Abstract
Cells and tissues display a remarkable range of plasticity and tissue-patterning activities that are emergent of complex signaling dynamics within their microenvironments. These properties, which when operating normally guide embryogenesis and regeneration, become highly disordered in diseases such as cancer. While morphogens and other molecular factors help determine the shapes of tissues and their patterned cellular organization, the parallel contributions of biophysical control mechanisms must be considered to accurately predict and model important processes such as growth, maturation, injury, repair, and senescence. We now know that mechanical, optical, electric, and electromagnetic signals are integral to cellular plasticity and tissue patterning. Because biophysical modalities underly interactions between cells and their extracellular matrices, including cell cycle, metabolism, migration, and differentiation, their applications as tuning dials for regenerative and anti-cancer therapies are being rapidly exploited. Despite this, the importance of cellular communication through biophysical signaling remains disproportionately underrepresented in the literature. Here, we provide a review of biophysical signaling modalities and known mechanisms that initiate, modulate, or inhibit plasticity and tissue patterning in models of regeneration and cancer. We also discuss current approaches in biomedical engineering that harness biophysical control mechanisms to model, characterize, diagnose, and treat disease states.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organisms achieve and maintain multicellularity by promoting cooperation and mediating conflict within groups of cells, thus prioritizing the collective over its units [1]. How do multicellular systems determine which individual cells should proliferate, specialize, or die in service of the group? This challenge is best illustrated by ontogenetic development, which involves the transition from a single cell to a unified organism comprising billions or trillions of cells. Embryonic cells must display sufficient plasticity to generate, prune, and remodel dozens of specialized tissues during morphogenesis before suppressing these same mechanisms to achieve stable, long-term maintenance of form [2]. However, even the cells of mature organisms retain the potential to re-active latent plasticity and patterning programs to repair or regenerate damaged tissues while suppressing spontaneous and disordered growth including cancers [3,4,5]. Indeed, cancer and regeneration are related physiological processes with similar levels of plasticity and markedly different capacities to pattern cells into cooperative tissue structures [6,7,8]. While biomolecular controls of plasticity and patterning are frequently discussed in the literature in the context of cellular communication, less attention has been afforded to biophysical controls including mechanical, electrical, magnetic, and optical signals. Here, we provide a review of known biophysical control mechanisms of tissue plasticity and patterning with a focus on regeneration and cancer as representative model systems. We examine mechanical, optical, electrical, and magnetic signaling modalities as parallel communication channels within tissue microenvironments and their roles as determinants of cell state and fate.
Tissue plasticity
Plasticity is a property of cells that enables phenotypic changes without genetic mutation and is typically activated as a response to signals and cues within tissue microenvironments [4, 9, 10]. Embryonic organisms display significant plasticity, enabling groups of cells to reconfigure bodies with segments, compartments, layers, topologies, and pigmentation patterns. Unlike humans, organisms such as axolotls can re-activate latent developmental pathways to regrow appendages and re-pattern disorganized tissues [11]. This remarkable state of tissue plasticity is termed “regeneration”. To replace lost or damaged cells, a pool of progenitors must be amassed, which may involve altering the epigenetic state of resident cells [12]. These progenitors, while initially possessing a relatively high degree of plasticity, must then cease proliferation and differentiate into the required somatic cell types for tissue replacement. Cellular plasticity is, therefore, an important element of regenerative success [13]. Despite retaining the same genes that regulate development throughout the lifecycle, only a limited set of mature human tissues retain their intrinsic plasticity, including adipose tissue, connective tissue, and to a much lesser extent, neural tissues [14, 15]. Consequently, humans suffer significantly and often permanently from lacerations, burns, limb loss, degenerative diseases, and other morbidities.
When cellular plasticity subverts key safeguards and becomes unrestrained, as is the case with cancer, tissue architecture can become irreversibly unstable as cells proliferate uncontrollably, invade foreign tissues, and contribute to widespread dysfunction and increased mortality [16]. The suppression of pro-regenerative pathways may have been selected due to their physiological overlap with tumorigenesis [17, 18], which threatens stable multicellularity. Indeed, maintaining low levels of plasticity would greatly favor the long-term structural stability of the organism’s cellular collective over the immortality of individual cancer cells [19]. Interestingly, when cancer cells are introduced into the microenvironments of embryonic or regenerating tissues, they become assimilated and differentiate into stable, somatic cells, suggesting that the microenvironment contains key regulatory signals that control plasticity [20,21,22]. What types of signals cause multicellular systems to “switch” their plasticity toward embryonic, regenerative, and carcinogenic states (Fig. 1)? Understanding how plasticity is activated or suppressed is integral to the development of regenerative therapies with significant clinical applications for dementia, stroke, spinal cord repair, heart disease, and limb loss.
Tissue states differ across dimensions of plasticity and patterning. A two-dimensional model of tissue state with low or high levels of plasticity and patterning accommodates tumor formation and response to injury. Normal, healthy tissues display high degrees of patterning with low levels of plasticity. As patterning decreases, tissues become disorganized with benign growths that can become malignant and metastatic as plasticity increases. Similarly, following an injury, tissue expressing high degrees of plasticity and patterning can re-establish pre-injury phenotypes; however, if these processes are not sufficiently promoted, wounds heal imperfectly with indelible fibrotic scar tissue.
Tissue patterning
Tissue patterning involves the integration of repeatable elements within the structure of an organism, including pigmentation and body segmentation. Originally predicted by Turing [23], the discovery that spatial patterning in biochemical systems including tissues can spontaneously emerge from the diffusion of molecules and related interactions was a paradigm-shifting achievement in the life sciences. Indeed, we now know that living systems derive their unparalleled complexity from simple, repeatable events at the sub-cellular level [24, 25], forming repeatable segments, internal compartments, and unique topologies. However, without control mechanisms with which to guide patterning, multicellularity can suffer from inadequate coordination and ultimately collapse [26, 27]. Fortunately, cells and their combinatory structures provide all the necessary conditions to initiate and maintain stable patterning at multiple scales. They generate internal and external gradients, isolate electrochemical reactions within internal compartments, sense microenvironmental cues, self-destruct upon losing control of their basic functions, and communicate with each other using a suite of signals. Indeed, communication is central to multicellular life [28, 29], enabling the continuous flow of information between cells and within changing tissue microenvironments to orchestrate local and long-range tissue dynamics, including plasticity and patterning. While molecular signals including morphogens, hormones, and neurotransmitters are clear determinants of cell fate and behavior toward tissue patterning in both regeneration and cancer (e.g., Notch, Wingless-related integration site (WNT), etc.) [30], mounting evidence suggests that biophysical signals serve as equally important top-down regulators of tissue patterning that can interact and synergize with molecular pathways [31,32,33,34]. For example, gene expression can be induced by physically deforming cells such as fibroblasts and cardiomyocytes, which can in turn alter their structure and the biochemical composition of the surrounding microenvironment, thus modifying the impact of subsequent physical stimuli on the tissue, its cell population, and the surrounding extracellular matrix (ECM) [35].
Regeneration and cancer
Cancer is defined by its highly plastic state and disorganized patterning, imperfectly mirroring normal morphogenetic processes [36,37,38]. Some authors have suggested that cancer is fundamentally a developmental disorder characterized by a failure to suppress latent plasticity and maintain stable tissue patterns [39, 40]. The parallels between cellular processes in regeneration and cancer have long been recognized [6, 7] and the two states share many common signaling mechanisms [8]. Many of the mechanisms essential to drive the cellular plasticity required for regeneration are co-opted to promote neoplasia and cancer progression. For example, the microRNA miR-21 mediates downregulation of growth-suppressing proteins in healing tissues in the mammalian brain, skin, and liver, as well as limb and kidney regeneration in fish and salamanders [41]. It is also associated with neoplasia, permitting unchecked cell proliferation [42], and is elevated in heterogeneous tumors which are associated with reduced patient survival rate [43]. Another example is in the regulation of cell turnover in intestinal epithelium where, subsequent to injury, various intestinal cell populations are reprogrammed through mechanisms such as ECM protein-mediated yes-associated protein/transcriptional coactivator with PDZ-binding motif (YAP/TAZ) activation; however, chronic activation and promotion of this plasticity can lead to neoplasia [44]. This overlap in signaling pathways between regeneration and cancer extends to many biophysical processes involving regulation of plasticity, which is discussed in later sections.
Biophysical controls
In addition to biochemical signaling and molecular pathways, there are at least three biophysical modalities that allow cells to share information with each other and their surrounding microenvironments: biomechanical, bioelectrical, and bioelectromagnetic [45,46,47] (Table 1). These signaling phenomena offer several advantages over their chemical equivalents. First, they often rely on wave propagation through a medium, which increases signaling speed relative to the diffusion or active transport of molecules [48]. Second, biophysical signals are less dependent on proximity or localized interfaces; instead, they can travel longer distances (tissue to tissue), often without specialized conduits [49, 50]. Third, unlike typical ligand–receptor interactions, biophysical signals have spectral properties that increase communicative degrees of freedom [51]. Lastly, biophysical quanta may interact with multiple target structures (Fig. 2) simultaneously rather than sequentially binding and dissociating at receptor sites. To expand on the individual properties of different signals, here, we review each modality as a unique control mechanism underlying plasticity and patterning in regeneration and cancer.
Biophysical modalities, transduction mechanisms, and effects. Photonic, mechanical, electric, and electromagnetic modalities constitute the major biophysical control mechanisms of cellular communication. Each modality is governed by organelles, molecules, and specialized interactions that transduce physical energies into electrochemical signals and their downstream cellular correlates. The effects of biophysical controls on tissue patterning and plasticity include the cell proliferation, migration, and differentiation toward the formation of polar tissues with intrinsic gradients and complex morphologies that contribute to carcinogenesis and regeneration
Biomechanical controls
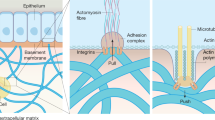
The ability for a tissue or organ to regenerate is dependent on many factors present in the post-injury microenvironment, including biomechanical properties of the ECM such as stress, strain, shear flow, stiffness, and topographical cues [52]. In mammals, the default response to injury is scarring or fibrosis, with the newly deposited ECM structures presenting a significant barrier to regeneration [53]. Fibrotic tissues increase the stiffness of the microenvironment, which disrupts cellular polarity, inhibits regeneration, and promotes malignancy [54]. Indeed, scars are ideal substrates to initiate tumor growth as stiff ECM induces angiogenesis, promotes hypoxia, and inhibits anti-tumor immunity [54]. Non-mammalian species such as axolotls, however, can mitigate these barriers by upregulating matrix metalloproteinases (MMPs), which remodel the otherwise inhospitable ECM, and by initiating signaling pathways to help direct cells to dedifferentiate, migrate, and replace damaged or lost tissues [55]. Thus, the ECM provides biophysical cues to regulate cellular phenotypes and plasticity in several ways from mechanoreception to integrin signaling, ligand binding, biochemical cues, and more [56,57,58]. Cells can sense mechanical cues via surface receptors such as integrins that adhere to ECM ligands and transmit signals through cytoskeletal elements, facilitating transduction of biomechanical signals into downstream molecular activation. Once transduced, mechanical signals propagate through cytoskeletal filaments and culminate at the nuclear membrane, resulting in changes in histone methylation and thus changes to chromatin architecture and the epigenetic state of a cell [59].
ECM composition and adhesion
ECM composition refers to the molecular network of proteins and proteoglycans that make up the natural scaffold of the tissue microenvironment. Modification of the ECM composition as part of the injury response can either promote or inhibit regeneration. Cells interact with surrounding ECM components through various surface receptors leading to activation of downstream intracellular pathways. Following injury in regeneration-competent species such as zebrafish, mechanical waves across tissues signal the position of wounds [60] and pro-regenerative proteins such as laminin and fibronectin are upregulated whereas collagen IV, a major component of fibrotic scars, is downregulated [61]. This contrasts with non-regenerative species, including humans, that experience scarring following injury, characterized by the differentiation of fibroblasts into ECM-synthesizing myofibroblasts [62]. Modulation of ECM protein composition can both maintain a desired cell phenotype (e.g., chondrocytes cultured on decellularized cartilage ECM) [63], and induce dedifferentiation (e.g., secretion of fibronectin, collagens, and hyaluronic acid by cardiac fibroblasts to promote dedifferentiation of cardiomyocytes after injury in regenerative-competent species) [64]. The ECM composition can also change with age; the composition of the cartilage matrix of the rabbit ear changes over time and corresponds to a loss of morphological plasticity in older animals when compared with the cartilage of immature animals [65].
The presence of excessive collagen and fibrinogen changes the composition of the injury microenvironment which elicits important cellular responses including alterations in plasticity and phenotype. For example, following injury to the liver, manipulating the microenvironment to decrease laminin and total ECM concentration leads to increased hepatocyte differentiation to promote liver regeneration [66]. Although plasticity is often thought of as desirable during regeneration to promote processes such as dedifferentiation, epithelial to mesenchymal transition (EMT), and redifferentiation, increasing plasticity can also lead to loss of regenerative ability. In joints, injury or osteoarthritis results in degradation of cartilage including loss of collagen, aggrecan, and other proteoglycans that in turn modulates the plasticity of chondrocytes, promoting their dedifferentiation into a chronic fibrogenic phenotype [67]. Indeed, cell plasticity can become self-limiting if induced differentiation promotes terminal or otherwise anti-regenerative phenotypes. Interestingly, in Duchenne muscular dystrophy, it has been observed that, through a transforming growth factor (TGF)β-dependent pathway, dystrophic muscle cells increase in plasticity with aging to adopt a multipotent, fibrogenic fate [68]. These cells then contribute to the decline in the ability of muscle to regenerate associated with the disease progression, demonstrating that increased cellular plasticity is not always linked to a pro-regenerative state [68]. Understanding the complex relationship between ECM composition and plasticity of important cell types can help develop targeted strategies for manipulation of the ECM in a way that will promote the desired plasticity (or perhaps suppress unwanted plasticity) to achieve regeneration. However, without effective tissue patterning, plasticity is a morphologically aimless process that may not always benefit the organism.
As with normal cell types, tumor cells interact with and are regulated by the surrounding tissues, cells, and ECM, which are collectively known as the tumor microenvironment (TME) [69]. The physical and biomechanical properties of the TME can alter tumor behavior, and tumor cells in turn alter the TME to promote their survival and cancer progression. Mesenchymal stromal cells can alter the stiffness and molecular composition of the TME by secreting cytokines like vascular epithelial growth factor (VEGF) to stimulate metastasis and migration [70]. This suggests that much like how regenerating cells can modulate their environment to promote growth, tumors respond to factors in the TME and alter their properties to promote cancer progression.
The secondary microenvironment of colonized metastatic sites represents another important feature of cancer that is influenced by biophysical properties. It has long been noted that certain cancers are more likely to metastasize to specific organs and that this may be related to physical bottlenecks like anatomical proximity and circulation patterns [71, 72]. In addition, much like in the primary tumor site, cancer cells shape the biophysical properties of the secondary site into a pro-cancer niche that can promote survival and colonization of metastatic cells and overall cancer progression [73]. Interestingly, some cancer cells can send long-distance signals (tissue level, centimeter scale) to macrophages in the secondary site that will favorably alter the ECM microenvironment to prime it for arrival of invading cancer cells [74].
In contrast to their normal counterparts, cancer cells can abnormally alter the TME to promote their survival and progression [75, 76]. Tumor cells drive cancer-associated fibroblasts (CAFs) to alter the ECM via TGF-β, Notch1, and WNT pathways, and crosstalk between these cells and the ECM generates tumor heterogeneity [77]. Cancer cells themselves can also directly interact with the surrounding ECM, exerting traction forces that have been demonstrated to generate dense collagen bundles between cells in a non-transient fashion, such that the bundles remain even after the traction is gone [78]. Furthermore, the tumor ECM is typically described as more aligned than normal tissue counterparts, where protein polymers display marked group polarity [79]. Through aberrant signaling and mechanical forces in CAFs and the cancer cells themselves, the ECM is remodeled to produce parallel organization of the stromal ECM fibrils [78]. This increase in alignment is believed to play an important role in cancer EMT [80].
ECM stiffness, stress, and topography
One major difference between scar tissue and regenerative-competent tissue is ECM stiffness, where scars are defined by fibrotic regions of disorganized, rigid collagen polymers [81]. Increased ECM stiffness following injury is cited as a central factor in driving scar formation over regeneration; for example, artificially increasing the load on mouse skin increases fibrosis through a focal adhesion kinase (FAK)-dependent mechanism [82]. Stiffness of the ECM translates into changes in the cell by modulating cellular adhesion and cytoskeletal tension, which in turn influences mechanosensitive ion channels [83]. Integrin-mediated mechanotransduction of stiffness cues results in phosphorylation of transcriptional regulators YAP and TAZ which translocate to the nucleus in response to increasing stiffness [84].
The stiffness of the ECM can affect the plasticity of many cell types via mechanoreceptor-mediated mechanisms. Regulation of stemness has been linked to changes in actin force [85]. For example, softening the culture substrate in vitro results in reprogramming of various stable cell lines via actin and tension-dependent upregulation of stemness-associated genes like Oct4 and Nanog5. The effect of matrix stiffness on cell plasticity in vitro has been extensively reported (10,37). Mesenchymal stem cells (MSCs) and other cell types can be directed into neurogenic, myogenic, or osteogenic lineages in vitro in the absence of soluble reprogramming factors simply by modulating ECM stiffness [86]. In MSCs, this modulation was found to be reliant on tropomyosin (TPM)1, a mechanosensitive differentiation regulator [87]. Furthermore, MSCs can undergo chromatin remodeling to upregulate pluripotency genes like Oct4, Nanog, and Sox2 in response to low-stiffness media (1.5–15 kPa) [85]. In vitro, mouse vascular smooth muscle cells can modulate their plasticity by switching between a dedifferentiated proliferative state, or a terminally differentiated contractile state depending on the stiffness of the ECM (low or high respectively) in a mechanoreceptor-mediated Rac/Rho-YAP/TAZ mechanism [88], and increased ECM stiffness upregulates RhoA and YAP/TAZ to inhibition expression of pro-regenerative markers in peripheral Schwann cells [89].
Available evidence of stiffness as a modulator of cellular plasticity in vivo is more limited. The African spiny mouse is a valuable model for its unique scar-free dermal regeneration [90]. Interestingly, their skin lacks α-smooth muscle actin, and is reported to be 20 times weaker than that of Mus musculus [90]. This reduced stiffness of the ECM is thought to be more permissive to scar-free healing, reminiscent of fetal mouse dermal healing. However, to date it is unknown if this example of low stiffness and scar-free healing is linked to regulation of cellular plasticity. Regeneration of zebrafish fins is partially dependent on viscous sheer stress and the resulting internal tension of the fin, which provides signals for guiding fin regeneration after amputation [91]. These natural examples of the control of regeneration through ECM stiffness and other biomechanical cues offer clues as to how we might bioengineer the tissue microenvironments of non-regenerative species to promote regeneration.
In tumors, the TME possesses elevated interstitial fluid pressure (IFP) due to the presence of leaky vasculature and deposition of excess ECM proteins causing local retention of fluid [92, 93]. This increased IFP leads to elevated shear stress acting on TME cells to drive a multitude of plasticity-related downstream changes, including induction of EMT. Research using several cancer cell lines has demonstrated that EMT is elevated in response to elevated shear stress [94, 95] with evidence in MCF7 breast cancer cells that it can also promote a cancer stem cell (CSC)-like phenotype [96]. Shear stress is not only a factor in the TME but also during cancer metastasis. A study by Cognart et al. [97] modeled the effect of shear stress on breast CSCs as they circulated in the vasculature during metastasis, finding that the inclusion of circulation induced significant changes in gene expression, particularly EMT markers [97].
Osteosarcoma cells have been observed to respond to ECM stiffness via an integrin-mediated FAK signaling pathway [63, 70], which promotes migration/invasion and angiogenesis [98]. In addition, high-stiffness ECM can drive the EMT, cell invasion, and metastasis in breast cancer cells by promoting ligand-independent phosphorylation of EphA2, which recruits and activates Lyn kinase, resulting in phosphorylation of Twist1, a pro-EMT protein [99].
In one of the most well-studied examples of the role of matrix biophysical properties in cancer, ECM stiffness has been found to play a role in the onset of neoplasia. Cancers can arise when cells lose their ability to sense the rigidity of their local ECM via interference with mechanoreception pathways, such that cancer cells become incapable of responding to mechanosensory growth inhibition [100]. When expression of cytoskeletal components such as tropomyosin is induced in cancer cells, rigidity sensing is restored and growth is inhibited [101]. Conversely, inhibiting other components of the rigidity-sensing complex promotes transformed growth of cells into neoplasia [102]. Understanding the cues that trigger this change in phenotype and transformation to a more plastic state may contribute to the development of therapeutics that can target these pathways to inhibit neoplasia at an early stage.
Bioelectric controls
Cell membranes are semi-permeable boundaries across which physiological ions are transported to generate chemical energy [103], activate secondary messengers [104], initiate transcription [105], mobilize cytoskeletal changes [106], induce mitosis [107], as well as conduct local and long-range signaling [108]. While membrane potential (Vmem) is most often associated with excitable cells such as neurons in the brain and cardiomyocytes in the heart, electrical signaling is a generalized feature of most cells [109]. Indeed, cellular respiration by mitochondria is effectively a bioelectric phenomenon as the canonical reactions are contingent upon the activities of ion channels and pumps [103, 110]. Oogenesis, cell growth, and proliferation are all associated with sharp influxes of calcium ions [111], which, in addition to their myriad chemical interactions, carry considerable positive charge and the ability to rapidly depolarize membranes, which are typically hyperpolarized at rest. Because calcium is also a well-known second messenger that promotes the expression of morphogens (e.g., Nodal, sonic hedgehog (SHH), bone morphogenic protein (BMP), TGF-β) [112], and disruptions of bioelectric networks have been linked to impaired body planning, it is worth considering the role of bioelectricity in tissue plasticity and patterning in the context of regeneration [113]. Similarly, the bioelectric states of cancer cells and the tumors they form differ markedly from healthy, somatic counterparts [114]; however, they share significant overlap with those of embryonic and regenerating tissues. Hallmarks include significantly depolarized Vmem, electrical isolation from local cell populations, and aberrant ion channel profiles [115], which can drive gene expression, inhibit apoptosis, and elevate a dysregulated plasticity, favoring a tumorigenic state.
Membrane potential
The functional role of Vmem has been a subject of intense debate in several subfields of biology. One major area of contention is the classification of Vmem as either an active participant in cell and tissue dynamics or as an epiphenomenon with little physiological relevance beyond representing a reporter of cell states. Examining the range of resting potentials associated with most cell types (− 10 to − 90 mV), it is clear that Vmem predicts proliferation and differentiation potential [114]. For example, somatic cells are generally hyperpolarized at rest (− 50 to − 90 mV) with transient depolarizations and re-polarization events. Among somatic cells, the most non-proliferative and terminally differentiated cells display the greatest membrane polarity, such as cardiomyocytes (− 90 mV) and neurons (− 70 mV)—these tissues are also the most resistant to regeneration and are often post-mitotic [114]. However, highly regenerative liver cells and stem cells as well as cancer cells generally display depolarized Vmem at rest (0 to − 50 mV) [114]. During malignant transformation and proliferation, Vmem depolarizes significantly [116]. On the bases of these observations alone, it is difficult to determine the possibility of cause-and-effect relationships. However, systematic manipulations of Vmem have been used to biophysically control cell function. Indeed, mitosis can be inhibited by altering the extracellular medium and forcibly hyperpolarizing cells [117]. Interestingly, otherwise terminally differentiated mature neurons can be induced to divide under chronic depolarizing conditions [118]. While neurons regularly undergo acute depolarization events over a few milliseconds termed “action potentials” (which are also accompanied by mechanical waves) [119], only chronic depolarization generates the mitotic phenotype. Stem cells are also known to alter their secretions, shapes, and migration trajectories when activated by bioelectric signals [117, 120, 121]. Notably, the types of channels that drive Vmem fluctuations are relevant to biological outcomes due to their unique association with specific ionic currents as well as their densities and intrinsic time constants. A cell’s complex “ion channel profile” is, therefore, critical to its function.
The resting Vmem of proliferative cells, including cancer cells, is significantly depolarized relative to differentiated cells [114]. Quantitatively, the discriminant threshold between these two populations is approximately − 36 mV [122]; however, cancer cells display even greater depolarizations than their non-cancerous, proliferative counterparts [114, 122]. This is consistent with the observations that stem cell differentiation is dependent on hyperpolarization and inhibited by depolarization [123]. As was described in a comprehensive review by Yang and Brackenbury [116], depolarized resting Vmem in cancer is maintained by ion channel profiles that elevate, among other parameters, intracellular Na+ rather than K+, ultimately favoring cell proliferation and migration over differentiation. Interestingly, facilitating anion transport in cancer stem cells hyperpolarizes Vmem, triggers differentiation, and promotes cell death [124]. However, Vmem is not static in cancer cells and transient hyperpolarization events may be necessary to initiate cell cycle progression [125, 126]. Finally, because tumors are characterized by disorganized cytoarchitectures that often lack polarity, it is likely that meso-scale bioelectric networks are significantly disrupted in addition to abnormalities within individual cells. Dynamic, multi-scale models of bioelectricity are needed to parse these contributions, inform experimentation, and guide clinical applications.
Ion channel profiles
Ion channels define the bioelectric states of cells. Several stimuli and environmental conditions can trigger ionic current flow including electrochemical gradients (e.g., potassium leak channels), Vmem fluctuations (e.g., voltage-gated calcium channels), molecular ligands [e.g., ionotropic channels such as N-methyl-D-aspartate receptors (NMDARs)], biomechanical signals (e.g., piezo channels), proton concentrations (e.g., acid-sensing ion channels or ASICs), photons (e.g., opsins), and other energy sources [127]. Variation of ion channel profiles—the unique combinations of ion channels embedded within the membranes of distinct cell types—can significantly impact cell fate and behavior [128]. For example, in response to experimentally lesioning rat brains, astrocytes lacking inward-rectifying potassium (K+ Kir) channels were induced to proliferate, forming scar tissues that impeded regenerative potential [129]; however, astrocytes that could re-polarize their membranes did not respond similarly. Indeed, ion channel profiles may play a critical role in lineage reprogramming [130].
Several ion channel-dependent mechanisms of proliferation, migration, and suppressed differentiation in cancer cells have been described in the literature [131, 132]. Blocking several types of Na+, K+, and Cl− channels can modulate the cancer state [20, 115]. In many cases, calcium (Ca2+) influx represents a convergent point for signaling [133]. Inward-rectifying (e.g., Kir) and depolarizing channels represent an important dimension of this dynamic [134]. As Vmem shifts toward a depolarized state, the resting potential approaches the activation threshold for voltage-gated calcium channels, which can trigger cell migration, cell cycle progression, and proliferation. Paradoxically, hyperpolarizing channels can generate favorable electrochemical gradients for Ca2+ influx with the same net effect as direct depolarization. However, forced expression of hyperpolarizing ion channels can significantly reduce oncogene-induced tumorigenesis [20, 115]. Overexpression of Kir 4.1 channels in gliomas, which are normally absent in tumor cells of glial origin, hyperpolarize Vmem, suppress growth, and promote differentiation into somatic cells [135]. Genes regulating differentiation are responsive to Vmem fluctuations and depolarization may serve to maintain cells in an undifferentiated state [136], which can be ideal as a transient response to injury in the case of regeneration but highly disruptive within normal tissues as is the case with cancer.
At the intersection of biomechanics and bioelectrics, piezo channels (piezo1 and piezo2) are cation-permeable mechanotransducers that are expressed in many different tissues from sensory neurons to kidney mesangial cells, and skeletal myotubes, where they regulate differentiation, migration, and proliferation [137]. Responding to shear flow, stretching, stiffness, topology, compression, and osmotic stress, piezo channels are an important part of the overall ion channel profile, where they transduce mechanical forces within the cytoskeleton or throughout the cell membrane to impact the bioelectric dynamics of regenerative and cancerous tissues. Piezo channels are overexpressed in several cancers, where influxes of calcium drive tumor progression [138], glioma aggression [139], and metastasis [140]. Ca2+-permeable piezo channels are known to inhibit axonal regeneration in Drosophila [141]; however, they have been implicated in neural stem cell differentiation by way of myosin II activation [142]. While piezo channels are considered an important interface with which to harness ECM stiffness modulation for improved cancer therapy [143], there are likely many unexplored applications. Indeed, the emergence of novel mechanostimulus-driven cancer therapeutics and functionalized materials to locally modulate shear forces, compression, and tension within the tissue microenvironment may indicate a trend in this direction [144].
Gap junctions
Gap junctions (GJs) are specialized channels that connect the intracellular spaces of adjacent cells, allowing the passage of ions as well as small molecules (< 1.5 kDa) including inositol trisphosphate (IP3), glucose, ATP, peptides, siRNAs, and amino acids [145]. GJs are important players in bioelectric networks because all physiological ions can pass through GJs. As cells coupled by GJs share a continuous plasma membrane, they display highly responsive electrotonic signaling capacities. Thus, large tissue areas can be synchronized by GJs, sharing Vmem to generate meso-scale and long-range tissue polarity associated with left–right patterning and the establishment of body axes [146, 147]. Normal developmental processes including embryogenesis and the activities of stem cells are critically dependent on GJ-related events such as calcium waves [148, 149]. For example, tissue patterning dynamics including the directional outgrowth of feathers in developing chicks, as well as the precise spacing between their limb buds, are determined by Ca2+ and GJ-mediated depolarizing waves that trigger mesenchymal stem cells to differentiate [150]. Indeed, factors associated with pluripotency (e.g., nanog, sox2, Oct4) are known to activate the expression of GJs to maintain embryonic stem cell phenotypes [151]. Further, morphogens such as BMP are active participants as modulators of GJ intercellular communication [152, 153]. Consistent with the role of GPs as mediators of plasticity and patterning, blocking or altering GJ function can cause teratogenic injuries, characterized by highly disorganized tissue patterning [154].
As regeneration is fundamentally a transient recapitulation of developmental pathways, it is unsurprising that blocking electronic signaling can also impact the ability for tissues to re-pattern themselves after an injury. Liver regeneration is marked by transient changes in GJ densities and sizes within cell membranes [155]. When GJs become dysfunctional in the highly regenerative liver, hepatic cancer becomes more likely to develop [156]. Even organs with low regenerative potential are impacted by GJ function. When bone-marrow-derived progenitor cells (BMPCs) are injected into damaged heart tissues for myocardium regeneration, the establishment of GJs between resident cardiomyocytes and BMPCs promotes plasticity, triggering differentiation along the cardiogenic fate lineage [157]. Patterning of regenerated tissues is also affected by GJs. Indeed, bisected flatworms display atypical morphologies including multiple head regions upon regeneration when GJ communication is blocked or subject to loss-of-function manipulations [158]. One proposed mechanism, which is consistent with the hypothesis that bioelectricity is a key determinant of regeneration, suggests that GJs work to re-establish and stabilize tissue polarity after an injury [159]. That is, without a capacity to rapidly synchronize the bioelectric networks of tissues, re-patterning fails due to a breakdown of biophysical communication among cells.
Gap junctions are also important regulators of cell fate and behavior in cancer. When GJs are blocked or downregulated in animal models, tumors spontaneously form as evidenced by pharmacological assays and knockout studies with mice [160, 161]. In the absence of GJs, as cells become electrically isolated from each other, the likelihood of tumor formation increases markedly [74]. This is consistent with the observation that many types of tumor cells are deficient in connexin expression [162]. Furthermore, transfection of cancer cells with connexins restores GJ signaling and suppresses tumor growth [163]. Interestingly, the relationship between cancer and GJs may be reciprocal as oncogenes such as Src can regulate GJ communication, providing a potential mechanism for positive feedback loops of increased bioelectric dysregulation [164, 165].
Acidification
Bioelectric signaling arises from disparities of charge across membranes, often driving ionic currents along electrochemical gradients or gating specialized channels that respond to Vmem. Notably, ion channels can respond either directly (e.g., ASICs) or indirectly to local pH changes in the extracellular environment [166]. Thus, chemical changes within the regenerative microenvironment may feedback into biophysical modulators as the extracellular concentration of positively charged hydrogen ions (H+) increases [167]. Indeed, acidification has recently been identified as a key factor in early stages of the regeneration process. Mammalian livers are capable of excellent regeneration, and rat models have demonstrated that post-hepatectomy there exists a transient acidification (with peaks around 3 h post-amputation) of hepatocytes proximal to the amputation plane, which promotes ectopic ATP synthase activity in these cells [168]. Acidification of lysosomes post-injury has also been observed in zebrafish fins, and inhibition of this process, which can be achieved by systemic glucocorticoid treatment, prevents the activation of downstream regenerative processes such as growth factor expression and blastema formation [169, 170]. Although the mechanistic details by which this process occurs have not yet been fully elucidated, it highlights that this sudden yet transient drop in pH plays an important role in the regenerative process. Perhaps the most direct link between pH as a modulator of bioelectric states in regeneration is the involvement of proton flux. For example, V-ATPase H+ pumps are upregulated at the cell surface in amputated Xenopus tails, where they drive the efflux of protons at the wound edge, generating increased polarity across the tissue relative to a distal depolarized region [171]. Other known contributors to regeneration, including potassium and calcium ion channels, are modulated by the electrochemical gradients formed by local pH changes [107]. In cancer, acidification of the TME has been shown to enhance invasion, propagation, and drug resistance, as well as cell survival and aggression in osteosarcomas, operating via a calf intestinal alkaline phosphatase (cIAP)/TNF receptor-associated factor (TRAF)/ nuclear factor (NF)-κB pathway [70]. Because H+ is a well-known activator of TRP channels [172], which are generally permeable to cations and thus contribute to depolarization, local acidification of tissues should be considered as a potential modulator of cell states including in cancer.
Bioelectromagnetic controls
Electromagnetic (EM) signals are receiving increased attention as relevant biophysical modulators of tissue plasticity and patterning [173]. Cells shuttle ions across their membranes, generating current with associated electric and magnetic fields. Unlike molecular signals, EM signals are not constrained by chemical bottlenecks such as diffusion, enzymatic degradation, and local concentration gradients. Cellular EM fields are detected by neighboring cells and are known modulators of bioelectric signaling [174]. EM fields can re-orient the cytoskeleton and selectively activate ion channels [175, 176]. Perhaps most relevant to regeneration are the well-characterized galvanotactic gradients that direct cell migration within tissues [176]. Their relevance is evidenced by the contemporary use of applied electric gradients as guides for oriented tissue regeneration and growth [177,178,179]. Further, biomedical techniques that employ more intense EM fields such as electropermeabilization extend the utility of EM as a tool for targeted tissue re-patterning [180, 181]. Beyond EM fields, biologically generated photon emissions (termed ultraweak photon emissions (UPEs) or “biophotons”) have been linked to mitogenic processes such as wound healing and optical signals such as pulsed light have attracted attention as potential mediators of regeneration [182, 183]—even in classically anti-regenerative tissues such as the brain [184].
Can electromagnetic factors influence cancer? There is a well-known, long-standing association between childhood leukemia and EM field exposure from high voltage powerlines [185,186,187]. There is also evidence that indicates EM radiation from cellphones may contribute to increased incidence of brain cancer and other pathologies [188, 189]. Considering EM fields affect cell migration, proliferation, and gene expression in vitro [190,191,192], it would be unsurprising to discover a defined mechanisms relating EM fields, tissue plasticity, and patterning that could drive or inhibit carcinogenesis. However, it is likely that EM field pattern (frequency modulation) and intensity (amplitude modulation) are critically important determinants of outcomes because many sources of EM radiation are not carcinogenic [193, 194]. Indeed, EM-based therapies have been developed which involve applications of electric or magnetic fields, direct electric current, and even pulsed light to treat cancer [195, 196]. Cancers, of all sub-types, emit UPEs with fingerprint-like spectral profiles (wavelength) and release patterns that are distinct from healthy tissues [197, 198]. Thus, light may be used as a biomarker of disease states including as a diagnostic marker of cancer. Importantly, EM factors do not represent an intrinsic hazard or toxin—nor do they represent a panacea. Rather, the preponderance of evidence suggests that EM factors influence fundamental biological processes that either directly or indirectly affect cell fate and behavior. Therefore, as our understanding of EM signaling increases, it becomes increasingly likely that many biomedical applications will soon follow.
Electric and magnetic fields
Electric and magnetic fields are distinct components of a shared EM modality that can interact with biological systems. While static charges such as meso-scale tissue polarity can generate electric fields, moving charges associated with ionic currents at the micro-scale are needed to generate magnetic fields. Thus, different bio-EM interactions are expected at different scales. Endogenous EM field emissions can be detected at the surface of the skin, tracking changes associated with growth, maturation, and regeneration of tissues [175, 199, 200]. Skin wounds in mammals generate endogenous electric field responses lateral to the wound center with intensities of ~ 150 mV/mm [201, 202]. Very weak currents (µA/cm2) can even be detected leading from the wound edge to its center, with the potential to guide cell populations to and from the injury site [203]. Cell migration along electric fields (galvanotaxis) is a well-documented phenomenon, where the direction is typically toward the positively charged cathode; however, some cell types including macrophages can be induced to migrate toward the anode [204]. Directional migration is possible because the intracellular space holds an intrinsically negative charge due to the presence of anionic molecules such as DNA, RNA, and phosphorylated proteins, and this bias toward polarized resting states is further reinforced by hyperpolarizing ion channels. However, the cell can also be considered an independent polar object, with inherent asymmetries (e.g., microtubule-organizing center location, ion channel distribution, nucleus location, etc.) that may contribute to its local EM-based response patterns. Indeed, because free-floating microtubules spontaneously align with electric fields in vitro [205], their role as endogenous EM biosensors must be considered seriously. As cells form tissues with specific orientations, endogenous EM field complexity likely increases, contributing to multiform EM landscape within the body. There is now overwhelming evidence that endogenous EM fields orchestrate brain function including coherent oscillations and memory-forming functions associated with synaptic plasticity [206,207,208].
Applied magnetic fields and electric fields have been used to initiate, enhance, and accelerate wound healing [209, 210]. In rats, low-milliTesla (mT)-range, time-varying magnetic fields have been used to heal cutaneous wounds while diabetic wounds have been treated with higher intensity (180 mT) static magnetic fields [211]. Electric fields have been used to guide stem cell migration for neural regeneration and nerve regrowth can be enhanced with weak currents (10 µA/cm2) and field strengths approximating 100 mV/cm [212]. Interestingly, low-frequency (< 100 Hz) EM field applications preferentially differentiated neural stem cells into astrocytes or neurons [213], suggesting a potential frequency-dependent role of EM fields in lineage programming. There is also evidence to suggest that direct applications of electric current can help regenerate bone and even spinal cord tissues [214, 215]. Electroacupuncture has even been used to stimulate peripheral nerve regeneration [216, 217]. Because life on Earth is constantly immersed within both natural and artificial EM fields including the geomagnetic field as well as those from high voltage power lines and other electronic circuits, it is worth considering how environmental influences may affect wound healing and regeneration.
EM fields and applied currents are known modulators of cancer, with both pro- and anti-carcinogenic properties that are critically dependent on the frequency and amplitude of the applied signal. Interestingly, endogenous EM fields have been observed in malignant tissues [218]. The direction and magnitude of EM fields across tumors are highly variable due to the disorganized nature of the cytoarchitecture [219]. Because different cell types display unique responses to different EM field thresholds, effects of applied fields are expected to be highly context-dependent. High-intensity (300 kV/cm) nanosecond-pulsed electric fields have been used to inhibit tumor growth, likely by apoptosis [220, 221]. However, low-intensity (1–2 V/cm) applications have also proved effective at similar and other frequencies, particularly as a targeted treatment of glioblastoma [222, 223]. The tumor-promoting effects of ambient magnetic field conditions associated with commercial electrical systems and lighting, with alternating frequencies within the 50–60 Hz are not clear as mixed results suggest unidentified complex interactions are likely [224]. Magnetic fields may promote or co-promote tumor growth under certain conditions [225]. Indeed, very weak (100 µT), time-varying magnetic fields enhanced mammary tumor growth in female rats exposed to a chemical carcinogen (7,12-dimethylbenz(a)anthracene or DMBA) [226]. Cancer-related gene expression was found to increase in cells exposed to low-mT magnetic fields for 24 h [227, 228]. Static magnetic fields can stimulate the secretion of ECM components, trigger specific types of calcium receptors, and modulate cytokine release [229, 230]. Alternating and/or pulsed magnetic fields induce current across tissues with minimal heating below 100 kHz. In addition, interactions between EM signals and other components of the tissue microenvironment can be quite complex. Galvanotactic experiments with brain tumor-initiating cells demonstrate that, upon exposure to direct current electric fields, migration toward the anode or cathode is dependent upon the substrate of the ECM (e.g., laminin, collagen, hyaluronan) and whether it was 2D (monolayer) or 3D (hydrogel)—a biophysical interaction between EM and mechanical factors involving topology [231]. Therefore, applied fields for therapeutic interventions are expected to interact with the tissue microenvironment.
Optical signaling
Spontaneous UPEs, with intensities approximating 10−15 W/cm2 and wavelengths ranging from 200 to 1300 nm, have been measured as endogenous EM-related signals from cells and tissues [232, 233]. Their fluctuations are linked to cell cycle progression, microtubule dynamics, cellular respiration, neurotransmission, and Vmem fluctuations among other correlates [234]. UPEs are generated by several biochemical mechanisms including lipid, nucleic acid, and protein oxidation reactions, mediated by reactive oxygen (ROS) and nitrogen species (RNS) [235, 236]. Thus, light emissions are readily observed in stressed, aging, diseased, or highly metabolic systems [237, 238]. As excited electrons return to ground state, photons are released with emission frequencies and patterns reflective of molecular events within the cell [239]. Though optical signaling among cells has not received significant attention, the widespread expression of non-visual opsins (e.g., OPN3, OPN5) in tissues of the eyes, skin, brain, testis, spinal cord, lung, liver, and kidney among others [240] suggest that they may serve a physiological role. In fact, light-based communication among neurons is an active area of investigation and inspiring the development of novel technologies to non-invasively measure biophysical states [241,242,243].
Optical signaling is perhaps the most understudied biophysical modality in the field of regenerative biology. However, UPEs have been investigated as correlates of wound healing in animals. In one study, light emissions with wavelengths between 230 and 700 nm were detected with unique signatures associated with inflammation and proliferation phases in rodent models [183]. Light emissions have also been used to track bone growth and fibroblast differentiation [244]. Interestingly, artificial sunlight irradiation of skin fibroblasts induces ultraweak photon emission and may even be transiently stored by cells [245]. Light therapies, involving wavelengths within the infrared and visual spectra, have been used in recent years to improve healing in retinal, vascular, and dermal tissues, and wavelength-specific light exposures may even induce patterned angiogenesis [245]. There is a current need to explore the role of UPEs in regeneration as well as potential light-based therapies and their underlying mechanisms.
Endogenous photon emissions are coupled to many classic cancer biomarkers. UPEs correlate with stress indicators such as ROS and RNS, microtubule dynamics, cell cycle progression, and gene transcription [235, 236]. Notably, wavelength-dependent signatures of UPE emissions (using filters for 420 nm, 620 nm, and 950 nm), including the ratio of infrared-to-ultraviolet emission counts, can discriminate malignant and non-malignant cancer cells and are predicted to converge with the spectral properties of delocalized electrons from linearized peptide chains in proteins associated with gene expression [246]. It may even be possible to discriminate cancerous and non-cancerous cells using very narrow-band filters for UPEs around 500 nm [247]. Further, when melanoma cells die, the spectral characteristics of their UPEs shift markedly and respond with specificity to different biochemical activators and blockers [248]. Mechanical factors may also influence biophoton emissions. Indeed, photon emission from tumor cells can be enhanced by 3 MHz ultrasonic stimulation (mechanical vibration) with distinct changes relative to healthy liver and spleen tissue [249]. Like many other cells, cancer cells express non-visual photoreceptors such as Opn3, which exhibits optimal absorption around 460–470 nm—values that are well within the range of endogenous UPE emission spectra [240]. Therefore, endogenous optical signaling in cancer is a relevant phenomenon that should be further explored.
The use of applied light as an external control of cell states has become increasingly popular. While many approaches incorporate the use of nanoparticles, porphyrins, and other photoactive molecules that act as guides for therapeutic exposures of light [250], simple exposure to light itself may be sufficient to modulate cancer [251]. A systematic analysis of laser light irradiation (intensity of 20 J/cm2) with multiple wavelengths (λ = 410 nm, 488 nm, 630 nm, 635 nm, 640 nm, 805 nm, and 1,064 nm) demonstrated that the mitotic rate of tumor and normal cells could be modulated by wavelength-specific light [252]; however, similar techniques with lower intensity had different biomodulative effects. Using blue LEDs (465 nm), which converge with the spectral characteristics of Opn3, investigators have demonstrated the ability to induce autophagy in colon cancer cells [253]. Similar conditions are associated with the inhibition of colon cancer and the promotion of cancer-associated fibroblasts [254], which can drive tissue microenvironment reorganization associated with tumors.
Engineered applications of biophysical control
As the biophysical dimensions of cellular communication and their roles as regulators of tissue dynamics have gained recognition, biomedical engineers have harnessed the underlying principles to design novel techniques to reprogram, re-pattern, and replace tissues (Fig. 3). Bioengineered in vitro models allow for the isolation and examination of discreet biophysical mechanisms in well-defined materials that mimic physiological environments. As more devices that allow for precise control of these important biophysical properties in vitro are developed, we can begin to identify and isolate cues to translate their use into in vivo models to further understand plasticity and patterning as products of cellular communication.
Bioengineered applications of biophysical controls. (Left) In vitro models can be constructed and tested by tuning properties that simulate the biophysics of tissue microenvironments. (Right) Diseases and injuries (red) can be mitigated, inhibited, or reversed by applications of electric fields, light, ECM-simulating scaffolds, compounds that modulate bioelectricity, or by magnetic field exposures
Surface functionalization of materials
The development of surface functionalization techniques allows an unprecedented control over biomaterial properties such as ECM ligand type, density, or patterning, and stiffness [255]. For example, Smith et al. [256] achieved direct reprogramming of mouse fibroblasts into cardiomyocyte-like cells using an engineered poly(ethylene glycol) (PEG) hydrogel presenting various concentrations and density of laminin and RDG peptide [256]. One particularly compelling use of surface functionalization to study and manipulate cellular plasticity is the creation of patterns and gradients using custom hydrogels and microfluidic devices. During embryo development, there are many important ECM protein and stiffness gradients that drive morphogenesis. For example, in the embryonic mouse limb bud, an ECM stiffness gradient matched by fibronectin expression directs MSC migration during limb development (i.e., durotaxis) [257]. Indeed, much of the work investigating the effect of biophysical gradients has focused on cell migration, but this may also prove to be an important factor to incorporate into developing strategies to promote regeneration through modulation of plasticity.
Topographical patterning
Topographical patterning is also an important bioengineering strategy to modulate cell fate and plasticity. Using various techniques [258], nano- and micro-topographical cues have been developed for in vitro systems that mimic the topography of natural tissue to study its effect on cells, or to promote a certain phenotype or reprogramming of cells. For example, fabrication of parallel microgrooves and nanofibers can direct cell morphology and enhance reprogramming efficiency of mouse fibroblasts through mechano-modulation of the epigenetic state [259]. Many gastrointestinal and colorectal cancers are difficult to model in vitro, as the in vivo structures are tubular, which is not well-represented by 2D epithelial culture. However, representative intestinal organoids can be generated using lipid bilayer-supported droplet networks to form 3D tubular structures [260, 261]. Similarly, to recapitulate the unique structure of cortical folding associated with developing brain tissues, 4D bioprinting techniques have been combined with cell-infused “smart” materials that can be activated to change their shapes by pulses of infrared light [262]. Furthermore, etched surfaces and microfluidic conduits have been developed to guide both neurites and vascular structures to generate polarized tissue structures that can more precisely mimic their natural templates [263]. The continued development of these kinds of 3D culture systems will provide accurate in vitro models that could mimic the ECM or cell–cell interactions present in vivo.
Tunable flow and shear stress
Bioengineered platforms can also be used to study the biophysical cues that influence cellular plasticity by incorporating properties such as fluid shear stress, ECM stiffness, and electric fields. Controlling ECM stiffness in vitro is accomplished in many ways, such as tuning the substrate concentrations [e.g., of polydimethylsiloxane (PDMS)], or chemical or UV crosslinking [264]. To mimic biophysical phenomena, cells may experience in vivo such as flow-induced shear stress, various in vitro devices have been developed to artificially introduce shear force into a system. Van Haaften et al. [264] created a physiologically relevant 3D model of hemodynamic loading to study the contribution of shear stress and cyclic stretch on tissue regeneration in vascular grafts. They reported that when human macrophages and myofibroblasts were cultured in this bioreactor, the effects of shear stress included modulation of myofibroblast phenotype [265]. Low interstitial flow has also been investigated for wound healing applications [266], for example, to induce fibroblast to myofibroblast differentiation in an α1β1 integrin-dependent process in vitro [267]. Fluid shear stress has also been studied in cancer using innovative bioengineering techniques, including the integration of perfusion and flow within microfluidic and bioreactor platforms [97, 268]. More work is needed to determine how the manipulation of shear stress in vitro may translate to strategies to induce tissue regeneration.
Decellularized ECM
The use of decellularized ECM from the organ or tissue is an alternative means by which in vivo biophysical properties such as 3D architecture, stiffness or composition can be retained. Once decellularized, this ECM can then be digested and resuspended into a hydrogel or seeded directly with the cells of interest and maintained in culture to study the effects of the ECM properties on cell phenotype and plasticity. Decellularized ECM can also be used to drive the differentiation of seeded cells for transplantation back into the body. One of the most commonly used reconstituted ECM substrates is Matrigel, which is naturally secreted by Engelbreth-Holm-Swarm mouse tumor cells. Interestingly, at microscopic scales, this material displays marked mechanical heterogeneity with punctate regions of increased stiffness relative to the bulk [269], consistent with the natural tumor microenvironment [270]. Hydrogels constructed with varying degrees of Matrigel density were shown to control stiffness-dependent YAP localization in human pluripotent stem cells with downstream effects on cell fate [271]. Other hydrogels, fabricated from decellularized ECM from various cardiovascular tissues and seeded with adipose stromal cells (ASCs), influence cell differentiation differently, likely due to differences in ECM composition of the tissues [272]. Similarly, scaffolds composed of decellularized meningeal tissue were shown to promote the adherence, differentiation, and viability of neural precursor cells [273].
Modulation of bioelectric activity
Investigators are now exploring the possibility of inhibiting tumor growth by targeting ion channels. Blockades of serotonin-gated Na+/K+ channels hyperpolarize colorectal cancer cells in vitro with downstream proapoptotic effects and cell cycle arrest [274]. Similar effects have been achieved using antiepileptic drugs that block voltage-gated sodium channels [275]. Regeneration may also be affected by transient sodium currents and mediated by voltage-gated channels as was demonstrated by tail regrowth and patterning experiments in a Xenopus model [276]. As drug delivery methods continue to advance, including the development of wearables with timed release of drugs at the wound interface [277], the possibility of restoring form and function with bioelectric modulators becomes increasingly likely.
While voltage-gated calcium channels can be markedly underexpressed across several types of cancer including brain, lung, kidney, and breast [278], it was recently demonstrated that voltage-gated calcium channel (VGCC) blockades can inhibit tumor growth with synergistic effects when combined with chemotherapies [279]. Interestingly, some authors have reported increased risk factors of cancer in hypertensive patients using VGCCs [280], indicating a more complex relationship requiring further study.
Localized electrical stimulation of the wound site by implanted EM field generators—also known as electroceuticals—is an emerging technology with promising effects on regeneration. Building on seminal experiments in the 1970s, platinum and silver-wired electrodes coupled to embedded resistors were recently implanted within the stumps of amputated rat limbs to deliver low-voltage DC stimulation to enhance regeneration [281]. The stimulation-based treatment induced significant regrowth of bone, cartilage, and vasculature while suppressing the formation of neuromas—indicating significant re-patterning potential. Several studies have demonstrated similarly promising effects on peripheral nerve regeneration with growth-associated gene expression [282, 283]. Earlier works also demonstrated that direct current (0.6 mA), applied for 15 min per day over a period of 9 consecutive days, could be used to reliably generate necrotic lesions within tumors [284]. Similarly brief, low-intensity currents delivered to cancerous tissues were demonstrated to be safe with regard to the surrounding healthy tissues [285]. Recent efforts have demonstrated that vascular normalization, which involves a suppression of pathological angiogenesis and promotes the effects of anti-cancer drugs, can be achieved by wireless electrical stimulation of tumors using polarized ferroelectric nanoparticles; effects which were attributed to disruptions of intracellular Ca2+ gradients and endothelial nitric oxide synthase expression [286].
Materials can also be used to focus or amplify endogenous bioelectric signaling for tissue engineering purposes. Indeed, recognizing the role of the microenvironment on electrical signal propagation, neural stem cell differentiation was modulated within custom scaffolds by increasing the electrical conductivity of the substrate (poly(3,4-ethylenedioxythiophene), polystyrene sulfonate or “PEDOT:PSS”) to build models of the central nervous system in vitro [287].
Applied electric and magnetic fields
To model electrotaxis in vitro, there is a growing number of researchers developing devices to generate electric fields to modulate tissue dynamics [288]. While it is well established that electric fields can drive migration of many different cell types [289, 290], the latest data indicate that EM field stimulation at low (30–300 kHz) and extremely low (3–30 Hz) frequencies can modulate the fate and behavior of stem cells including effects on proliferation, differentiation, cell cycle progression, viability, morphology, and metabolism [291]. It was recently demonstrated that low-intensity (1.5 mT) pulsed EM fields were able to stimulate secretome activation, enhancing myogenesis with orientation-specific effects [292]. There is also considerable evidence that EM fields re-pattern tissues in wound repair, with significant regulation of inflammatory pathways, enhanced proliferation and tubulization of endothelial cells to generate new vasculature, and marked re-epithelialization with collagen deposition [209]. Indeed, regenerative therapies are being developed which involve the use of EM fields to trigger free ion release (e.g., Ca2+, Na+, K+) in cells and, in turn, downstream signaling cascades to proliferate and differentiate mesenchymal stem cells [293]. EM fields have already been used to upregulate the expression of osteogenic factors (e.g., BMP-2, osteopontin, COL1, and ALP) for bone regeneration with effects on several cell types [294, 295].
An emerging alternative to classic anti-cancer drugs is the use of tumor-treating fields. Non-ionizing radio frequency (RF) radiation has been used to locally heat cancerous tissues (> 45 °C) for direct ablation or sensitization to other treatments; however, because needles must be inserted directly into the tumor, applications are limited, the effect is highly localized, and the technique is invasive [296]. Alternatively, ferromagnetic nanoparticles can be injected into the tumor site and excited with kHz-to-THz-range RF pulses to ablate tumors. However, low-frequency, weak-intensity (µT) EM fields can also inhibit tumor growth by inducing calcium ion influx and, potentially, several downstream molecular targets including cyclin expression [297]. In addition to affecting cells directly, applied EM fields can change the environment around the tumor itself, including effects on ECM remodeling and modulation of inflammatory response [298]. These fields can play multiple roles, from producing an environment which is less compatible with tumor development and spread, to increasing the absorption rate of specific chemotherapeutic or radiopharmaceutical drugs [299]. Interestingly, magnetic fields have been shown to suppress the growth of some tumors [300] and promote others [301]; however, the frequency, amplitude, and pattern of the applied EMF are critical. Unlike molecular signals, EM fields are not limited to the activation of a limited set of specific receptor sub-types; rather, they can activate several parallel endogenous bioelectric currents and conserved intracellular pathways which are shared among most or all cells. Therefore, the bioelectromagnetic modality is widely applicable as a biophysical mechanism of cellular control.
Photobiomodulation
Photobiomodulation is gaining attention as a technique to program tissue plasticity and patterning. All cells express light-sensitive molecules, from enzymes (e.g., cytochrome c oxidase) to light-gated ion channels (e.g., TRP channels), and G-protein coupled receptors (e.g., opsins) [302]. Even amino acids such as L-tryptophan can be photoactivated [303]. In many cases, biophotonic interactions are redox-mediated phenomena with the potential to initiate transcription via ROS, nitric oxide (NO), cyclic adenosine monophosphate (cAMP), and other secondary mediators [304]. It is, therefore, unsurprising that, in search of therapeutic applications, researchers have explored the effects of applied light on stem cell function including differentiation, proliferation, gene expression, cytokine release, morphogen release, metabolic function, and bioelectricity [305]. Encouragingly, photobiomodulation has demonstrated clinically relevant effects in wound healing, vascularization, tissue repair following heart attack, and other ischemic conditions, neurodegeneration, and other pathologies [306].
Healing and repair can be stimulated by photobiomodulation by triggering inflammatory activations including the release of interleukins, tumor necrosis factor (TNF), and several growth factors [307]. Infrared (830 nm), low-level laser light (50 J/cm2) exposures triggered significant bone regrowth and re-patterning due to activation of mitogen-activated protein kinase (MAPK) and β-catenin pathways and downstream osteogenic gene expression of BMP-4 and RUNX-2 [308]. Skin regeneration and vascularization have also been stimulated by photobiomodulation, including the activation of VEGF and pericyte mobilization [309]. Similarly, regeneration of peripheral nerves including sciatic, facial, fibular, and vagus nerve has been demonstrated using photobiomodulation within the red-to-infrared component of the EM spectrum [310]. As clinical applications have become viable, implantable devices have been engineered to deliver light-based therapies directly to tissues including uses in dentistry; however, there is a significant need to improve dose and other parameters [311]. Nevertheless, implanted photobiomodulators have been used to accelerate orthodontic movement in molar intrusions [312] and periodontal bone healing [313]. Similar implants made from biocompatible optical fibers composed of poly(L-lactic acid) produced similar effects when delivering green light to experimental femoral bone injuries in rodents [314].
While cancer cells emit light signatures that are diagnostically relevant [197, 198, 315], the impact of photobiomodulation on cancer and its therapeutic potential is less clear. A recent systematic review of 67 studies examining the effects of photobiomodulation on tumor growth, recurrence rate, proliferation, differentiation, and survival demonstrated that light-based therapies is generally safe; however, outcomes are often mixed and likely dependent on tissue-specific factors [316]. Tumor regression following light exposure has been reported in several animal models [317, 318] with proposed mechanisms including immune system activation [307]. Interestingly, photobiomodulation can sensitize tumors to irradiation, increasing tumor necrosis while protecting normal tissue [319]. It has also been used to prevent and manage toxicity and side effects associated with traditional cancer treatments including neuropathy, oral mucositis, and lymphedema [320]. However, results from photobiomodulation therapies in animal models of subcutaneous melanoma [321], anaplastic thyroid cancer [322], and a cheek pouch model of carcinogenesis [323] indicate that tumors exposed to low-intensity (< 5 W/cm2) light with wavelengths of approximately 660 nm may exacerbate tumor growth and contribute to poor differentiation of cytoarchitecture. Therefore, there is a timely need to identify the determinants of photobiomodulation responses and the isolation of therapeutic applications which may be used to non-invasively control tissue dynamics.
Conclusion
Consistent with their shared molecular pathways, regenerative and cancerous tissues display common biophysical regulators of plasticity and patterning (or mispatterning). With mechanical forces, it seems that, in general, increased ECM stiffness promotes cancer cell induction, tumor growth, and metastasis while inhibiting regenerative potential in favor of scar formation. While it may be tempting to plot cancer and regeneration at opposite points on a continuum, they share several bioelectric signatures including a significantly depolarized resting membrane potential. Both systems, which involve cell migration toward different ends, are responsive to electric field gradients that guide outgrowths. Differentiation, cell division, and migration are also regulated by ambient and applied light in both regeneration and cancer. As discussed, tissue patterns associated with embryogenesis and recapitulated during regeneration may be stabilized by gap junctional networks, where disruptions result in teratogenic phenotypes or malignancies. Perhaps most clear is the previously underestimated interaction between molecular and physical modalities within the extended microenvironment, where mechanical, electrical, or electromagnetic signaling converge upon gene expression and factor release with feedbacks to modulate the environment in turn. Top-down biophysical control mechanisms are intrinsically linked to the ECM composition, structural topography, and cytoarchitecture of the microenvironment. Recognition of this is evidenced by parallel trends in regenerative medicine and oncology toward the modulation of tissue microenvironments to program physiological outcomes bypassing the micromanagement of individual cells. Our review of the literature suggests that biophysical phenomena are receiving increased attention as determinants of cell state and fate; however, there is a need to further integrate molecular and biophysical factors in consideration of their meaningful interactions.
While traditional cell culture methods provide conditions to isolate individual variables to better understand cell signaling, the interconnectedness of several molecular and biophysical signaling modalities demands the development of model systems with increased physiological relevance. Three-dimensional cell culture techniques incorporate customizable ECM structures in vitro, enabling the assessment of cell–substrate interactions which are central to biophysical signaling. Current tissue engineering applications in animal models are beginning to enable such integration. Indeed, by combining topographical alignment, chemical cues, and electric fields, it was recently demonstrated that myofibroblast could be differentiated from dermal fibroblasts and tuned to enhance wound healing with pronounced matrix reconstruction [324]. The full-circle integration of ECM-embedded cues, cell mobilization, and microenvironmental remodeling that is now possible provides a foundation for novel pro-regenerative and anti-cancer therapies.
As 3D tissue cultures have become more accessible tools, it has become possible to recapitulate the relationships between matrix geometry and bioelectricity or the impact of tissue orientation on electromagnetic field effects within tractable and scalable platforms. Novel questions can now be addressed: Are long-range bioelectric signals impacted by the polarization of ECM polymers? Can optical signaling be disrupted by ECM composition? Can tumorigenic microenvironments be remodeled by combinations of biophysical signaling patterns? In addition to enabling unprecedented experiments, 3D tissues also permit the integration of implantable materials for enhanced translation of engineered biophysical interventions. To fully harness the potential of biophysical control over tissue plasticity and patterning, 3D tissue culture systems can be used to systematically disentangle determinant factors in ways that were previously impractical when restricted to the use of animal models of cell culture with monolayers. Once the physicochemical complexities of living systems are better understood, the full spectrum of cellular communication will become available to bioengineers in search of means to medically restore or stabilize form and function in the clinical setting.
Data availability
None.
References
Michod RE, Roze D (2001) Cooperation and conflict in the evolution of multicellularity. Heredity 86(1):1–7
Smiley P, Levin M (2022) Competition for finite resources as coordination mechanism for morphogenesis: an evolutionary algorithm study of digital embryogeny. Biosystems 221:104762
Edgell TC, Neufeld CJ (2008) Experimental evidence for latent developmental plasticity: intertidal whelks respond to a native but not an introduced predator. Biol Lett 4(4):385–387
Pisco AO, Huang S (2015) Non-genetic cancer cell plasticity and therapy-induced stemness in tumour relapse: ‘What does not kill me strengthens me.’ Br J Cancer 112(11):1725–1732
Quintanal-Villalonga Á, Chan JM, Yu HA, Pe’er D, Sawyers CL, Sen T, Rudin CM (2020) Lineage plasticity in cancer: a shared pathway of therapeutic resistance. Nat Rev Clin Oncol 17(6):360–371
Donaldson DJ, Mason JM (1975) Cancer-related aspects of regeneration research: a review. Growth 39(4):475–496
Tsonis PA (1983) Effects of carcinogens on regenerating and non-regenerating limbs in amphibia. Anticancer Res 3(3):195–202
Pesic M, Greten FR (2016) Inflammation and cancer: tissue regeneration gone awry. Curr Opin Cell Biol 43:55–61
Huang S (2021) Reconciling non-genetic plasticity with somatic evolution in cancer. Trends Cancer 7(4):309–322
Taddei ML, Giannoni E, Comito G, Chiarugi P (2013) Microenvironment and tumor cell plasticity: an easy way out. Cancer Lett 341(1):80–96
Kragl M, Knapp D, Nacu E, Khattak S, Maden M, Epperlein HH, Tanaka EM (2009) Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature 460(7251):60–65
Sámano C, González-Barrios R, Castro-Azpíroz M, Torres-García D, Ocampo-Cervantes JA, Otero-Negrete J, Soto-Reyes E (2021) Genomics and epigenomics of axolotl regeneration. Int J Dev Biol 65(7–9):465–474
Galliot B, Ghila L (2010) Cell plasticity in homeostasis and regeneration. Mol Reprod Dev 77(10):837–855
Gritti A, Vescovi AL, Galli R (2002) Adult neural stem cells: plasticity and developmental potential. J Physiol-Paris 96(1–2):81–90
Sakers A, De Siqueira MK, Seale P, Villanueva CJ (2022) Adipose-tissue plasticity in health and disease. Cell 185(3):419–446
Friedl P, Alexander S (2011) Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147(5):992–1009
Pearson BJ, Alvarado AS (2008) Regeneration, stem cells, and the evolution of tumor suppression. Cold Spring Harb Symp Quant Biol 73:565–572
Pomerantz JH, Blau HM (2013) Tumor suppressors: enhancers or suppressors of regeneration? Development 140(12):2502–2512
Erenpreisa J, Cragg MS (2013) Three steps to the immortality of cancer cells: senescence, polyploidy and self-renewal. Cancer Cell Int 13(1):1–12
Chernet B, Levin M (2013) Endogenous voltage potentials and the microenvironment: bioelectric signals that reveal, induce and normalize cancer. J Clin Exp Oncol Suppl 1:S1-002. https://doi.org/10.4172/2324-9110.S1-002
Postovit LM, Margaryan NV, Seftor EA, Kirschmann DA, Lipavsky A, Wheaton WW, Abbott DE, Seftor RE, Hendrix MJ (2008) Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad Sci 105(11):4329–4334
Rose SM, Wallingford HM (1948) Transformation of renal tumors of frogs to normal tissues in regenerating limbs of salamanders. Science (New York, NY) 107(2784):457–457
Turing A (1952) The chemical basis of morphogenesis. Philos Trans R Soc Lond Ser B Biol Sci 237(641):37–72
Ellis GF, Kopel J (2019) The dynamical emergence of biology from physics: branching causation via biomolecules. Front Physiol 9:1966
Pross A (2005) On the emergence of biological complexity: life as a kinetic state of matter. Origins Life Evol Biosph 35:151–166
Cervera J, Manzanares JA, Mafe S, Levin M (2019) Synchronization of bioelectric oscillations in networks of nonexcitable cells: from single-cell to multicellular states. J Phys Chem B 123(18):3924–3934
Wrenn E, Huang Y, Cheung K (2021) Collective metastasis: coordinating the multicellular voyage. Clin Exp Metas 38:373–399
Dunny GM, Brickman TJ, Dworkin M (2008) Multicellular behavior in bacteria: communication, cooperation, competition and cheating. BioEssays 30(4):296–298
Mian IS, Rose C (2011) Communication theory and multicellular biology. Integr Biol 3(4):350–367
Muñoz-Descalzo S, de Navascues J, Arias AM (2012) Wnt-Notch signalling: an integrated mechanism regulating transitions between cell states. BioEssays 34(2):110–118
Cervera J, Alcaraz A, Mafe S (2016) Bioelectrical signals and ion channels in the modeling of multicellular patterns and cancer biophysics. Sci Rep 6(1):20403
Keung AJ, Healy KE, Kumar S, Schaffer DV (2010) Biophysics and dynamics of natural and engineered stem cell microenvironments. Wiley Interdiscip Rev Syst Biol Med 2(1):49–64
Levin M, Stevenson CG (2012) Regulation of cell behavior and tissue patterning by bioelectrical signals: challenges and opportunities for biomedical engineering. Annu Rev Biomed Eng 14:295–323
Moore D, Walker SI, Levin M (2017) Cancer as a disorder of patterning information: computational and biophysical perspectives on the cancer problem. Converg Sci Phys Oncol 3(4):043001
Saucerman JJ, Tan PM, Buchholz KS, McCulloch AD, Omens JH (2019) Mechanical regulation of gene expression in cardiac myocytes and fibroblasts. Nat Rev Cardiol 16(6):361–378
Clark WH (1995) The nature of cancer: morphogenesis and progressive (self)- disorganization in neoplastic development and progression. Acta Oncol 34(1):3–21
Faurobert E, Bouin AP, Albiges-Rizo C (2015) Microenvironment, tumor cell plasticity, and cancer. Curr Opin Oncol 27(1):64–70
Lee G, Hall RR III, Ahmed AU (2016) Cancer stem cells: cellular plasticity, niche, and its clinical relevance. J Stem Cell Res Ther 6(10):363
Dean M (1998) Cancer as a complex developmental disorder—nineteenth Cornelius P. Rhoads Memorial Award Lecture. Cancer Res 58(24):5633–5636
Feinberg AP (2007) Phenotypic plasticity and the epigenetics of human disease. Nature 447(7143):433–440
Holman EC, Campbell LJ, Hines J, Crews CM (2012) Microarray analysis of microRNA expression during axolotl limb regeneration. PLoS ONE 7:e41804
Pfeffer SR, Yang CH, Pfeffer LM (2015) The role of miR-21 in cancer. Drug Dev Res 76(6):270–277
Zhao Q, Chen S, Zhu Z, Yu L, Ren Y, Jiang M, Weng J, Li B (2018) MiR-21 promotes EGF-induced pancreatic cancer cell proliferation by targeting Spry2. Cell Death Dis. https://doi.org/10.1038/s41419-018-1182-9
Eggington HR, Mulholland EJ, Leedham SJ (2022) Morphogen regulation of stem cell plasticity in intestinal regeneration and carcinogenesis. Dev Dyn 251(1):61–74
Levin M (2003) Bioelectromagnetics in morphogenesis. Bioelectromagnetics 24(5):295–315
Luo R, Liang Y, Yang J, Feng H, Chen Y, Jiang X, Zhang Z, Liu J, Bai Y, Xue J, Chao S, Xi Y, Liu X, Wang E, Luo D, Li Z, Zhang J (2023) Reshaping the endogenous electric field to boost wound repair via electrogenerative dressing. Adv Mater 35:2208395
Shieh AC (2011) Biomechanical forces shape the tumor microenvironment. Ann Biomed Eng 39:1379–1389
Cherubini C, Filippi S, Nardinocchi P, Teresi L (2008) An electromechanical model of cardiac tissue: constitutive issues and electrophysiological effects. Prog Biophys Mol Biol 97(2–3):562–573
Serra-Picamal X, Conte V, Vincent R, Anon E, Tambe DT, Bazellieres E, Butler JP, Fredberg JJ, Trepat X (2012) Mechanical waves during tissue expansion. Nat Phys 8(8):628–634
Zinner M, Lukonin I, Liberali P (2020) Design principles of tissue organisation: How single cells coordinate across scales. Curr Opin Cell Biol 67:37–45
Levine H, Ben-Jacob E (2004) Physical schemata underlying biological pattern formation—examples, issues and strategies. Phys Biol 1(2):P14
Urbanczyk M, Layland SL, Schenke-Layland K (2020) The role of extracellular matrix in biomechanics and its impact on bioengineering of cells and 3D tissues. Matrix Biol 85:1–14
Iismaa SE, Kaidonis X, Nicks AM, Bogush N, Kikuchi K, Naqvi N, Harvey RP, Husain A, Graham RM (2018) Comparative regenerative mechanisms across different mammalian tissues. NPJ Regen Med 3(1):6
Piersma B, Hayward MK, Weaver VM (2020) Fibrosis and cancer: a strained relationship. Biochim Biophys Acta: BBA Rev Cancer 1873(2):188356
Yang EV, Gardiner DM, Carlson MR, Nugas CA, Bryant SV (1999) Expression of Mmp-9 and related matrix metalloproteinase genes during axolotl limb regeneration. Dev Dyn 216(1):2–9
Gasiorowski JZ, Murphy CJ, Nealey PF (2013) Biophysical cues and cell behavior: the big impact of little things. Annu Rev Biomed Eng 15:155–176
Tang RZ, Liu XQ (2023) Biophysical cues of in vitro biomaterials-based artificial extracellular matrix guide cancer cell plasticity. Mater Today Bio 19:100607
Walma DAC, Yamada KM (2020) The extracellular matrix in development. Development 147(10):dev175596
Nemec S, Kilian KA (2021) Materials control of the epigenetics underlying cell plasticity. Nat Rev Mater 6(1):69–83
De Leon MP, Wen FL, Paylaga GJ, Wang YT, Roan HY, Wang CH, Hsiao CD, Lin KH, Chen CH (2023) Mechanical waves identify the amputation position during wound healing in the amputated zebrafish tailfin. Nat Phys 19:1362–1370
Garcia-Puig A, Mosquera JL, Jiménez-Delgado S, García-Pastor C, Jorba I, Navajas D, Canals F, Raya A (2019) Proteomics analysis of extracellular matrix remodeling during zebrafish heart regeneration*[S]. Mol Cell Proteomics 18(9):1745–1755
Darby IA, Laverdet B, Bonté F, Desmoulière A (2014) Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol 7:301–311
Jiang T, Zhao J, Yu S, Mao Z, Gao C, Zhu Y, Mao C, Zheng L (2019) Untangling the response of bone tumor cells and bone forming cells to matrix stiffness and adhesion ligand density by means of hydrogels. Biomaterials 188:130–143
Hortells L, Johansen AKZ, Yutzey KE (2019) Cardiac fibroblasts and the extracellular matrix in regenerative and nonregenerative hearts. J Cardiovasc Dev Dis 6(3):29
Chen L, Li C, He A, Tong H, Lu X, Yang R, Chen X, Wu X, Wang X, Wang S, Ma J, Fu Y, Zhang T (2023) Changes of age-related auricular cartilage plasticity and biomechanical property in a rabbit model. Laryngoscope 133(1):88–94
Español-Suñer R, Carpentier R, Van Hul N, Legry V, Achouri Y, Cordi S, Jacquemin P, Lemaigre F, Leclercq IA (2012) Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology 143(6):1564–1575
Varela-Eirin M, Loureiro J, Fonseca E, Corrochano S, Caeiro JR, Collado M, Mayan MD (2018) Cartilage regeneration and ageing: targeting cellular plasticity in osteoarthritis. Ageing Res Rev 42:56–71. https://doi.org/10.1016/j.arr.2017.12.006
Pessina P, Kharraz Y, Jardí M, Fukada SI, Serrano AL, Perdiguero E, Muñoz-Cánoves P (2015) Fibrogenic cell plasticity blunts tissue regeneration and aggravates muscular dystrophy. Stem Cell Rep 4(6):1046–1060
Wu T, Dai Y (2017) Tumor microenvironment and therapeutic response. Cancer Lett 387:61–68
Lin Z, Fan Z, Zhang X, Wan J, Liu T (2020) Cellular plasticity and drug resistance in sarcoma. Life Sci 263:118589
Cunningham JE, Jurj AL, Oman L, Stonerock AE, Nitcheva DK, Cupples TE (2006) Is risk of axillary lymph node metastasis associated with proximity of breast cancer to the skin? Breast Cancer Res Treat 100:319–328
Merkher Y, Weihs D (2017) Proximity of metastatic cells enhances their mechanobiological invasiveness. Ann Biomed Eng 45:1399–1406
Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y (2020) Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther 5(1):28
McMillen P, Oudin MJ, Levin M, Payne SL (2021) Beyond neurons: long distance communication in development and cancer. Front Cell Dev Biol 9:739024
Dehne N, Mora J, Namgaladze D, Weigert A, Brüne B (2017) Cancer cell and macrophage cross-talk in the tumor microenvironment. Curr Opin Pharmacol 35:12–19
Quail DF, Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19(11):1423–1437
Kim HJ, Yang K, Kim K, Lee YJ, Lee S, Ahn SY, Ahn YH, Kang JL (2022) Reprogramming of cancer-associated fibroblasts by apoptotic cancer cells inhibits lung metastasis via notch1-WISP-1 signaling. Cell Mol Immunol 19(12):1373–1391
Kim J, Feng J, Jones CA, Mao X, Sander LM, Levine H, Sun B (2017) Stress-induced plasticity of dynamic collagen networks. Nat Commun. https://doi.org/10.1038/s41467-017-01011-7
He X, Lee B, Jiang Y (2016) Cell-ECM interactions in tumor invasion. Syst Biol Tumor Microenviron Quant Model Simul 936:73–91
Ray A, Provenzano PP (2021) Aligned forces: origins and mechanisms of cancer dissemination guided by extracellular matrix architecture. Curr Opin Cell Biol 72:63–71
Rippa AL, Kalabusheva EP, Vorotelyak EA (2019) Regeneration of dermis: scarring and cells involved. Cells 8(6):607
Wong VW, Rustad KC, Akaishi S, Sorkin M, Glotzbach JP, Januszyk M, Nelson ER, Levi K, Paterno J, Vial IN, Kuang AA, Longaker MT, Gurtner GC (2012) Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med 18(1):148–152
Saraswathibhatla A, Indana D, Chaudhuri O (2023) Cell–extracellular matrix mechanotransduction in 3D. Nat Rev Mol Cell Biol 24(495):516
Ross TD, Coon BG, Yun S, Baeyens N, Tanaka K, Ouyang M, Schwartz MA (2013) Integrins in mechanotransduction. Curr Opin Cell Biol 25(5):613–618
Gerardo H, Lima A, Carvalho J, Ramos JR, Couceiro S, Travasso RD, Pires das Neves R, Grãos M (2019) Soft culture substrates favor stem-like cellular phenotype and facilitate reprogramming of human mesenchymal stem/stromal cells (hMSCs) through mechanotransduction. Sci Rep 9(1):1–18
Steward AJ, Kelly DJ (2015) Mechanical regulation of mesenchymal stem cell differentiation. J Anat 227(6):717–731
Brielle S, Bavli D, Motzik A, Kan-Tor Y, Sun X, Kozulin C, Avni B, Ram O, Buxboim A (2021) Delineating the heterogeneity of matrix-directed differentiation toward soft and stiff tissue lineages via single-cell profiling. Proc Natl Acad Sci 118(19):e2016322118
Talwar S, Kant A, Xu T, Shenoy VB, Assoian RK (2021) Mechanosensitive smooth muscle cell phenotypic plasticity emerging from a null state and the balance between Rac and Rho. Cell Rep 35(3):109019. https://doi.org/10.1016/j.celrep.2021.109019
Xu Z, Orkwis JA, Harris GM (2021) Cell shape and matrix stiffness impact Schwann cell plasticity via YAP/TAZ and Rho GTPases. Int J Mol Sci 22(9):4821
Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M (2012) Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 489(7417):561–565
Dagenais P, Blanchoud S, Pury D, Pfefferli C, Aegerter-Wilmsen T, Aegerter CM, Jaźwińska A (2021) Hydrodynamic stress and phenotypic plasticity of the zebrafish regenerating fin. J Exp Biol 224(15):jeb242309
Ferretti S, Allegrini PR, Becquet MM, McSheehy PM (2009) Tumor interstitial fluid pressure as an early-response marker for anticancer therapeutics. Neoplasia 11(9):874–881
Yeldag G, Rice A, del Río Hernández A (2018) Chemoresistance and the self- maintaining tumor microenvironment. Cancers 10(12):471
Liu S, Zhou FY, Shen Y, Zhang Y, Yin H, Zeng Y, Liu J, Yan Z, Liu X (2016) Fluid shear stress induced epithelial-mesenchymal transition (EMT) in Hep-2 cells. Oncotarget 7:32876–32892
Risvi I, Gurkan U, Tasoglu S, Alagic N, Celli J, Mensah L, Mai Z, Demirci U, Hasan T (2013) Flow induces epithelial-mesenchymal transition, cellular heterogeneity and biomarker modulation in 3D ovarian cancer nodules. Proc Natl Acad Sci USA 110:E1974–E1983
Triantafillu U, Park S, Klaassen N, Raddatz A, Kim Y (2017) Fluid shear stress induces cancer stem cell-like phenotype in MCF7 breast cancer cell line without inducing epithelial to mesenchymal transition. Int J Oncol 50:993–1001
Cognart HA, Viovy JL, Villard C (2020) Fluid shear stress coupled with narrow constrictions induce cell type-dependent morphological and molecular changes in SK-BR-3 and MDA-MB-231 cells. Sci Rep 10(1):1–14
Zhao X, Guan JL (2011) Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev 63(8):610–615
Fattet L, Jung HY, Matsumoto MW, Aubol BE, Kumar A, Adams JA, Chen AC, Sah RL, Engler AJ, Pasquale EB, Yang J (2020) Matrix rigidity controls epithelial-mesenchymal plasticity and tumor metastasis via a mechanoresponsive EPHA2/LYN complex. Dev Cell. https://doi.org/10.1016/j.devcel.2020.05.031
Lin H-H, Lin H-K, Lin I-H, Chiou Y-W, Chen H-W, Liu C-Y, Harn HI-C, Chiu W-T, Wang Y-K, Shen M-R, Tang M-J (2015) Mechanical phenotype of cancer cells: cell softening and loss of stiffness sensing. Oncotarget 6(25):20946
Wolfenson H, Meacci G, Liu S, Stachowiak MR, Iskratsch T, Ghassemi S, Roca-Cusachs P, O’Shaughnessy B, Hone J, Sheetz MP (2016) Tropomyosin controls sarcomere-like contractions for rigidity sensing and suppressing growth on soft matrices. Nat Cell Biol 18(1):33–42
Yang B, Wolfenson H, Chung VY, Nakazawa N, Liu S, Hu J, Huang RY, Sheetz MP (2020) Stopping transformed cancer cell growth by rigidity sensing. Nat Mater 19(2):239–250
Belevich I, Verkhovsky MI, Wikström M (2006) Proton-coupled electron transfer drives the proton pump of cytochrome c oxidase. Nature 440(7085):829–832
Levin M (2014) Endogenous bioelectrical networks store non-genetic patterning information during development and regeneration. J Physiol 592(11):2295–2305
Cervera J, Meseguer S, Mafe S (2016) The interplay between genetic and bioelectrical signaling permits a spatial regionalisation of membrane potentials in model multicellular ensembles. Sci Rep 6(1):35201
Chifflet S, Hernández JA (2012) The plasma membrane potential and the organization of the actin cytoskeleton of epithelial cells. Int J Cell Biol 2012:1–13
Blackiston DJ, McLaughlin KA, Levin M (2009) Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle 8(21):3527–3536
Chernet BT, Fields C, Levin M (2015) Long-range gap junctional signaling controls oncogene-mediated tumorigenesis in Xenopus laevis embryos. Front Physiol 5:519
Levin M (2021) Bioelectric signaling: Reprogrammable circuits underlying embryogenesis, regeneration, and cancer. Cell 184(8):1971–1989
O’Rourke B, Cortassa S, Aon MA (2005) Mitochondrial ion channels: gatekeepers of life and death. Physiology 20(5):303–315
Whitaker M (2006) Calcium at fertilization and in early development. Physiol Rev 86(1):25–88
Huizar F, Soundarrajan D, Paravitorghabeh R, Zartman J (2020) Interplay between morphogen-directed positional information systems and physiological signaling. Dev Dyn 249(3):328–341
Pietak A, Levin M (2018) Bioelectrical control of positional information in development and regeneration: a review of conceptual and computational advances. Prog Biophys Mol Biol 137:52–68
Levin M (2012) Molecular bioelectricity in developmental biology: new tools and recent discoveries: control of cell behavior and pattern formation by transmembrane potential gradients. BioEssays 34(3):205–217
Lobikin M, Chernet B, Lobo D, Levin M (2012) Resting potential, oncogene-induced tumorigenesis, and metastasis: the bioelectric basis of cancer in vivo. Phys Biol 9(6):065002
Yang M, Brackenbury WJ (2013) Membrane potential and cancer progression. Front Physiol 4:185
Sundelacruz S, Levin M, Kaplan DL (2009) Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev Rep 5:231–246
Cone CD Jr, Cone CM (1976) Induction of mitosis in mature neurons in central nervous system by sustained depolarization. Science 192(4235):155–158
El Hady A, Machta BB (2015) Mechanical surface waves accompany action potential propagation. Nat Commun 6(1):6697
Aprea J, Calegari F (2012) Bioelectric state and cell cycle control of mammalian neural stem cells. Stem Cells Int 2012:816049. https://doi.org/10.1155/2012/816049
Petsakou A, Perrimon N (2022) Bioelectric regulation of intestinal stem cells. Trends Cell Biol 33(555):567
Srivastava P, Kane A, Harrison C, Levin M (2021) A meta-analysis of bioelectric data in cancer, embryogenesis, and regeneration. Bioelectricity 3(1):42–67
Pchelintseva E, Djamgoz MB (2018) Mesenchymal stem cell differentiation: control by calcium-activated potassium channels. J Cell Physiol 233(5):3755–3768
Soto-Cerrato V, Manuel-Manresa P, Hernando E, Calabuig-Fariñas S, Martínez-Romero A, Fernández-Dueñas V, Sahlholm K, Knöpfel T, García-Valverde M, Rodilla AM, Jantus-Lewintre E, Farràs R, Ciruela F, Pérez-Tomás R, Quesada R (2015) Facilitated anion transport induces hyperpolarization of the cell membrane that triggers differentiation and cell death in cancer stem cells. J Am Chem Soc 137(50):15892–15898
Strobl JS, Wonderlin WF, Flynn DC (1995) Mitogenic signal transduction in human breast cancer cells. Gen Pharmacol Vasc Syst 26(8):1643–1649
Wonderlin WF, Woodfork KA, Strobl JS (1995) Changes in membrane potential during the progression of MCF-7 human mammary tumor cells through the cell cycle. J Cell Physiol 165(1):177–185
Prevarskaya N, Skryma R, Shuba Y (2010) Ion channels and the hallmarks of cancer. Trends Mol Med 16(3):107–121
Bates E (2015) Ion channels in development and cancer. Annu Rev Cell Dev Biol 31:231–247
Sontheimer H, Fernandez-Marques E (1997) Ion channel expression and function in astrocytic scars. In: Jeserich G, Althaus HH, Richter-Landsberg C, Heumann R (eds) Molecular signaling and regulation in glial cells: a key to remyelination and functional repair. Springer, Berlin, pp 101–113
Mustard J, Levin M (2014) Bioelectrical mechanisms for programming growth and form: taming physiological networks for soft body robotics. Soft Robot 1(3):169–191
Fiske JL, Fomin VP, Brown ML, Duncan RL, Sikes RA (2006) Voltage-sensitive ion channels and cancer. Cancer Metastasis Rev 25:493–500
Kunzelmann K (2005) Ion channels and cancer. J Membr Biol 205:159–173
Wu L, Lian W, Zhao L (2021) Calcium signaling in cancer progression and therapy. FEBS J 288(21):6187–6205
Zhang C, Guo J (2023) Diverse functions of the inward-rectifying potassium channel Kir5. 1 and its relationship with human diseases. Front Physiol 14:302
Higashimori H, Sontheimer H (2007) Role of Kir4. 1 channels in growth control of glia. Glia 55(16):1668–1679
Pai VP, Martyniuk CJ, Echeverri K, Sundelacruz S, Kaplan DL, Levin M (2016) Genome-wide analysis reveals conserved transcriptional responses downstream of resting potential change in Xenopus embryos, axolotl regeneration, and human mesenchymal cell differentiation. Regeneration 3(1):3–25
Volkers L, Mechioukhi Y, Coste B (2015) Piezo channels: from structure to function. Pflügers Archiv Eur J Physiol 467:95–99
Pethő Z, Najder K, Bulk E, Schwab A (2019) Mechanosensitive ion channels push cancer progression. Cell Calcium 80:79–90
Chen X, Wanggou S, Bodalia A, Zhu M, Dong W, Fan JJ, Yin WC, Min H-K, Hu M, Draghici D, Dou W, Li F, Coutinho FJ, Whetstone H, Kushida MM, Dirks PB, Song Y, Hui C-C, Sun Y, Wang L-Y, Li X, Huang X (2018) A feedforward mechanism mediated by mechanosensitive ion channel PIEZO1 and tissue mechanics promotes glioma aggression. Neuron 100(4):799–815
Dombroski JA, Hope JM, Sarna NS, King MR (2021) Channeling the force: Piezo1 mechanotransduction in cancer metastasis. Cells 10(11):2815
Song Y, Li D, Farrelly O, Miles L, Li F, Kim SE, Lo TY, Wang F, Li T, Thompson-Peer KL, Gong J, Murthy SE, Coste B, Yakubovich N, Patapoutian A, Xiang Y, Rompolas P, Jan LY, Jan YN (2019) The mechanosensitive ion channel piezo inhibits axon regeneration. Neuron 102(2):373–389
Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DT, Bernardis E, Flanagan LA, Tombola F (2014) Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci 111(45):16148–16153
Jiang Y, Zhang H, Wang J, Liu Y, Luo T, Hua H (2022) Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J Hematol Oncol 15(1):34
An J, Hong H, Won M, Rha H, Ding Q, Kang N, Kang H, Kim JS (2023) Mechanical stimuli-driven cancer therapeutics. Chem Soc Rev 52(1):30–46
Decrock E, De Bock M, Wang N, Gadicherla AK, Bol M, Delvaeye T, Vandenabeele P, Vinken M, Bultynck G, Krysko DV, Leybaert L (2013) IP3, a small molecule with a powerful message. Biochim Biophys Acta: BBA Mol Cell Res 1833(7):1772–1786
Levin M, Mercola M (1998) Gap junctions are involved in the early generation of left–right asymmetry. Dev Biol 203(1):90–105
Oviedo NJ, Levin M (2007) Gap junctions provide new links in left-right patterning. Cell 129(4):645–647
Elias LA, Kriegstein AR (2008) Gap junctions: multifaceted regulators of embryonic cortical development. Trends Neurosci 31(5):243–250
Ho KY, Khadilkar RJ, Carr RL, Tanentzapf G (2021) A gap-junction-mediated, calcium-signaling network controls blood progenitor fate decisions in hematopoiesis. Curr Biol 31(21):4697–4712
Matta C, Zakany R (2013) Calcium signalling in chondrogenesis: implications for cartilage repair. Front Biosci Sch 5(1):305–324
Wong RC, Pera MF, Pébay A (2008) Role of gap junctions in embryonic and somatic stem cells. Stem Cell Rev 4:283–292
Schalper KA, Riquelme MA, Brañes MC, Martínez AD, Vega JL, Berthoud VM, Bennett MV, Saez JC (2012) Modulation of gap junction channels and hemichannels by growth factors. Mol BioSyst 8(3):685–698
Zhang W, Green C, Stott NS (2002) Bone morphogenetic protein-2 modulation of chondrogenic differentiation in vitro involves gap junction-mediated intercellular communication. J Cell Physiol 193(2):233–243
Upham BL (2011) Role of integrative signaling through gap junctions in toxicology. Curr Protoc Toxicol 47(1):2–18
Kren BT, Kumar NM, Wang SQ, Gilula NB, Steer CJ (1993) Differential regulation of multiple gap junction transcripts and proteins during rat liver regeneration. J Cell Biol 123(3):707–718
Hernández-Guerra M, Hadjihambi A, Jalan R (2019) Gap junctions in liver disease: implications for pathogenesis and therapy. J Hepatol 70(4):759–772
Hosoda T, Kajstura J, Leri A, Anversa P (2010) Mechanisms of myocardial regeneration. Circ J 74(1):13–17
Nogi T, Levin M (2005) Characterization of innexin gene expression and functional roles of gap-junctional communication in planarian regeneration. Dev Biol 287(2):314–335
Shi J, Barakat M, Chen D, Chen L (2018) Bicellular tight junctions and wound healing. Int J Mol Sci 19(12):3862
Livny O, Kaplan I, Reifen R, Polak-Charcon S, Madar Z, Schwartz B (2002) Lycopene inhibits proliferation and enhances gap-junction communication of KB-1 human oral tumor cells. J Nutr 132(12):3754–3759
Temme A, Buchmann A, Gabriel HD, Nelles E, Schwarz M, Willecke K (1997) High incidence of spontaneous and chemically induced liver tumors in mice deficient for connexin32. Curr Biol 7(9):713–716
King TJ, Bertram JS (2005) Connexins as targets for cancer chemoprevention and chemotherapy. Biochim Biophys Acta BBA Biomembr 1719(1–2):146–160
Mesnil M (2002) Connexins and cancer. Biol Cell 94(7–8):493–500
Pahujaa M, Anikin M, Goldberg GS (2007) Phosphorylation of connexin43 induced by Src: regulation of gap junctional communication between transformed cells. Exp Cell Res 313(20):4083–4090
Solan JL, Lampe PD (2014) Specific Cx43 phosphorylation events regulate gap junction turnover in vivo. FEBS Lett 588(8):1423–1429
DeCoursey TE (2018) Voltage and pH sensing by the voltage-gated proton channel, HV1. J R Soc Interface 15(141):20180108
Borrelli C, Buckley CT (2020) Synergistic effects of acidic ph and pro- inflammatory cytokines il-1β and tnf-α for cell-based intervertebral disc regeneration. Appl Sci 10(24):9009
Gnoni A, Giannoccaro C, Taurino F, Papa S, Zanotti F (2011) Ecto- FOF1 ATP synthase is associated with intracellular acidification induced by partial hepatectomy in rat hepatocytes. FEBS J 278:373–374
Schmidt JR, Geurtzen K, von Bergen M, Schubert K, Knopf F (2019) Glucocorticoid treatment leads to aberrant ion and macromolecular transport in regenerating zebrafish fins. Front Endocrinol 10:674
Takayama K, Muto A, Kikuchi Y (2018) Leucine/glutamine and V-atpase/lysosomal acidification via mtorc1 activation are required for position-dependent regeneration. Sci Rep. https://doi.org/10.1038/s41598-018-26664-2
Adams DS, Masi A, Levin M (2007) H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development 134(1323):1335
Venkatachalam K, Montell C (2007) TRP channels. Annu Rev Biochem 76:387–417
Levin M (2013) Reprogramming cells and tissue patterning via bioelectrical pathways: molecular mechanisms and biomedical opportunities. Wiley Interdiscip Rev Syst Biol Med 5(6):657–676
Anastassiou CA, Perin R, Markram H, Koch C (2011) Ephaptic coupling of cortical neurons. Nat Neurosci 14(2):217–223
Funk RH (2015) Endogenous electric fields as guiding cue for cell migration. Front Physiol 6:143
Nuccitelli R (2003) A role for endogenous electric fields in wound healing. Curr Top Dev Biol 58(2):1–26
Li Y, Weiss M, Yao L (2014) Directed migration of embryonic stem cell- derived neural cells in an applied electric field. Stem Cell Rev Rep 10:653–662
Messerli MA, Graham DM (2011) Extracellular electrical fields direct wound healing and regeneration. Biol Bull 221(1):79–92
Rajabi AH, Jaffe M, Arinzeh TL (2015) Piezoelectric materials for tissue regeneration: a review. Acta Biomater 24:12–23
Lin RZ, Ho CT, Liu CH, Chang HY (2006) Dielectrophoresis based-cell patterning for tissue engineering. Biotechnol J Healthc Nutr Technol 1(9):949–957
Yahya WNW, Kadri NA, Ibrahim F (2014) Cell patterning for liver tissue engineering via dielectrophoretic mechanisms. Sensors 14(7):11714–11734
Devaraj B, Usa M, Inaba H (1997) Biophotons: ultraweak light emission from living systems. Curr Opin Solid State Mater Sci 2(2):188–193
Jinno K, Hirata T, Watanabe A, Hatakeyama S, Akiyama T, Takahashi G, Kobayashi M, Mori A (2020) Extremely weak light emission during the healing process by biophotons emitted from wounds irradiated with atmospheric pressure plasma. Jpn J Appl Phys 59(10):100904
Moro C, Liebert A, Hamilton C, Pasqual N, Jeffery G, Stone J, Mitrofanis J (2022) The code of light: do neurons generate light to communicate and repair? Neural Regen Res 17(6):1251
Brabant C, Geerinck A, Beaudart C, Tirelli E, Geuzaine C, Bruyère O (2022) Exposure to magnetic fields and childhood leukemia: a systematic review and meta-analysis of case-control and cohort studies. Rev Environ Health 38:229–253
Pedersen C, Raaschou-Nielsen O, Rod NH, Frei P, Poulsen AH, Johansen C, Schüz J (2014) Distance from residence to power line and risk of childhood leukemia: a population-based case–control study in Denmark. Cancer Causes Control 25:171–177
Zhao L, Liu X, Wang C, Yan K, Lin X, Li S, Bao H, Liu X (2014) Magnetic fields exposure and childhood leukemia risk: a meta-analysis based on 11,699 cases and 13,194 controls. Leuk Res 38(3):269–274
French PW, Penny R, Laurence JA, McKenzie DR (2001) Mobile phones, heat shock proteins and cancer. Differentiation 67(4–5):93–97
Havas M (2017) When theory and observation collide: can non-ionizing radiation cause cancer? Environ Pollut 221:501–505
Aaron RK, Boyan BD, Ciombor DM, Schwartz Z, Simon BJ (2004) Stimulation of growth factor synthesis by electric and electromagnetic fields. Clin Orthop Relat Res 419:30–37
Fan W, Qian F, Ma Q, Zhang P, Chen T, Chen C, Zhang Y, Deng P, Zhou Z, Yu Z (2015) 50Hz electromagnetic field exposure promotes proliferation and cytokine production of bone marrow mesenchymal stem cells. Int J Clin Exp Med 8(5):7394
Wu S, Yu Q, Sun Y, Tian J (2018) Synergistic effect of a LPEMF and SPIONs on BMMSC proliferation, directional migration, and osteoblastogenesis. Am J Transl Res 10(5):1431
Goodman R, Blank M (2002) Insights into electromagnetic interaction mechanisms. J Cell Physiol 192(1):16–22
Kocaman A, Altun G, Kaplan AA, Deniz ÖG, Yurt KK, Kaplan S (2018) Genotoxic and carcinogenic effects of non-ionizing electromagnetic fields. Environ Res 163:71–79
Hamblin MR (2018) Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol 94(2):199–212
Xu W, Xie X, Wu H, Wang X, Cai J, Xu Z, E, S. (2022) Pulsed electromagnetic therapy in cancer treatment: progress and outlook. View 3:20220029
Murugan NJ, Rouleau N, Karbowski LM, Persinger MA (2018) Biophotonic markers of malignancy: discriminating cancers using wavelength-specific biophotons. Biochem Biophys Rep 13:7–11
Takeda M, Kobayashi M, Takayama M, Suzuki S, Ishida T, Ohnuki K, Moriya T, Ohuchi N (2004) Biophoton detection as a novel technique for cancer imaging. Cancer Sci 95(8):656–661
Burr HS, Harvey SC, Taffel M (1938) Bio-electric correlates of wound healing. Yale J Biol Med 11(2):103
Robinson KR, Messerli MA (2003) Left/right, up/down: the role of endogenous electrical fields as directional signals in development, repair and invasion. BioEssays 25(8):759–766
Sta. Iglesia DD, Vanable JW (1998) Endogenous lateral electric fields around bovine corneal lesions are necessary for and can enhance normal rates of wound healing. Wound Repair Regen 6(6):531–542
Zhao M (2009) Electrical fields in wound healing—an overriding signal that directs cell migration. Semin Cell Dev Biol 20(6):674–682
Shen Y, Pfluger T, Ferreira F, Liang J, Navedo MF, Zeng Q, Reid B, Zhao M (2016) Diabetic cornea wounds produce significantly weaker electric signals that may contribute to impaired healing. Sci Rep 6(1):26525
Sun YH, Luxardi G, Xu G, Zhu K, Reid B, Guo BP, Lebrilla CB, Maverakis E, Zhao M (2022) Surface glycans regulate salmonella infection-dependent directional switch in macrophage galvanotaxis independent of NanH. Infect Immun 90(1):e00516-e521
Vassilev PM, Dronzine RT, Vassileva MP, Georgiev GA (1982) Parallel arrays of microtubules formed in electric and magnetic fields. Biosci Rep 2(12):1025–1029
Chiang CC, Shivacharan RS, Wei X, Gonzalez-Reyes LE, Durand DM (2019) Slow periodic activity in the longitudinal hippocampal slice can self-propagate non-synaptically by a mechanism consistent with ephaptic coupling. J Physiol 597(1):249–269
Fröhlich F (2022) Endogenous and exogenous electric fields as modifiers of brain activity: rational design of noninvasive brain stimulation with transcranial alternating current stimulation. Dialogues Clin Neurosci 16(93):102
Pinotsis DA, Miller EK (2023) In vivo ephaptic coupling allows memory network formation. Cereb Cortex 33:9877–9895
Costin GE, Birlea SA, Norris DA (2012) Trends in wound repair: cellular and molecular basis of regenerative therapy using electromagnetic fields. Curr Mol Med 12(1):14–26
Henry SL, Concannon MJ, Yee GJ (2008) The effect of magnetic fields on wound healing: experimental study and review of the literature. Eplasty 8:e40
Jing D, Shen G, Cai J, Li F, Huang J, Wang Y, Xu Q, Tang C, Luo E (2010) Effects of 180 mT static magnetic fields on diabetic wound healing in rats. Bioelectromagnetics 31(8):640–648
Li L, El-Hayek YH, Liu B, Chen Y, Gomez E, Wu X, Ning K, Li L, Chang N, Zhang L, Wang Z, Hu X, Wan Q (2008) Direct-current electrical field guides neuronal stem/progenitor cell migration. Stem Cells 26(8):2193–2200
Cheng H, Huang Y, Yue H, Fan Y (2021) Electrical stimulation promotes stem cell neural differentiation in tissue engineering. Stem Cells Int 2021:1–14
Ramirez-Vick JE (2013) Biophysical stimulation for bone regeneration. JSM Biotechnol Biomed Eng 1(2):1014
Yao L, Li Y (2016) The role of direct current electric field-guided stem cell migration in neural regeneration. Stem Cell Rev Rep 12:365–375
Hoang NS, Sar C, Valmier J, Sieso V, Scamps F (2012) Electro- acupuncture on functional peripheral nerve regeneration in mice: a behavioural study. BMC Complement Altern Med 12(1):1–9
Liu YP, Luo ZR, Wang C, Cai H, Zhao TT, Li H, Shao SJ, Guo HD (2020) Electroacupuncture promoted nerve repair after peripheral nerve injury by regulating miR-1b and its target brain-derived neurotrophic factor. Front Neurosci 14:525144
Nuccitelli R (2011) Measuring endogenous electric fields. Physiol Bioelectr Dev Tissue Regen Cancer 1:1–16
Golberg A, Bruinsma BG, Uygun BE, Yarmush ML (2015) Tissue heterogeneity in structure and conductivity contribute to cell survival during irreversible electroporation ablation by “electric field sinks.” Sci Rep 5(1):8485
Beebe SJ, Fox PM, Rec LJ, Somers K, Stark RH, Schoenbach KH (2002) Nanosecond pulsed electric field (nsPEF) effects on cells and tissues: apoptosis induction and tumor growth inhibition. IEEE Trans Plasma Sci 30(1):286–292
Beebe SJ, Fox PM, Rec LJ, Willis LK, Schoenbach KH (2003) Nanosecond, high-intensity pulsed electric fields induce apoptosis in human cells. FASEB J 17(11):1–23
Kinzel A, Ambrogi M, Varshaver M, Kirson ED (2019) Tumor treating fields for glioblastoma treatment: patient satisfaction and compliance with the second-generation Optune® system. Clin Med Insights Oncol 13:1179554918825449
Wenger C, Salvador R, Basser PJ, Miranda PC (2016) Improving tumor treating fields treatment efficacy in patients with glioblastoma using personalized array layouts. Int J Radiat Oncol* Biol* Phys 94(5):1137–1143
Zipse DW (1993) Health effects of extremely low-frequency (50 and 60 Hz) electric and magnetic fields. IEEE Trans Ind Appl 29(2):447–458
Zhang G, Liu X, Liu Y, Zhang S, Yu T, Chai X, He J, Yin D, Zhang C (2023) The effect of magnetic fields on tumor occurrence and progression: recent advances. Prog Biophys Mol Biol 179:50
Baum A, Mevissen M, Kamino K, Mohr U, Löscher W (1995) A histopathological study on alterations in DMBA-induced mammary carcinogenesis in rats with 50 Hz, 100 μT magnetic field exposure. Carcinogenesis 16(1):119–125
Holmberg B (1995) Magnetic fields and cancer: animal and cellular evidence-an overview. Environ Health Perspect 103(Suppl 2):63–67
Chang CY, Lew WZ, Feng SW, Wu CL, Wang HH, Hsieh SC, Huang HM (2020) Static magnetic field-enhanced osteogenic differentiation of human umbilical cord-derived mesenchymal stem cells via matrix vesicle secretion. Int J Radiat Biol 96(9):1207–1217
Manikonda PK, Rajendra P, Devendranath D, Gunasekaran B, Aradhya RSS, Sashidhar RB, Subramanyam C (2007) Influence of extremely low frequency magnetic fields on Ca2+ signaling and NMDA receptor functions in rat hippocampus. Neurosci Lett 413(2):145–149
Huang YJ, Hoffmann G, Wheeler B, Schiapparelli P, Quinones-Hinojosa A, Searson P (2016) Cellular microenvironment modulates the galvanotaxis of brain tumor initiating cells. Sci Rep 6(1):1–10
Popp FA (2003) Properties of biophotons and their theoretical implications. Indian J Exp Biol 41:391–402
Rahnama M, Tuszynski JA, Bokkon I, Cifra M, Sardar P, Salari V (2011) Emission of mitochondrial biophotons and their effect on electrical activity of membrane via microtubules. J Integr Neurosci 10(01):65–88
Jiin-Ju C (2008) Physical properties of biophotons and their biological functions. Indian J Exp Biol 46(5):371
Bókkon I, Salari V, Tuszynski JA, Antal I (2010) Estimation of the number of biophotons involved in the visual perception of a single-object image: biophoton intensity can be considerably higher inside cells than outside. J Photochem Photobiol B 100(3):160–166
Pospíšil P, Prasad A, Rác M (2014) Role of reactive oxygen species in ultra-weak photon emission in biological systems. J Photochem Photobiol B 139:11–23
Guo J, Zhu G, Li L, Liu H, Liang S (2017) Ultraweak photon emission in strawberry fruit during ripening and aging is related to energy level. Open Life Sci 12(1):393–398
Zhao X, van Wijk E, Yan Y, van Wijk R, Yang H, Zhang Y, Wang J (2016) Ultra-weak photon emission of hands in aging prediction. J Photochem Photobiol B 162:529–534
Popp FA, Nagl W, Li KH, Scholz W, Weingärtner O, Wolf R (1984) Biophoton emission: new evidence for coherence and DNA as source. Cell Biophys 6:33–52
Andrabi M, Upton B, Lang RA, Vemaraju S (2023) An expanding role for nonvisual opsins in extraocular light sensing physiology. Annu Rev Vis Sci 9:245–267
Kumar S, Boone K, Tuszyński J, Barclay P, Simon C (2016) Possible existence of optical communication channels in the brain. Sci Rep 6(1):1–13
Sun Y, Wang C, Dai J (2010) Biophotons as neural communication signals demonstrated by in situ biophoton autography. Photochem Photobiol Sci 9(3):315–322
Zarkeshian P, Kumar S, Tuszynski J, Barclay P, Simon C (2017) Are there optical communication channels in the brain? arXiv:1708.08887
Niggli HJ, Applegate LA (2003) Biophotons: ultraweak photons in cells. In: Popp FA, Beloussov L (eds) Integrative biophysics. Springer, Dordrecht, pp 361–385. https://doi.org/10.1007/978-94-017-0373-4_11
Niggli HJ (1993) Artificial sunlight irradiation induces ultraweak photon emission in human skin fibroblasts. J Photochem Photobiol B 18(2–3):281–285
Dungel P, Hartinger J, Chaudary S, Slezak P, Hofmann A, Hausner T, Strassl M, Wintner E, Redl H, Mittermayr R (2014) Low level light therapy by LED of different wavelength induces angiogenesis and improves ischemic wound healing. Lasers Surg Med 46(10):773–780
Murugan NJ, Karbowski LM, Dotta BT, Vares DAE, Saroka KS, Lafrenie RM, Persinger MA (2016) Differentiation of malignant compared to non- malignant cells by their bio-photon emissions may only require a specific filter around 500 nm. J Cancer Sci Ther 8:170–171
Dotta BT, Murugan NJ, Karbowski LM, Lafrenie RM, Persinger MA (2014) Shifting wavelengths of ultraweak photon emissions from dying melanoma cells: their chemical enhancement and blocking are predicted by Cosic’s theory of resonant recognition model for macromolecules. Naturwissenschaften 101:87–94
Kim H, Ahn S, Kim J (2007) Biophoton emission induced by ultrasonic irradiation. In: World Congress on medical physics and biomedical engineering 2006: August 27–September 1, 2006 COEX Seoul, Korea “imaging the future medicine”. Springer, Berlin, pp 1277–1280
Huang H, Song W, Rieffel J, Lovell JF (2015) Emerging applications of porphyrins in photomedicine. Front Phys 3:23
de Freitas LF, Hamblin MR (2016) Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 22(3):348–364
Sroka R, Schaffer M, Fuchs C, Pongratz T, Schrader-Reichard U, Busch M, Schaffer PM, Dühmke E, Baumgartner R (1999) Effects on the mitosis of normal and tumor cells induced by light treatment of different wavelengths. Lasers Surg Med 25(3):263–271
Yoshimoto T, Morine Y, Takasu C, Feng R, Ikemoto T, Yoshikawa K, Iwahashi S, Saito Y, Kashihara H, Akutagawa M, Emoto T, Kinouchi Y, Shimada M (2018) Blue light-emitting diodes induce autophagy in colon cancer cells by Opsin 3. Ann Gastroenterol Surg 2(2):154–161
Matsumoto N, Yoshikawa K, Shimada M, Kurita N, Sato H, Iwata T, Higashijima J, Chikakiyo M, Nishi M, Kashihara H, Takasu C, Eto S, Takahashi A, Akutagawa M, Emoto T (2014) Effect of light irradiation by light emitting diode on colon cancer cells. Anticancer Res 34(9):4709–4716
Wieszczycka K, Staszak K, Woźniak-Budych MJ, Litowczenko J, Maciejewska BM, Jurga S (2021) Surface functionalization—the way for advanced applications of smart materials. Coord Chem Rev 436:213846
Smith AW, Hoyne JD, Nguyen PK, McCreedy DA, Aly H, Efimov IR, Rentschler S, Elbert DL (2013) Direct reprogramming of mouse fibroblasts to cardiomyocyte-like cells using Yamanaka factors on engineered poly (ethylene glycol)(PEG) hydrogels. Biomaterials 34(28):6559–6571
Zhu M, Tao H, Samani M, Luo M, Wang X, Hopyan S, Sun Y (2020) Spatial mapping of tissue properties in vivo reveals a 3D stiffness gradient in the mouse limb bud. Proc Natl Acad Sci 117(9):4781–4791. https://doi.org/10.1073/pnas.1912656117
Nabizadeh Z, Nasrollahzadeh M, Daemi H, Eslaminejad MB, Shabani AA, Dadashpour M, Mirmohammadkhani M, Nasrabadi D (2022) Micro-and nanotechnology in biomedical engineering for cartilage tissue regeneration in osteoarthritis. Beilstein J Nanotechnol 13(1):363–389
Downing TL, Soto J, Morez C, Houssin T, Fritz A, Yuan F, Chu J, Patel S, Schaffer DV, Li S (2013) Biophysical regulation of epigenetic state and cell reprogramming. Nat Mater 12(12):1154–1162
Devarasetty M, Skardal A, Cowdrick K, Marini F, Soker S (2017) Bioengineered submucosal organoids for in vitro modeling of colorectal cancer. Tissue Eng Part A 23(19–20):1026–1041
Zhou L, Ruiz-Puig C, Jacobs BA, Han X, Lisle R, Bayley H, Lu X (2021) Bioengineered gastrointestinal tissues with fibroblast-induced shapes. Adv Func Mater 31(6):2007514
Cui H, Miao S, Esworthy T, Lee SJ, Zhou X, Hann SY, Webster TJ, Harris BT, Zhang LG (2019) A novel near-infrared light responsive 4D printed nanoarchitecture with dynamically and remotely controllable transformation. Nano Res 12:1381–1388
Sundararaghavan HG, Masand SN, Shreiber DI (2011) Microfluidic generation of haptotactic gradients through 3D collagen gels for enhanced neurite growth. J Neurotrauma 28(11):2377–2387
Yeh YC, Corbin EA, Caliari SR, Ouyang L, Vega SL, Truitt R, Han L, Margulies KB, Burdick JA (2017) Mechanically dynamic PDMS substrates to investigate changing cell environments. Biomaterials 145:23–32
van Haaften EE, Wissing TB, Kurniawan NA, Smits AI, Bouten CV (2020) Human in vitro model mimicking material-driven vascular regeneration reveals how cyclic stretch and shear stress differentially modulate inflammation and matrix deposition. Adv Biosyst 4(6):1900249
Wahlsten A, Rütsche D, Nanni M, Giampietro C, Biedermann T, Reichmann E, Mazza E (2021) Mechanical stimulation induces rapid fibroblast proliferation and accelerates the early maturation of human skin substitutes. Biomaterials 273:120779
Ng CP, Hinz B, Swartz MA (2005) Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J Cell Sci 118(20):4731–4739
Novak CM, Horst EN, Taylor CC, Liu CZ, Mehta G (2019) Fluid shear stress stimulates breast cancer cells to display invasive and chemoresistant phenotypes while upregulating PLAU in a 3D bioreactor. Biotechnol Bioeng 116(11):3084–3097
Reed J, Walczak WJ, Petzold ON, Gimzewski JK (2009) In situ mechanical interferometry of matrigel films. Langmuir 25(1):36–39
Emon B, Bauer J, Jain Y, Jung B, Saif T (2018) Biophysics of tumor microenvironment and cancer metastasis-a mini review. Comput Struct Biotechnol J 16:279–287
Lee S, Stanton AE, Tong X, Yang F (2019) Hydrogels with enhanced protein conjugation efficiency reveal stiffness-induced YAP localization in stem cells depends on biochemical cues. Biomaterials 202:26–34
Liguori GR, Liguori TT, de Moraes SR, Sinkunas V, Terlizzi V, van Dongen JA, Sharma PK, Moreira LF, Harmsen MC (2020) Molecular and biomechanical clues from cardiac tissue decellularized extracellular matrix drive stromal cell plasticity. Front Bioeng Biotechnol 8:520
Vishwakarma SK, Lakkireddy C, Bardia A, Paspala SAB, Khan AA (2019) Engineering bio-mimetic humanized neurological constructs using acellularized scaffolds of cryopreserved meningeal tissues. Mater Sci Eng C 102:34–44
Ataee R, Ajdary S, Zarrindast M, Rezayat M, Shokrgozar MA, Ataee A (2010) Y25130 hydrochloride, a selective 5HT3 receptor antagonist has potent antimitogenic and apoptotic effect on HT29 colorectal cancer cell line. Eur J Cancer Prev 19(2):138–143
Yang M, Kozminski DJ, Wold LA, Modak R, Calhoun JD, Isom LL, Brackenbury WJ (2012) Therapeutic potential for phenytoin: targeting Nav1. 5 sodium channels to reduce migration and invasion in metastatic breast cancer. Breast Cancer Res Treat 134(2):603–615
Tseng AS, Beane WS, Lemire JM, Masi A, Levin M (2010) Induction of vertebrate regeneration by a transient sodium current. J Neurosci 30(39):13192–13200
Murugan NJ, Vigran HJ, Miller KA, Golding A, Pham QL, Sperry MM, Rasmussen-Ivey C, Kane AW, Kaplan DL, Levin M (2022) Acute multidrug delivery via a wearable bioreactor facilitates long-term limb regeneration and functional recovery in adult Xenopus laevis. Sci Adv 8(4):eabj2164
Phan NN, Wang CY, Chen CF, Sun Z, Lai MD, Lin YC (2017) Voltage-gated calcium channels: novel targets for cancer therapy. Oncol Lett 14(2):2059–2074
Bhargava A, Saha S (2019) T-Type voltage gated calcium channels: a target in breast cancer? Breast Cancer Res Treat 173:11–21
Pahor M, Guralnik JM, Salive ME, Corti MC, Carbonin P, Havlik RJ (1996) Do calcium channel blockers increase the risk of cancer? Am J Hypertens 9(7):695–699
Leppik LP, Froemel D, Slavici A, Ovadia ZN, Hudak L, Henrich D, Marzi I, Barker JH (2015) Effects of electrical stimulation on rat limb regeneration, a new look at an old model. Sci Rep 5(1):18353
Geremia NM, Gordon T, Brushart TM, Al-Majed AA, Verge VM (2007) Electrical stimulation promotes sensory neuron regeneration and growth- associated gene expression. Exp Neurol 205(2):347–359
Willand MP, Nguyen MA, Borschel GH, Gordon T (2016) Electrical stimulation to promote peripheral nerve regeneration. Neurorehabil Neural Repair 30(5):490–496
Miklavcic D, Sersa G, Novaković S, Rebersek S (1990) Tumor bioelectric potential and its possible exploitation for tumor growth retardation. J Bioelectr 9(2):133–149
Taylor TV, Engler P, Pullan BR, Holt S (1994) Ablation of neoplasia by direct current. Br J Cancer 70(2):342–345
Li C, Xiao C, Zhan L, Zhang Z, Xing J, Zhai J, Zhou Z, Tan G, Piao J, Zhou Y, Qi S, Wang Z, Yu P, Ning C (2022) Wireless electrical stimulation at the nanoscale interface induces tumor vascular normalization. Bioact Mater 18:399–408
Sordini L, Garrudo FF, Rodrigues CA, Linhardt RJ, Cabral JM, Ferreira FC, Morgado J (2021) Effect of electrical stimulation conditions on neural stem cells differentiation on cross-linked PEDOT: PSS films. Front Bioeng Biotechnol 9:591838
Zhu K, Hum NR, Reid B, Sun Q, Loots GG, Zhao M (2020) Electric fields at breast cancer and cancer cell collective galvanotaxis. Sci Rep 10(1):1–11
Lange F, Venus J, Shams Esfand Abady D, Porath K, Einsle A, Sellmann T, Neubert V, Reichart G, Linnebacher M, Köhling R, Kirschstein T (2022) Galvanotactic migration of glioblastoma and brain metastases cells. Life 12(4):580
Kemkemer R, Naggay BK, Schmidt TB, Ende K (2020) Development of a multi-well-chip for studying 2D and 3D tumor cell migration and spheroid growth in electrical fields. Curr Dir Biomed Eng 6(3):164–167
Maziarz A, Kocan B, Bester M, Budzik S, Cholewa M, Ochiya T, Banas A (2016) How electromagnetic fields can influence adult stem cells: positive and negative impacts. Stem Cell Res Ther 7(1):1–12
Wong CJK, Tai YK, Yap JLY, Fong CHH, Loo LSW, Kukumberg M, Fröhlich J, Zhang S, Li JZ, Wang J-W, Rufaihah AJ, Franco-Obregón A (2022) Brief exposure to directionally-specific pulsed electromagnetic fields stimulates extracellular vesicle release and is antagonized by streptomycin: a potential regenerative medicine and food industry paradigm. Biomaterials 287:121658
Hamid HA, Sarmadi VH, Prasad V, Ramasamy R, Miskon A (2022) Electromagnetic field exposure as a plausible approach to enhance the proliferation and differentiation of mesenchymal stem cells in clinically relevant scenarios. J Zhejiang Univ Sci B 23(1):42–57
Azadian E, Arjmand B, Khodaii Z, Ardeshirylajimi A (2019) A comprehensive overview on utilizing electromagnetic fields in bone regenerative medicine. Electromagn Biol Med 38(1):1–20
Dixon DT, Gomillion CT (2021) Conductive scaffolds for bone tissue engineering: current state and future outlook. J Funct Biomater 13(1):1
Jimenez H, Blackman C, Lesser G, Debinski W, Chan M, Sharma S, Watabe K, Lo H-W, Thomas A, Godwin D, Blackstock W, Mudry A, Posey J, O’Connor R, Brezovich I, Bonin K, Kim-Shapiro D, Barbault A, Pasche B (2018) Use of non-ionizing electromagnetic fields for the treatment of cancer. Front Biosci Landmark 23(2):284–297
Buckner CA, Buckner AL, Koren SA, Persinger MA, Lafrenie RM (2015) Inhibition of cancer cell growth by exposure to a specific time-varying electromagnetic field involves T-type calcium channels. PLoS ONE 10(4):e0124136
Patruno A, Ferrone A, Costantini E, Franceschelli S, Pesce M, Speranza L, Amerio P, D’angelo C, Felaco M, Grilli A, Reale M (2018) Extremely low-frequency electromagnetic fields accelerates wound healing modulating MMP-9 and inflammatory cytokines. Cell Prolif 51(2):e12432
Tatarov I, Panda A, Petkov D, Kolappaswamy K, Thompson K, Kavirayani A, Lipsky MM, Elson E, Davis CC, Martin SS, DeTolla LJ (2011) Effect of magnetic fields on tumor growth and viability. Comp Med 61(4):339–345
Löscher W, Mevissen M, Lehmacher W, Stamm A (1993) Tumor promotion in a breast cancer model by exposure to a weak alternating magnetic field. Cancer Lett 71(1–3):75–81
Hamblin MR, Liebert A (2022) Photobiomodulation therapy mechanisms beyond cytochrome c oxidase. Photobiomodulation Photomed Laser Surg 40(2):75–77
Wong TW, Wu EC, Ko WC, Lee CC, Hor LI, Huang IH (2018) Photodynamic inactivation of methicillin-resistant Staphylococcus aureus by indocyanine green and near infrared light. Dermatol Sin 36(1):8–15
Hosseinpour S, Fekrazad R, Arany PR, Ye Q (2019) Molecular impacts of photobiomodulation on bone regeneration: a systematic review. Prog Biophys Mol Biol 149:147–159
Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR (2016) Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: role of intracellular calcium and light-gated ion channels. Sci Rep 6(1):33719
Hamblin MR (2017) Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys 4(3):337
Hamblin MR, Nelson ST, Strahan JR (2018) Photobiomodulation and cancer: what is the truth? Photomed Laser Surg 36(5):241–245
Fávaro-Pípi E, Ribeiro DA, Ribeiro JU, Bossini P, Oliveira P, Parizotto NA, Tim C, de Araújo HS, Renno ACM (2011) Low-level laser therapy induces differential expression of osteogenic genes during bone repair in rats. Photomed Laser Surg 29(5):311–317
do Valle IB, Prazeres PHDM, Mesquita RA, Silva TA, de Castro Oliveira HM, Castro PR, Freitas IDP, Oliveira SR, Gomes NA, de Oliveira RF, Marquiore LF, Macari S, do Amaral FA, Jácome-Santos H, Barcelos LS, Menezes GB, Marques MM, Birbrair A, Diniz IMA (2020) Photobiomodulation drives pericyte mobilization towards skin regeneration. Sci Rep 10(1):1–15
Rosso MPDO, Buchaim DV, Kawano N, Furlanette G, Pomini KT, Buchaim RL (2018) Photobiomodulation therapy (PBMT) in peripheral nerve regeneration: a systematic review. Bioengineering 5(2):44
Murakami-Malaquias-Silva F, Rosa EP, Almeida PA, Schalch TO, Tenis CA, Negreiros RM, Horliana RF, Garcez AS, Fernandes MUR, Tortamano A, Motta LJ, Bussadori SK, Horliana ACRT (2020) Evaluation of the effects of photobiomodulation on orthodontic movement of molar verticalization with mini-implant: a randomized double-blind protocol study. Medicine 99(13):e19430
Lopes CB, Pinheiro AL, Sathaiah S, Silva NSD, Salgado MA (2007) Infrared laser photobiomodulation (λ 830 nm) on bone tissue around dental implants: a Raman spectroscopy and scanning electronic microscopy study in rabbits. Photomed Laser Surg 25(2):96–101
Jiang Y, Qi W, Zhang Q, Liu H, Zhang J, Du N, Nazempour R, Su Y, Fu R, Zhang K, Lyu P, Dong F, Yin L, Sheng X, Wang Y (2020) Green light-based photobiomodulation with an implantable and biodegradable fiber for bone regeneration. Small Methods 4(7):1900879
Hossu M, Ma L, Zou X, Chen W (2013) Enhancement of biophoton emission of prostate cancer cells by Ag nanoparticles. Cancer Nanotechnol 4(1):21–26
Bensadoun R-J, Epstein JB, Nair RG, Barasch A, Raber-Durlacher JE, Migliorati C, Genot-Klastersky M-T, Treister N, Arany P, Lodewijckx J, Robijns J, World Association for Laser Therapy (WALT) (2020) Safety and efficacy of photobiomodulation therapy in oncology: a systematic review. Cancer Med 9(22):8279–8300
Lu C, Zhou F, Wu S, Liu L, Xing D (2016) Phototherapy-induced antitumor immunity: long-term tumor suppression effects via photoinactivation of respiratory chain oxidase-triggered superoxide anion burst. Antioxid Redox Signal 24(5):249–262
Ottaviani G, Martinelli V, Rupel K, Caronni N, Naseem A, Zandonà L, Perinetti G, Gobbo M, Di Lenarda R, Bussani R, Benvenuti F, Giacca M, Biasotto M, Zacchigna S (2016) Laser therapy inhibits tumor growth in mice by promoting immune surveillance and vessel normalization. EBioMedicine 11:165–172
de Faria CM, Barrera-Patiño CP, Santana JP, de Avó LRDS, Bagnato VS (2021) Tumor radiosensitization by photobiomodulation. J Photochem Photobiol B 225:112349
de Pauli Paglioni M, Araújo ALD, Arboleda LPA, Palmier NR, Fonsêca JM, Gomes-Silva W, Madrid-Troconis CC, Silveira FM, Martins MD, Faria KM, Ribeiro ACP, Brandão TB, Lopes MA, Leme AFP, Migliorati CA, Santos-Silva AR (2019) Tumor safety and side effects of photobiomodulation therapy used for prevention and management of cancer treatment toxicities. A systematic review. Oral Oncol 93:21–28
Frigo L, Luppi JS, Favero GM, Maria DA, Penna SC, Bjordal JM, Bensadoun RJ, Lopes-Martins RA (2009) The effect of low-level laser irradiation (In-Ga-Al-AsP-660 nm) on melanoma in vitro and in vivo. BMC Cancer 9(1):1–8
Rhee YH, Moon JH, Choi SH, Ahn JC (2016) Low-level laser therapy promoted aggressive proliferation and angiogenesis through decreasing of transforming growth factor-β1 and increasing of Akt/Hypoxia inducible factor-1α in anaplastic thyroid cancer. Photomed Laser Surg 34(6):229–235
de C Monteiro JS, Pinheiro ALB, de Oliveira SC, Aciole GT, Sousa JA, Cangussu MC, Dos Santos JN (2011) Influence of laser phototherapy (λ660 nm) on the outcome of oral chemical carcinogenesis on the hamster cheek pouch model: histological study. Photomed Laser Surg 29(11):741–745
Ko UH, Choi J, Choung J, Moon S, Shin JH (2019) Physicochemically tuned myofibroblasts for wound healing strategy. Sci Rep 9(1):16070
Rhee YH, Moon JH, Choi SH, Ahn JC (2016) Low-level laser therapy promoted aggressive proliferation and angiogenesis through decreasing of transforming growth factor-β1 and increasing of Akt/Hypoxia inducible factor-1α in anaplastic thyroid cancer. Photomed Laser Surg 34(6):229–235
de C. Monteiro, JS, Pinheiro ALB, de Oliveira SC, Aciole GT, Sousa JA, Cangussu MC, Dos Santos JN (2011) Influence of laser phototherapy (λ660 nm) on the outcome of oral chemical carcinogenesis on the hamster cheek pouch model: histological study. Photomed Laser Surg 29(11):741–745
Ko UH, Choi J, Choung J, Moon S, Shin JH (2019) Physicochemically tuned myofibroblasts for wound healing strategy. Sci Rep 9(1):16070
Acknowledgements
The authors express sincere gratitude to Lauren Hugdahl for the schematic illustrations in the presented figures.
Funding
The authors acknowledge support from the National Sciences and Engineering Research Council of Canada (NSERC), Discovery Grant RGPIN-2021-03783 (to NJM), Discovery Grant RGPIN- 2022-04162 (to NR), and Discovery Grant RGPIN-2022-03260 (to SLP).
Author information
Authors and Affiliations
Contributions
NJM, SLP, NR equally contributed to the review conception and design. All authors contributed equally to the writing of this review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval and consent to participate
This manuscript does not require ethics approval or participation consent.
Consent for publication
All authors have read and approved its submission to this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Murugan, N.J., Cariba, S., Abeygunawardena, S. et al. Biophysical control of plasticity and patterning in regeneration and cancer. Cell. Mol. Life Sci. 81, 9 (2024). https://doi.org/10.1007/s00018-023-05054-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-05054-6