failure, including adult respiratory distress syndrome (ARDS) [1–5], as a salvage therapy for patients with profound gas-exchange abnormalities despite positive-pressure ventilation. Additionally, partial extracorporeal support systems have been suggested for less severe respiratory failure as an adjunct to invasive mechanical ventilation (MV) for patients who have excessively high inspiratory airway pressures or who are unable to tolerate volume-limited and pressure-limited strategies. These devices predominately remove carbon dioxide (CO2) from the blood and provide limited oxygenation [6–8]. Such systems are often classified as extracorporeal carbon dioxide removal (ECCO2R) systems and cannot provide complete respiratory support. VV-ECMO and ECCO2R may now be considered management options for chronic end-stage respiratory failure where MV is contraindicated or undesirable; for example, as a bridge to lung transplantation in patients with cystic fibrosis who need to perform airway clearance techniques for sputum retention [9, 10]. ECCO2R has also been described for chronic obstructive pulmonary disease patients with prolonged weaning of invasive MV [11]. Venoarterial (VA)-ECMO is a rapidly deployable treatment option for temporary circulatory assistance in patients with cardiogenic shock or refractory cardiac arrest [12–14] secondary to a large number of acute and chronic cardiac illnesses.
MV management during VV-ECMO and VA-ECMO has received scant attention to date despite high-level evidence to support low-tidal-volume ventilation strategies to improve survival [15, 16]. The design of randomized controlled trials of ECMO in ARDS did not use standardized protective ventilation in the interventional arm [8, 17] or in the control arm [3], which could have jeopardized the success of the ECMO treatment in these trials. MV settings may have important implications in both modes of ECMO (that is, VV-ECMO and VA-ECMO). Patients with the most severe forms of lung injury are likely to be particularly susceptible to ventilator-associated lung injury. Limiting stress and strain with a volume-limited and pressure-limited protective ventilation strategy beyond that recommended for patients with ARDS could provide additional benefit during ECMO support [4, 18, 19]. For patients with severe cardiac failure supported with VA-ECMO, pulmonary artery blood flow may be severely reduced and the maintenance of normal alveolar ventilation might lead to severe over-ventilation of the lungs [20]. Positive airway pressure settings will also affect right and left ventricular load in both VV-ECMO and VA-ECMO [21].
Brief guidelines for the use of ECMO [22] and expert points of views [3, 23] have been published, mostly during the recent influenza A(H1N1) pandemic [24]. These publications are based on clinician preference, experience of centers with high case volumes, previous randomized trials [3] and local resource availability.
While there are extensive reviews on ECMO management [23, 25–29], there is a significant knowledge gap in understanding the benefits and risks of MV during ECMO. Unlike previous reviews on ECMO [23, 27, 29], this review will focus on MV during ECMO. The purpose is to highlight the interactions between MV, ECMO and the pathophysiology of severe acute respiratory and cardiac failure. A second purpose is to provide evidence of the risks associated with MV during ECMO. Additionally, this review will summarize current guidelines, describe new strategies advocated for MV, provide evidence-based criteria that can be used for MV during ECMO and discuss what future studies are needed to address the evidence gap in this area.
Physiological considerations and possible mechanisms for harm and benefit of mechanical ventilation during venovenous extracorporeal membrane oxygenation
Nonpulmonary gas exchange: how much gas exchange can extracorporeal membrane oxygenation provide?
The extent of nonpulmonary gas exchange required during ECMO is directly related to the limitation of pulmonary gas exchange. The amount of oxygen supplied to the patient by the ECMO circuit is limited by the maximal oxygen delivery of the membrane (that is, membrane outlet–inlet oxygen content). The current generation of ECMO membranes can deliver up to 450 ml oxygen/minute [30]. Actual patient oxygen delivery from an ECMO circuit is affected by the rate of circuit blood flow, the hemoglobin concentration and the oxyhemoglobin saturation of the venous blood (partly reflecting the level of recirculation). Of note, with VV-ECMO the circuit blood flow is related to both the inflow cannula diameter and the cardiac output [31]. The CO2 content in blood is higher than the oxygen content, and is rapidly diffusible. CO2 transfer provided by current membranes may exceed 450 ml/minute depending on the ratio of gas to blood flow in the membrane and the CO2 partial pressure. Higher sweep gas flow and higher CO2 partial pressure in the oxygenator blood result in greater CO2 clearance. CO2 removal is therefore easily controlled with sweep gas flow settings [32].
Minimizing ventilator-induced lung injury
MV can activate inflammation and worsen the pulmonary damage of the underlying disease, leading to ventilator-induced lung injury (VILI) [33]. Three possible causal mechanisms of VILI may be modifiable with the use of ECMO.
First is the alveolar strain, which represents the amount of aerated lung receiving ventilation [34, 35]. In 2000, the ARDS Network published a multicenter randomized clinical trial where a strategy aimed at maintaining plateau pressure ≤30 cmH2O with an initial tidal volume of ≈ 6 ml/kg predicted body weight (PBW) was compared with traditional ventilation treatment that involved an initial tidal volume of ≈ 12 ml/kg PBW [15]. The protective ventilation, which minimizes the alveolar strain physiological concept, was associated with a decreased mortality of 22%. Patients at many centers who have received ECMO for severe ARDS have a very low arterial partial pressure of oxygen/fraction of inspired oxygen ratio (≈50 mmHg) [1, 4] and a very high acute injury score [1, 4]. In addition, these patients have a very small area of normally aerated alveoli located in the nondependent lung, a large consolidated or nonaerated region located in the dependent lung along the vertical axis [36–38] and frequent infiltration of all of the four lung’s quadrants on chest radiographs [1]. As the aerated compartment receives the largest part of the tidal volume [37, 39], these severely unwell patients with a large amount of collapsed lung may be exposed to VILI despite low-tidal-volume ventilation strategies [40]. Limitation of the alveolar strain is a major concern of patients with ARDS receiving MV during ECMO.
A second mechanism of VILI is due to repeated intra-tidal alveolar opening and closing (atelectrauma), defined as the amount of collapsed lung tissue that is re-opened during inspiration and re-collapsed during expiration [41–43]. The challenge is to find the right ventilator settings to avoid intra-tidal alveolar opening and closing while limiting the risk of alveolar overdistension or strain [44]. Combining a low tidal volume with high levels of positive end-expiratory pressures (PEEP) appears to be important. Caironi and colleagues showed similar alveolar strain after application of 15 cmH2O PEEP in two distinct groups of 34 ARDS/acute lung injury patients (that is, higher vs. lower percentage of potentially recruitable lung groups) [41], suggesting that the beneficial impact of reducing intra-tidal alveolar opening and closing by increasing PEEP prevailed over the effects of increasing alveolar strain. Of note, despite improving oxygenation [45, 46] and reducing the duration of MV [46], a strategy for setting PEEP aimed at increasing alveolar recruitment while limiting hyperinflation did not significantly reduce mortality in ARDS [45–47].
Finally, oxygen lung toxicity from a high fraction of inspired oxygen in lung areas with a low ventilation–perfusion ratio might alone cause reabsorption atelectasis [48–51]. Such areas are frequent in ARDS, and Aboab and colleagues showed in mechanically ventilated patients with acute lung injury that the breathing of pure oxygen leads to derecruitment, which is prevented by high PEEP [52]. The challenge of MV settings with ECMO, particularly when lung function is severely impaired, is to minimize these pitfalls.
Physiological considerations and possible mechanisms for harm and benefit of mechanical ventilation during venoarterial extracorporeal membrane oxygenation
Patients with cardiac failure receiving VA-ECMO often have abnormal lung function that may be associated with ARDS. Considerations from the previous section may also apply to this group. However, the major cardiovascular effect associated with PEEP is reduction in cardiac output. Although the effect of PEEP on cardiac output is complex, the decrease is caused predominantly by decreasing the right ventricular preload and direct heart–lung interaction [53]. By increasing the intrathoracic pressure, PEEP can increase pulmonary vascular resistance, which may cause right ventricular overload and reduced left ventricular compliance. Patients who have received VA-ECMO with predominately right ventricular failure can be adversely affected by high PEEP [54, 55]. Conversely, patients with predominately left ventricular failure supported with VA-ECMO may develop pulmonary edema despite adequate systemic support and often benefit from the application of high PEEP [34].
Additionally, VA-ECMO may dramatically reduce pulmonary blood flow as a result of pulmonary shunting. If normal lung ventilation is maintained in this setting, severe local alkalosis might result. To date, this potential deleterious effect has not been widely described and clinical consequences are still unknown. However, some authors have suggested that decreased lung perfusion with VA-ECMO may accelerate pulmonary vascular thrombosis in the presence of severe lung injury [17, 20].
Evidence and current recommendations
To date, animal data, observational studies and previous randomized trials may give a physiologic rationale to promote ultra-protective ventilation during ECMO.
Mechanical ventilation settings: tidal volume and plateau pressure limitation
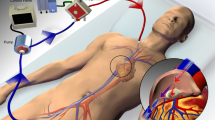
The main objectives of MV during ECMO for patients with severe acute respiratory failure are summarized in Figure 1. However, multiple approaches to ventilation could be acceptable [29]. By directly removing CO2 from the blood, ECMO enables lung-protective ventilation. Without ECMO, difficulty maintaining adequate alveolar ventilation is one limitation to the use of a protective ventilation strategy – exposing patients to potential side effects of subsequent hypercapnia, such as intracranial pressure elevation, pulmonary hypertension, depressed myocardial contractility and a reduction in renal blood flow [56, 57].
Specifications of mechanical ventilation with extracorporeal membrane oxygenation for patients with severe acute respiratory failure. ECMO, extracorporeal membrane oxygenation; FiO2, fraction of inspired oxygen; NAVA, neurally adjusted ventilator assist; PBW, predicted body weight; PEEP, positive end-expiratory pressure.
Using tidal volume <4 ml/kg PBW is thus recommended with ECMO [29] and is often referred to imprecisely as lung rest or ultra-protective ventilation [3, 7]. The concept of ultra-protective MV was suggested and investigated in animal studies [58]. Using a rat model of acid-induced lung injury, Frank and colleagues showed that a tidal volume reduction from 12 to 6 to 3 ml/kg, with the same level of PEEP (10 cmH2O), decreased pulmonary edema and lung injury, and increased protection of the alveolar epithelium [58]. In addition, post-hoc analysis of the ARDS Network data in a multivariable logistic regression model showed that lower tidal volume assignment and lower plateau pressure quartile were significant predictors of lower mortality [59]. Favorable outcome of patients with ARDS treated with a strong tidal volume reduction until 1.9 ml/kg PBW with ECMO [19] and with ECCO2R [18] have been reported. Terragni and colleagues used a system that predominately removed CO2 to reduce tidal volume <6 ml/kg PBW and observed a reduction of pulmonary cytokine concentration [60]. However, survival benefit from ultra-protective lung ventilation was not seen in a recent multicenter randomized controlled trial [6]. Bein and colleagues compared a very low tidal volume ventilation strategy (≈ 3 ml/kg PBW) combined with ECCO2R to lower tidal ventilation (≈ 6 ml/kg PBW) without the extracorporeal device in 79 patients with ARDS. The number of ventilator-free days at day 60 and the mortality rates were not significantly different between the two study groups. However, promising results were found in patients with severe hypoxemia with a lower number of ventilator-free days at 60 days in the control group [6]. In addition, Pham and colleagues recently showed, in a cohort of 123 patients with influenza A(H1N1)-induced ARDS, that a higher plateau pressure on the first day of VV-ECMO for acute respiratory failure was significantly associated with ICU death (odds ratio = 1.33, 95% confidence interval = 1.14 to 1.59, P <0.01) [4]. It is worth noting that the ultra-protective ventilation strategy was associated with the use of high PEEP levels in all cases [6, 18, 60].
Using a pressure control approach with tight limitation of the peak inspiratory pressure between 20 and 25 cmH2O has been suggested to be beneficial [3]. Depending on the severity of the lung disease, ultra-protective ventilation with plateau pressure limitation may lead to apneic ventilation (that is, no tidal volume) for several days. This may be particularly evident with pediatric patients [61, 62], and in some cases the reduction of the tidal volume to achieve limited ventilation pressure strategy is considered so important that the result is insufficient ventilation to maintain adequate oxygen delivery. In this case, a third venous ECMO cannula to increase ECMO blood flow may be utilized rather than increasing the inspiratory pressure, which may negate the beneficial effect of the ECMO. Despite its feasibility reported on animal studies [63], pediatric studies [64] and in our daily adult practice, the long-term effect of apneic ventilation is unknown. A very low tidal volume results in dead space ventilation only. In our opinion, this must be combined with a high level of PEEP, to maintain convective ventilation for the elimination of alveolar nitrogen [63] and avoid alveolar collapse.
Although there are no large randomized studies focused on MV settings during ECMO in severe acute respiratory failure, it is reasonable, at this time, to advise an ultra-protective ventilation strategy with ECMO, based on a tidal volume reduction (that is, <4 ml/kg PBW) and on a plateau pressure reduction (that is, ≤25 cmH2O), provided lung recruitment with PEEP is sufficient.
For patients without ARDS treated by VA-ECMO, lung function is often abnormal. Cardiogenic pulmonary edema, postoperative lung damage and thoracic compliance reduction are frequently present after cardiac surgery, and these patients are at risk of developing ARDS. Targeting lower tidal volumes (6 to 8 ml/kg PBW) appears to decrease the incidence of adverse outcome, even without ARDS [65], and would appear to be reasonable in this population [22], as would a reduction in the respiratory rate during periods of minimal pulmonary artery blood flow. In addition, CO2 removal by VA-ECMO might allow a better tolerance of low tidal volume with less discomfort and dyspnea, and therefore less sedation – but this is an area for further research.
Mechanical ventilation settings: positive end-expiratory pressure
It is important to be aware that, despite the use of ECMO, decreasing tidal volume <4 ml/kg PBW may increase atelectasis and result in severe ventilation/perfusion mismatch unless PEEP is appropriately increased [66]. Higher PEEP levels are essential [18, 19, 63] – probably higher than suggested by the Extracorporeal Life Support Organization (ELSO) guidelines, which suggest a modest PEEP of 10 cmH2O [22] – while taking into account the risk of alveolar overdistension and increased alveolar strain [44]. In addition, higher PEEP levels, can also maintain convective ventilation for the elimination of alveolar nitrogen accumulated during apneic oxygenation with ECMO [63].
High PEEP levels may have an adverse effect on hemodynamics when the patient is managed in the VV-ECMO mode by inhibiting venous return [22]. Although heart function is partially or completely replaced by VA-ECMO, high PEEP levels might also exacerbate right ventricular dysfunction and delay heart recovery. Particular caution and frequent cardiac echography monitoring should thus be advised for patients with ARDS and moderate right failure treated with VV-ECMO.
Mechanical ventilation settings: fraction of inspired oxygen, respiratory rate
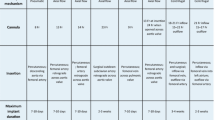
To limit pulmonary oxygen toxicity [52], the ventilator fraction of inspired oxygen should also be reduced to the minimal value to keep arterial saturation >85% [23]. Settings for the respiratory rate are debated, with some authors suggesting that rapid respiratory rates may increase mechanical lung stress [22]. Current expert opinions vary, and a broad range has been suggested from 4 to 30 cycles/minute (Table 1). In our opinion, the respiratory rate must be set to maintain pH and arterial CO2 partial pressure within normal ranges. A first approach could thus be to tailor the respiratory rate to the tidal volume and ECMO gas flow settings.
Mechanical ventilation settings: mode of mechanical ventilation
To date no study has compared different modes of MV during ECMO. The choice of mode used during ECMO must thus be guided by both physician usage pattern and local resource availability. However, an ultra-protective strategy should be advocated (Table 1).
The assist-controlled mode in pressure or volume seems to be commonly used during the initial phase of ARDS with ECMO, when patients are often deeply sedated and paralyzed and when alterations of lung compliance are greatest. With the target settings discussed previously, the pressure-controlled mode appears to be advocated, and is probably the most popular mode in the initial phase of ARDS with ECMO [3, 22]. While minimizing the potential for VILI with ultra-protective ventilation, the pressure-controlled mode allows daily monitoring of the tidal volume increase as the patient’s condition improves. As the lung compliance improves, it is possible to see the gradual increase in tidal volume from very negligible tidal volume (50 ml) to 6 ml/kg PBW (inferior limit for ECMO weaning) [22]. However, while pressure control ventilation is advocated, some authors have recommended including spontaneous breathing to allow diaphragm contraction. Spontaneous breathing in any phase of the mechanical ventilator cycle is possible during airway pressure release ventilation, a ventilation mode that periodically switches between two levels of continuous positive airway pressure [67]. Combined with spontaneous breathing, airway pressure release ventilation appeared to augment the distribution of ventilation to dependent lung regions [68], to decrease the workload on the respiratory muscle [69] and to increase systemic blood flow in patients with severe ARDS [70]. As a result, airway pressure release ventilation might be an alternative mode to conventional pressure-controlled MV in ARDS with ECMO [71, 72].
Prolonged controlled ventilation without diaphragmatic contraction may result in severe atrophy and increased duration of ventilatory support [73]. The pressure-assisted mode with spontaneous diaphragm contraction should therefore be used as soon as possible. Recent case reports have noticed the successful combination of neurally adjusted ventilatory assist and ECMO in patients with severely impaired lung function in the recovery phase [74, 75]. The automated protective ventilation permitted with this closed-loop ventilation mode [76] may improve patient–ventilator synchrony, particularly in patients with ARDS [75].
In the case of patients with hypercapnic respiratory failure due to chronic obstructive pulmonary disease, or chronic end-stage respiratory failure treated with VV-ECMO or ECCO2R, the findings of recent pilot studies have suggested that the optimal management is to wean from MV as soon as possible once ECMO has been established [9, 11, 77]. In this case, ECMO without invasive MV, named awake ECMO, seems feasible, is relevant and has been associated with good results [9, 11, 77].
Associated measures
To obtain high-flow ECMO sufficient to perform ultra-protective ventilation, ECMO cannulas with high diameter are essential [31]. Similarly, diuresis to dry weight [78] and restrictive transfusion strategies [31] should be attempted early in the patient’s management, despite volume expansion that may be needed at the initial phase of the disease. Every effort should then be made to achieve net negative fluid balance. In addition, tracheotomy is frequently done safely under ECMO in this population exposed to prolonged MV. Sedation and analgesia should be titrated as low as possible to conciliate protective ventilation with comfort and tolerance of the cannula.
Recommendations
Many retrospective studies of patients with ARDS mechanically ventilated during ECMO did not provide details of MV settings [1, 2, 79]. Table 1 summarizes the views of experts in the field in the form of the ELSO guidelines [22], MV network [24] and protocols from previous [3] or ongoing [72] prospective randomized trials. For brief MV management, ELSO recommends ‘reasonable initial ventilator settings during extracorporeal life support (ECLS) … with a respiratory frequency of 4 to 5 per minute, modest PEEP (e.g. 10 cm H2O), and low inflation pressure (e.g. 10 cm H2O above PEEP, or a peak inspiratory pressure (PIP) of 20 cm H2O. Once patients are stabilized and sedation can be lightened, spontaneous ventilation with pressure support ventilation can be considered’ [22].
Despite current use of heterogeneous modes of MV, an ultra-protective ventilation strategy for ECMO with acute respiratory failure is commonly suggested as best practice (Table 1). To what extent we should reduce both the tidal volume and the plateau pressure to allow lung rest remains unknown and is an area for future research. Similarly, the impact of PEEP and tidal volume on the timing of recovery of heart function in patients supported with VA-ECMO is unclear and is another important area of future research.
Evidence gap and future directions
Recent publications have suggested several directions forward.
No future for invasive mechanical ventilation with ECMO?
In the future, will we still need invasive MV with ECMO at all? As described previously, invasive MV is a potential cause of VILI and ventilator-associated pneumonia, which can further enhance the initial lung damage. Numerous centers have reported the strategy of employing ECMO as a bridge to lung transplantation [9, 80, 81] without invasive ventilation.
A recent pilot study has suggested that ECMO might be used for hypercapnic respiratory failure in chronic obstructive pulmonary disease patients as an alternative to non-invasive ventilation or in the case of non-invasive ventilation failure [11]. In a proof-of-concept study, 26 patients awaiting lung transplantation who developed end-stage respiratory failure and were supported with ECMO while awake (that is, no invasive ventilation) were retrospectively compared with a historical control group [9]. The control group was supported with invasive MV as a bridge to lung transplantation. Despite the same duration of assistance, the 6-month survival after transplantation was higher in the ECMO group. The main benefits of awake ECMO were avoiding the complications of prolonged intubation, MV and sedation, as well as maintaining active physical activity while receiving ECMO, which may have improved physical fitness prior to transplantation. The same strategy was also described as feasible and safe with VA-ECMO [82]. This is briefly suggested by the ELSO guidelines as: ‘… An alternative is to extubate the patient and allow spontaneous breathing with the patient awake’ [22].
Recent advances in ECMO technology and a better understanding of the respiratory drive, in particular the source of dyspnea and discomfort, might allow the use of ECMO as an alternative to invasive MV in selected patients with ARDS [83]. Hoeper and colleagues recently reported the feasibility of VV-ECMO in six awake, non-intubated, spontaneously breathing patients with ARDS [84]. Avoiding mechanical ventilation might be of particular interest in specific patient populations that are placed at high risk with invasive ventilation; for example, patients with immunosuppression or end-stage chronic lung disease [9, 11].
Trials focus on mechanical ventilation strategies with extracorporeal membrane oxygenation
Description of actual MV management with ECMO worldwide is warranted to give the basis to design future interventional trials. For instance, evaluation of the impact of an ultra-protective lung ventilation strategy with ECMO for ARDS by a large international randomized trial is now needed.
Monitoring mechanical ventilation settings prior to and during extracorporeal membrane oxygenation
As blood gas is a mix up of oxygen delivered both from the ECMO and from the native lung, monitoring the native lung during ECMO is very scanty. However, simple beside tools are available. Daily plateau pressure and compliance monitoring is a first, but imperfect, way to monitor native lung function. In addition, if a pressure mode is used, the daily monitoring of the tidal volume obtained may be valuable information. Although there is no published evidence, continuous measurement of end-tidal CO2 is used by some teams to monitor native lung improvement during the ECMO course. Moreover, MV during ECMO may lead to overdistension and under-recruitment depending on the degree of heterogeneity of regional compliance [44].
Assessment of regional lung mechanics is not easy with extremely severe ARDS prior to or during ECMO. Therefore, for technical and safety reasons, thoracic computed tomography could not be routinely recommended for these patients. Electrical impedance tomography could be considered as a MV monitoring tool prior and during ECMO for ARDS. Some authors have suggested that it could be a bedside tool to identify patients in whom lung protection and reversal of hypoxemia is not achievable with MV (that is, ECMO recipient), or to identify patients who would benefit from MV with lung recruitment maneuvers (that is, ECMO nonrecipient) [85]. Similarly, Grasso and colleagues suggested the use of transpulmonary pressure (assessed by esophageal balloon) as a bedside tool to assess physiological titration of PEEP [86]. Indeed, the authors demonstrated that 50% of patients with influenza A(H1N1)-associated ARDS referred to their unit for ECMO did not receive ECMO, because the transpulmonary pressure was lower than airway pressure alone. This unexpected lower value, mainly due to low chest wall compliance, allowed a safe increase in the PEEP level, which in turn increased oxygenation and avoided the use of ECMO. This individually tailored approach could be the future direction of research to select the ‘best’ candidates for ECMO, if used as a rescue therapy for severe acute hypoxemic respiratory failure.
Conclusions
Although the positive impact of a protective lung ventilation strategy on survival in ARDS has been clearly demonstrated, in patients receiving MV during ECMO there is limited evidence to guide practice. Based on actual and past randomized trials of ARDS with ECMO, an ultra-protective ventilation strategy that limits tidal volume to <4 ml/kg PBW, targets a very low plateau pressure (<25 cmH2O) and is supported by increased alveolar recruitment with PEEP may be the best option for clinicians managing these critically ill patients. As the use of ECMO increases internationally, future studies are urgently required to determine the best practice of MV during ECMO and its impact on patient-centered outcomes.
Abbreviations
- ARDS:
-
Adult respiratory distress syndrome
- CO2:
-
Carbon dioxide
- ECCO2R:
-
Extracorporeal carbon dioxide removal
- ECMO:
-
Extracorporeal membrane oxygenation
- ELSO:
-
Extracorporeal Life Support Organization
- MV:
-
Mechanical ventilation
- PBW:
-
Predicted body weight
- PEEP:
-
Positive end-expiratory pressure
- VA-ECMO:
-
Venoarterial extracorporeal membrane oxygenation
- VILI:
-
Ventilator-induced lung injury
- VV-ECMO:
-
Venovenous extracorporeal membrane oxygenation.
References
Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, Forrest P, Gattas D, Granger E, Herkes R, Jackson A, McGuinness S, Nair P, Pellegrino V, Pettila V, Plunkett B, Pye R, Torzillo P, Webb S, Wilson M, Ziegenfuss M: Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA 2009, 302: 1888-1895.
Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, Sadique MZ, Sekhon JS, McAuley DF, Firmin RK, Harvey C, Cordingley JJ, Price S, Vuylsteke A, Jenkins DP, Noble DW, Bloomfield R, Walsh TS, Perkins GD, Menon D, Taylor BL, Rowan KM: Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011, 306: 1659-1668. 10.1001/jama.2011.1471
Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D: Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009, 374: 1351-1363. 10.1016/S0140-6736(09)61069-2
Pham T, Combes A, Roze H, Chevret S, Mercat A, Roch A, Mourvillier B, Ara-Somohano C, Bastien O, Zogheib E, Clavel M, Constan A, Marie Richard JC, Brun-Buisson C, Brochard L: Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013, 187: 276-285. 10.1164/rccm.201205-0815OC
Schmidt M, Zogheib E, Roze H, Repesse X, Lebreton G, Luyt CE, Trouillet JL, Brechot N, Nieszkowska A, Dupont H, Ouattara A, Leprince P, Chastre J, Combes A: The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med 2013, 39: 1704-1713. 10.1007/s00134-013-3037-2
Bein T, Weber-Carstens S, Goldmann A, Muller T, Staudinger T, Brederlau J, Muellenbach R, Dembinski R, Graf BM, Wewalka M, Philipp A, Wernecke KD, Lubnow M, Slutsky AS: Lower tidal volume strategy (approximately 3 ml/kg) combined with extracorporeal CO(2) removal versus ‘conventional’ protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med 2013, 39: 847-856. 10.1007/s00134-012-2787-6
Gattinoni L, Pesenti A, Mascheroni D, Marcolin R, Fumagalli R, Rossi F, Iapichino G, Romagnoli G, Uziel L, Agostoni A, Kolobow T, Damia G: Low-frequency positive-pressure ventilation with extracorporeal CO2 removal in severe acute respiratory failure. JAMA 1986, 256: 881-886. 10.1001/jama.1986.03380070087025
Morris AH, Wallace CJ, Menlove RL, Clemmer TP, Orme JF Jr, Weaver LK, Dean NC, Thomas F, East TD, Pace NL, Suchyta MR, Beck E, Bombino M, Sittig DF, Bohm S, Hoffmann B, Becks H, Butler S, Pearl J, Rasmusson B: Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994, 149: 295-305. 10.1164/ajrccm.149.2.8306022
Fuehner T, Kuehn C, Hadem J, Wiesner O, Gottlieb J, Tudorache I, Olsson KM, Greer M, Sommer W, Welte T, Haverich A, Hoeper MM, Warnecke G: Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med 2012, 185: 763-768. 10.1164/rccm.201109-1599OC
Ricci D, Boffini M, Del Sorbo L, El Qarra S, Comoglio C, Ribezzo M, Bonato R, Ranieri VM, Rinaldi M: The use of CO2 removal devices in patients awaiting lung transplantation: an initial experience. Transplant Proc 2010, 42: 1255-1258. 10.1016/j.transproceed.2010.03.117
Burki NK, Mani RK, Herth FJ, Schmidt W, Teschler H, Bonin F, Becker H, Randerath WJ, Stieglitz S, Hagmeyer L, Priegnitz C, Pfeifer M, Blaas SH, Putensen C, Theuerkauf N, Quintel M, Moerer O: A novel extracorporeal CO(2) removal system: results of a pilot study of hypercapnic respiratory failure in patients with COPD. Chest 2013, 143: 678-686.
Chen YS, Chao A, Yu HY, Ko WJ, Wu IH, Chen RJ, Huang SC, Lin FY, Wang SS: Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. J Am Coll Cardiol 2003, 41: 197-203.
Magovern GJ Jr, Simpson KA: Extracorporeal membrane oxygenation for adult cardiac support: the Allegheny experience. Ann Thorac Surg 1999, 68: 655-661. 10.1016/S0003-4975(99)00581-0
Smedira NG, Moazami N, Golding CM, McCarthy PM, Apperson-Hansen C, Blackstone EH, Cosgrove DM 3rd: Clinical experience with 202 adults receiving extracorporeal membrane oxygenation for cardiac failure: survival at five years. J Thorac Cardiovasc Surg 2001, 122: 92-102. 10.1067/mtc.2001.114351
The Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000, 342: 1301-1308.
Burns KE, Adhikari NK, Slutsky AS, Guyatt GH, Villar J, Zhang H, Zhou Q, Cook DJ, Stewart TE, Meade MO: Pressure and volume limited ventilation for the ventilatory management of patients with acute lung injury: a systematic review and meta-analysis. PLoS One 2011, 6: e14623. 10.1371/journal.pone.0014623
Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, Morris AH, Peirce EC 2nd, Thomas AN, Proctor HJ, Drinker PA, Pratt PC, Bagniewski A, Miller RG Jr: Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979, 242: 2193-2196. 10.1001/jama.1979.03300200023016
Bein T, Zimmermann M, Hergeth K, Ramming M, Rupprecht L, Schlitt HJ, Slutsky AS: Pumpless extracorporeal removal of carbon dioxide combined with ventilation using low tidal volume and high positive end-expiratory pressure in a patient with severe acute respiratory distress syndrome. Anaesthesia 2009, 64: 195-198. 10.1111/j.1365-2044.2008.05735.x
Mauri T, Foti G, Zanella A, Bombino M, Confalonieri A, Patroniti N, Bellani G, Pesenti A: Long-term extracorporeal membrane oxygenation with minimal ventilatory support: a new paradigm for severe ARDS? Minerva Anestesiol 2012, 78: 385-389.
Ratliff JL, Hill JD, Fallat RJ, Parrot J, Tucker HJ: Complications associated with membrane lung support by venoarterial perfusion. Ann Thorac Surg 1975, 19: 537-539. 10.1016/S0003-4975(10)64429-3
Luecke T, Pelosi P: Clinical review: Positive end-expiratory pressure and cardiac output. Crit Care 2005, 9: 607-621. 10.1186/cc3877
Extracorporeal Life Support Organization: ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support and Patient Specific Supplements to the ELSO General Guidelines. Ann Arbor, MI; [http://elso.org/]
Combes A, Bacchetta M, Brodie D, Muller T, Pellegrino V: Extracorporeal membrane oxygenation for respiratory failure in adults. Curr Opin Crit Care 2012, 18: 99-104. 10.1097/MCC.0b013e32834ef412
Réseau Europeen de Recherche en Ventilation Artificielle (REVA) – Syndrome de Détresse Respiratoire Aiguë lié à la grippe A(H1N1)-2009 Recommandations pour l'assistance respiratoire [http://www.revaweb.org]
Joffe AR, Lequier L, Robertson CM: Pediatric outcomes after extracorporeal membrane oxygenation for cardiac disease and for cardiac arrest: a review. ASAIO J 2012, 58: 297-310. 10.1097/MAT.0b013e31825a21ff
Kar B, Basra SS, Shah NR, Loyalka P: Percutaneous circulatory support in cardiogenic shock: interventional bridge to recovery. Circulation 2012, 125: 1809-1817. 10.1161/CIRCULATIONAHA.111.040220
Combes A, Brechot N, Luyt CE, Schmidt M: What is the niche for extracorporeal membrane oxygenation in severe acute respiratory distress syndrome? Curr Opin Crit Care 2012, 18: 527-532. 10.1097/MCC.0b013e328357f090
Pitsis AA, Visouli AN: Mechanical assistance of the circulation during cardiogenic shock. Curr Opin Crit Care 2011, 17: 425-438. 10.1097/MCC.0b013e32834a75c1
Brodie D, Bacchetta M: Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 2011, 365: 1905-1914. 10.1056/NEJMct1103720
ECMO and Life Support Systems Quadrox PLS and Rotaflow Hardware and Accessories [http://www.maquet.com/content/Cardiopulmonary/Documents/Brochures/PLS_BROCHU_MCV-BR-40000145-EN-04_1010_EN_NONUS.pdf]
Schmidt M, Tachon G, Devilliers C, Muller G, Hekimian G, Brechot N, Merceron S, Luyt CE, Trouillet JL, Chastre J, Leprince P, Combes A: Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med 2013, 39: 838-846. 10.1007/s00134-012-2785-8
MacLaren G, Combes A, Bartlett RH: Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med 2012, 38: 210-220. 10.1007/s00134-011-2439-2
Dreyfuss D, Basset G, Soler P, Saumon G: Intermittent positive-pressure hyperventilation with high inflation pressures produces pulmonary microvascular injury in rats. Am Rev Respir Dis 1985, 132: 880-884.
Dreyfuss D, Soler P, Basset G, Saumon G: High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis 1988, 137: 1159-1164. 10.1164/ajrccm/137.5.1159
Gattinoni L, Pesenti A: The concept of ‘baby lung’. Intensive Care Med 2005, 31: 776-784. 10.1007/s00134-005-2627-z
Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G: Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 2006, 354: 1775-1786. 10.1056/NEJMoa052052
Gattinoni L, Caironi P, Pelosi P, Goodman LR: What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med 2001, 164: 1701-1711. 10.1164/ajrccm.164.9.2103121
Rouby JJ: A lung computed tomographic assessment of positive end-expiratory pressure-induced lung overdistension. Am J Respir Crit Care Med 2000, 161: 1396-1397.
Vieira SR, Puybasset L, Lu Q, Richecoeur J, Cluzel P, Coriat P, Rouby JJ: A scanographic assessment of pulmonary morphology in acute lung injury. Significance of the lower inflection point detected on the lung pressure–volume curve. Am J Respir Crit Care Med 1999, 159: 1612-1623. 10.1164/ajrccm.159.5.9805112
Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, Gandini G, Herrmann P, Mascia L, Quintel M, Slutsky AS, Gattinoni L, Ranieri VM: Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 2007, 175: 160-166. 10.1164/rccm.200607-915OC
Caironi P, Cressoni M, Chiumello D, Ranieri M, Quintel M, Russo SG, Cornejo R, Bugedo G, Carlesso E, Russo R, Caspani L, Gattinoni L: Lung opening and closing during ventilation of acute respiratory distress syndrome. Am J Respir Crit Care Med 2010, 181: 578-586. 10.1164/rccm.200905-0787OC
Mead J, Takishima T, Leith D: Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 1970, 28: 596-608.
Muscedere JG, Mullen JB, Gan K, Slutsky AS: Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 1994, 149: 1327-1334. 10.1164/ajrccm.149.5.8173774
Grasso S, Stripoli T, Sacchi M, Trerotoli P, Staffieri F, Franchini D, De Monte V, Valentini V, Pugliese P, Crovace A, Driessen B, Fiore T: Inhomogeneity of lung parenchyma during the open lung strategy: a computed tomography scan study. Am J Respir Crit Care Med 2009, 180: 415-423. 10.1164/rccm.200901-0156OC
Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, Davies AR, Hand LE, Zhou Q, Thabane L, Austin P, Lapinsky S, Baxter A, Russell J, Skrobik Y, Ronco JJ, Stewart TE: Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008, 299: 637-645. 10.1001/jama.299.6.637
Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, Lefrant JY, Prat G, Richecoeur J, Nieszkowska A, Gervais C, Baudot J, Bouadma L, Brochard L: Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008, 299: 646-655. 10.1001/jama.299.6.646
Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT: Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004, 351: 327-336.
Dantzker DR, Wagner PD, West JB: Proceedings: Instability of poorly ventilated lung units during oxygen breathing. J Physiol 1974, 242: 72P.
Rothen HU, Sporre B, Engberg G, Wegenius G, Reber A, Hedenstierna G: Prevention of atelectasis during general anaesthesia. Lancet 1995, 345: 1387-1391. 10.1016/S0140-6736(95)92595-3
Santos C, Ferrer M, Roca J, Torres A, Hernandez C, Rodriguez-Roisin R: Pulmonary gas exchange response to oxygen breathing in acute lung injury. Am J Respir Crit Care Med 2000, 161: 26-31. 10.1164/ajrccm.161.1.9902084
Suter PM, Fairley HB, Schlobohm RM: Shunt, lung volume and perfusion during short periods of ventilation with oxygen. Anesthesiology 1975, 43: 617-627. 10.1097/00000542-197512000-00003
Aboab J, Jonson B, Kouatchet A, Taille S, Niklason L, Brochard L: Effect of inspired oxygen fraction on alveolar derecruitment in acute respiratory distress syndrome. Intensive Care Med 2006, 32: 1979-1986. 10.1007/s00134-006-0382-4
Fessler HE: Heart-lung interactions: applications in the critically ill. Eur Respir J 1997, 10: 226-237. 10.1183/09031936.97.10010226
Jardin F: Acute leftward septal shift by lung recruitment maneuver. Intensive Care Med 2005, 31: 1148-1149. 10.1007/s00134-005-2733-y
Jardin F, Vieillard-Baron A: Monitoring of right-sided heart function. Curr Opin Crit Care 2005, 11: 271-279. 10.1097/01.ccx.0000158847.56107.55
Feihl F, Eckert P, Brimioulle S, Jacobs O, Schaller MD, Melot C, Naeije R: Permissive hypercapnia impairs pulmonary gas exchange in the acute respiratory distress syndrome. Am J Respir Crit Care Med 2000, 162: 209-215. 10.1164/ajrccm.162.1.9907119
Feihl F, Perret C: Permissive hypercapnia. How permissive should we be? Am J Respir Crit Care Med 1994, 150: 1722-1737. 10.1164/ajrccm.150.6.7952641
Frank JA, Gutierrez JA, Jones KD, Allen L, Dobbs L, Matthay MA: Low tidal volume reduces epithelial and endothelial injury in acid-injured rat lungs. Am J Respir Crit Care Med 2002, 165: 242-249. 10.1164/ajrccm.165.2.2108087
Hager DN, Krishnan JA, Hayden DL, Brower RG: Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med 2005, 172: 1241-1245. 10.1164/rccm.200501-048CP
Terragni PP, Del Sorbo L, Mascia L, Urbino R, Martin EL, Birocco A, Faggiano C, Quintel M, Gattinoni L, Ranieri VM: Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology 2009, 111: 826-835. 10.1097/ALN.0b013e3181b764d2
Kumpers P, Nickel N, Lukasz A, Golpon H, Westerkamp V, Olsson KM, Jonigk D, Maegel L, Bockmeyer CL, David S, Hoeper MM: Circulating angiopoietins in idiopathic pulmonary arterial hypertension. Eur Heart J 2010, 31: 2291-2300. 10.1093/eurheartj/ehq226
Zabrocki LA, Brogan TV, Statler KD, Poss WB, Rollins MD, Bratton SL: Extracorporeal membrane oxygenation for pediatric respiratory failure: survival and predictors of mortality. Critical Care Med 2011, 39: 364-370. 10.1097/CCM.0b013e3181fb7b35
Nielsen ND, Kjaergaard B, Koefoed-Nielsen J, Steensen CO, Larsson A: Apneic oxygenation combined with extracorporeal arteriovenous carbon dioxide removal provides sufficient gas exchange in experimental lung injury. ASAIO J 2008, 54: 401-405. 10.1097/MAT.0b013e31817e2b5f
Somaschini M, Bellan C, Locatelli G, Glauber M, Colombo A: Extracorporeal membrane oxygenation with veno-venous bypass and apneic oxygenation for treatment of severe neonatal respiratory failure. Int J Artif Organs 1995, 18: 574-578.
Serpa Neto A, Cardoso SO, Manetta JA, Pereira VG, Esposito DC, Pasqualucci Mde O, Damasceno MC, Schultz MJ: Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA 2012, 308: 1651-1659. 10.1001/jama.2012.13730
Dembinski R, Hochhausen N, Terbeck S, Uhlig S, Dassow C, Schneider M, Schachtrupp A, Henzler D, Rossaint R, Kuhlen R: Pumpless extracorporeal lung assist for protective mechanical ventilation in experimental lung injury. Crit Care Med 2007, 35: 2359-2366. 10.1097/01.CCM.0000281857.87354.A5
Hormann C, Baum M, Putensen C, Mutz NJ, Benzer H: Biphasic positive airway pressure (BIPAP) – a new mode of ventilatory support. Eur J Anaesthesiol 1994, 11: 37-42.
Yoshida T, Rinka H, Kaji A, Yoshimoto A, Arimoto H, Miyaichi T, Kan M: The impact of spontaneous ventilation on distribution of lung aeration in patients with acute respiratory distress syndrome: airway pressure release ventilation versus pressure support ventilation. Anesth Analg 2009, 109: 1892-1900. 10.1213/ANE.0b013e3181bbd918
Hering R, Zinserling J, Wrigge H, Varelmann D, Berg A, Kreyer S, Putensen C: Effects of spontaneous breathing during airway pressure release ventilation on respiratory work and muscle blood flow in experimental lung injury. Chest 2005, 128: 2991-2998. 10.1378/chest.128.4.2991
Putensen C, Mutz NJ, Putensen-Himmer G, Zinserling J: Spontaneous breathing during ventilatory support improves ventilation–perfusion distributions in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 1999, 159: 1241-1248. 10.1164/ajrccm.159.4.9806077
EOLIA Trial [http://www.clinicaltrials.gov/ct2/show/NCT01470703?term=EOLIA+ECMO&rank=1]
Combes A: Extracorporeal membrane oxygenation (ECMO) for severe acute respiratory distress syndrome (ARDS). The EOLIA (ECMO to rescue Lung Injury in severe ARDS) trial: a multicenter, international, randomized, controlled open trial. Reanimation 2011, 20: 49-61.
Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB: Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 2008, 358: 1327-1335. 10.1056/NEJMoa070447
Bein T, Osborn E, Hofmann HS, Zimmermann M, Philipp A, Schlitt HJ, Graf BM: Successful treatment of a severely injured soldier from Afghanistan with pumpless extracorporeal lung assist and neurally adjusted ventilatory support. Int J Emerg Med 2010, 3: 177-179. 10.1007/s12245-010-0192-x
Mauri T, Bellani G, Grasselli G, Confalonieri A, Rona R, Patroniti N, Pesenti A: Patient-ventilator interaction in ARDS patients with extremely low compliance undergoing ECMO: a novel approach based on diaphragm electrical activity. Intensive Care Med 2013, 39: 282-291. 10.1007/s00134-012-2755-1
Karagiannidis C, Lubnow M, Philipp A, Riegger GA, Schmid C, Pfeifer M, Mueller T: Autoregulation of ventilation with neurally adjusted ventilatory assist on extracorporeal lung support. Intensive Care Med 2010, 36: 2038-2044. 10.1007/s00134-010-1982-6
Del Sorbo L, Ranieri VM, Keshavjee S: Extracorporeal membrane oxygenation as ‘bridge’ to lung transplantation: what remains in order to make it standard of care? Am J Respir Crit Care Med 2012, 185: 699-701. 10.1164/rccm.201202-0193ED
Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL: Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006, 354: 2564-2575.
Patroniti N, Zangrillo A, Pappalardo F, Peris A, Cianchi G, Braschi A, Iotti GA, Arcadipane A, Panarello G, Ranieri VM, Terragni P, Antonelli M, Gattinoni L, Oleari F, Pesenti A: The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med 2011, 37: 1447-1457. 10.1007/s00134-011-2301-6
Lang G, Taghavi S, Aigner C, Renyi-Vamos F, Jaksch P, Augustin V, Nagayama K, Ghanim B, Klepetko W: Primary lung transplantation after bridge with extracorporeal membrane oxygenation: a plea for a shift in our paradigms for indications. Transplantation 2012, 93: 729-736. 10.1097/TP.0b013e318246f8e1
Mason DP, Thuita L, Nowicki ER, Murthy SC, Pettersson GB, Blackstone EH: Should lung transplantation be performed for patients on mechanical respiratory support? The US experience. J Thorac Cardiovasc Surg 2010, 139: 765-773. e1 10.1016/j.jtcvs.2009.09.031
Olsson KM, Simon A, Strueber M, Hadem J, Wiesner O, Gottlieb J, Fuehner T, Fischer S, Warnecke G, Kuhn C, Haverich A, Welte T, Hoeper MM: Extracorporeal membrane oxygenation in nonintubated patients as bridge to lung transplantation. Am J Transplant 2010, 10: 2173-2178. 10.1111/j.1600-6143.2010.03192.x
Wiesner O, Hadem J, Sommer W, Kuhn C, Welte T, Hoeper MM: Extracorporeal membrane oxygenation in a nonintubated patient with acute respiratory distress syndrome. Eur Respir J 2012, 40: 1296-1298. 10.1183/09031936.00076912
Hoeper MM, Wiesner O, Hadem J, Wahl O, Suhling H, Duesberg C, Sommer W, Warnecke G, Greer M, Boenisch O, Busch M, Kielstein JT, Schneider A, Haverich A, Welte T, Kuhn C: Extracorporeal membrane oxygenation instead of invasive mechanical ventilation in patients with acute respiratory distress syndrome. Intensive Care Med 2013, 39: 2056-2057. 10.1007/s00134-013-3052-3
Camporota L, Smith J, Barrett N, Beale R: Assessment of regional lung mechanics with electrical impedance tomography can determine the requirement for ECMO in patients with severe ARDS. Intensive Care Med 2012, 38: 2086-2087. 10.1007/s00134-012-2701-2
Grasso S, Terragni P, Birocco A, Urbino R, Del Sorbo L, Filippini C, Mascia L, Pesenti A, Zangrillo A, Gattinoni L, Ranieri VM: ECMO criteria for influenza A (H1N1)-associated ARDS: role of transpulmonary pressure. Intensive Care Med 2012, 38: 395-403. 10.1007/s00134-012-2490-7
Acknowledgements
MS was supported by the French Intensive Care Society (SRLF), the « Fonds de dotation Recherche en Santé Respiratoire, 2012 », the « Collège des Enseignants de Réanimation Médicale » and the « Fonds d’Etude et de Recherche du Corps Médical des Hôpitaux de Paris ».
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
AC is the primary investigator of the EOLIA trial, NCT01470703, a randomized trial of VV-ECMO supported in part by MAQUET. AC has received honoraria for lectures from MAQUET. The remaining authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Schmidt, M., Pellegrino, V., Combes, A. et al. Mechanical ventilation during extracorporeal membrane oxygenation. Crit Care 18, 203 (2014). https://doi.org/10.1186/cc13702
Published:
DOI: https://doi.org/10.1186/cc13702