Abstract
Ultraweak intrinsic bioluminescence of cancer cell is a noninvasive method of assessing bioenergetic status of the investigated cells. This weak biophoton emission generated by prostate cancer cells (PC3) was measured in the presence of Ag nanoparticles and its correlation with singlet oxygen production was investigated. The comparison between nanoparticles concentration, bioluminescence intensity, and cell survival showed that Ag nanoparticles do not significantly affect cell survival at used concentration but they increase cell bioluminescent processes. It was also confirmed that singlet oxygen contributes to biophoton emission, that Ag nanoparticles increase this contribution, and that there are secondary mechanisms independent of singlet oxygen by which Ag nanoparticles contribute to increased cellular bioluminescence, possibly through plasmon resonance enhancement of intrinsic fluorescence.
Similar content being viewed by others
1 Introduction
Luminescence investigation is a fundamental tool in cellular biology and a useful method in understanding molecular mechanisms of medical therapies. One of these methods is the measurement of intrinsic bioluminescence of living tissue, process also called biophoton emission (BPE) (Popp et al. 1988; Cohen and Popp 1997; Kobayashi and Inaba 2000; Chang 2008). The advantage of using BPE is that it monitors intrinsic processes of the investigated biological system versus the interaction of the system with an external stimulus, be it light in fluorescence, magnetic field in MRI, X-ray in CT, etc. It was demonstrated that all cells emits light during normal metabolic processes ( Kobayashi and Inaba 2000; Chang 2008). However, due to its extremely low intensity and its sensitivity to interaction with ambient light, this intrinsic bioluminescence was rarely used as a monitoring tool for cells’ physiology or pathology. The typical light emission is in the order of tens of photons per second per square centimeter of tissue hence the term biophoton is used more often and only very sensitive and very low noise phototomultipliers are used to record it. One of the emission’s mechanisms was correlated with the generation of metastable excited states by high energetic metabolic processes (cellular respiration, phagocytosis, mitosis, neural activity) and by oxidative reactions (Cilento 1988; Villablanca and Cilento 1985; Devaraj et al. 1997; Van Wijk et al. 2008). Most common identified excited molecules are intrinsic fluorophores, singlet oxygen, or excited carbonil (Villablanca and Cilento 1985). Other suggested sources are excitons in macromolecules, particularly DNA and collagen ( Popp et al. 1984; Brizhik et al. 2001; Brizhik 2008). Since all these mechanisms are intimately related to each cell’s functions, BPE analysis was proposed as a noninvasive descriptor at deep quantum level of biological systems (Cohen and Popp 1997; Chang 2008; Popp et al. 1994; Hossu and Rupert 2006) and it was suggested to be a global indicator of viability, reactivity, and health of a living organism (Popp et al. 1994; Bajpai 2003; Hossu and Rupert 2006).
Since cancer is one of the major causes of death any new in vivo and in vitro study could potentially reflect into life-saving protocols. Understanding molecular events including luminescence in cancer could provide helpful insights on the action mechanism of various therapies, whereas monitoring cells behavior through luminescence could also help in identifying specific points of intervention into cellular functions. Few attempts were performed to measure the intrinsic BPE of cancer cells (Grasso et al. 1992; Amano et al. 1995; Kim et al. 2005a, b, 2006) indentifying mostly the differences between cancer and normal tissues. Based on these results BPE was also proposed as a possible noninvasive imaging method to identify cancer (Kim et al. 2006; Takeda et al. 2004; Popp 2009).
On the other hand silver nanoparticles (AgNP) were extensively evaluated for their interaction with biological systems, mostly for their antimicrobial and antiseptic properties (Lansdown 2006; Chen and Schluesener 2008; Rai et al. 2009). AgNP’s low toxicity for normal cells and their intrinsic antimicrobial characteristics might be of benefit if they will also be used in cancer therapy (Nowack 2010). However, recent data show that in higher concentrations, AgNP may be cytotoxic (El Badawy et al. 2011; Hackenberg et al. 2011; AshaRani et al. 2009), effect that may be detrimental for normal physiology but helpful in oncology. It was also shown that in a plant system AgNP intensify BPE (Hossu et al. 2010) without interfering with normal plant curing mechanisms; however, no data are available for correlation between intrinsic BPE of cancer cells and AgNP. Therefore this BPE study of cancer cells in the presence of AgNP will provide a window into direct effect of these NPs onto high energetic cellular processes, without the interferences from external intervention. Since one major therapeutic mechanism in cancer is based on reactive oxygen species (ROS) mainly singlet oxygen (1O2) and 1O2 is also involved in BPE we specifically tested the correlation between BPE and 1O2 generation in the presence of AgNP.
2 Materials and methods
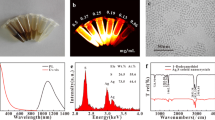
Chemicals used were of analytical grade from Sigma Chemical Co. (St. Louis, MO) and used without further purification. 1O2 chemiluminescent sensor: trans-1-(2′-Methoxyvinyl)pyrene (MVP) was purchased from Invitrogen (Carlsbad, CA) and diluted to a concentration of 10 μM before use. Sodium azide (NaN3) solution was prepared in 10 μM concentration. AgNP were prepared according to previously described methods by standard wet chemical synthesis based on reduction reactions (Huang et al. 2007; Zhang et al. 2008; Hossu et al. 2010). The initial dimensions of AgNPs were estimated to be 6–20 nm, verified by dynamic light scattering and transmission electron microscopy as shown in Fig. 1. They were kept in a separate dark storage chamber at room temperature. The initial concentration was estimated at 10−8 M (6 × 109 NP/μL) based on chemistry ratios and confirmed using absorption spectrometry.
A photon counting system (Hamamatsu Photonics K.K., Hamamatsu, Japan) was used to observe time-dependent photon emission intensity. The system is equipped with a H6180-1 photomultiplier tube (PMT) providing a maximum spectral response from 240 to 630 nm and a C8855 counting system, operating at room temperature. The gate time for collecting the photon signal from the PMT was set at 1 s. The measuring room was light proofed with dark materials and only a red radiological safe light was used during the manipulation of the chemicals and cells to minimize delayed luminescence. The PMT was placed inside a custom made dark chamber equipped with thermostat controlled heating pad and the dark count was measured at the beginning of each experiment to ensure that its value was at the level of the instrument noise, i.e., seven to nine counts per second (CPS). This insured that any signal recorded was from the sample and not due to transient changes of a residual light in the room or in the dark chamber. The distance between the PMT and the sample was 5 mm in all measurements. Each set of data consisting of up to 10,000 measurements was recorded using C8855’s operating software (Hamamatsu, Japan) and processed using Microsoft® Office Excel™ 2007 and OriginPro™ 8.5.0.
PC3 cell line was purchased from ATCC (American Type Culture Collection, Manassas, VA) and cultured using supplier’s media and instructions. For BPE measurements, the cells were subcultured in six-well plates using 1 mL of suspension, 1 × 105 cells/mL, in an MCO-18AIC Sanyo incubator (Sanyo North America, San Diego, CA) at 370C and 5 % CO2. The cells were left to reach 80 % confluence for 48 h. Various concentrations of AgNPs solution were added corresponding to a range of 10−10 to 10−9 M. For 1O2 evidence 10 μL of MVP or NaN3 were added to the cell wells immediately before measurements. All experiments were performed in triplicates and average results and standard deviation were presented.
Cytotoxicity was assessed by trypan blue dye exclusion and by colorimetric 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) cell viability assay. For this, an average of 103 cells/well were seeded into 96-well plates and allowed to grow for 48 h in the incubator. Thereafter the confluency was verified, the culture medium was removed and 100 μL of new media containing AgNP was added using stock solution and its 1:10 and 1:100 dillutions. Comparing with concentration used for BPE measurements, this is equivalent to 10−8 , 10−9, and 10−10 M concentrations in the BPE experiment, where we used 105 cells seeded per 1 mL. The solvent (PBS, PH 7.4) was used as blanks and fully vital cells that were not exposed to the nanoparticles as controls. After 24 h incubation 10 μl of MTT stock solution (5 mg/ml) was added to each well. Cell viability was measured 4 h later after the supernatant was removed and cells were lysed with 100 μl of acidic isopropanol (0.04 N HCl in isopropanol). Absorbance was recorded at 540 nm using a microplate reader (Fisher Scientific, Hudson, NH). The OD value of the control cells (nanoparticle-free medium) was taken as 1.0 and proportionate change in OD as affected by AgNP, MVP, and NaN3 were determined. All experiments were set up in triplicates and repeated twice for statistical analysis. Results were expressed as mean ± standard deviation.
3 Results and discussions
Measuring biophoton emission from various cell lines was seldom performed due to the low intensity and the presence of delayed luminescence (DL) after light exposure. Long-time measurements indicate that the average luminescence depends on the number of cells and the phase of growing (Takeda et al. 2004; Popp 2009). In the plateau phase, the average luminescence however is constant for hours. The most important interfering factor is the DL due to exposure to light during experimental procedures. However, sub-culturing the cells in a closed incubator inside a dark room and transferring the cells from the incubator to the detection area in an environment illuminated only with a red safe light minimized the exposure to light. In the same time, the illumination with red light provided the least amount of energy compared with shorter wavelengths or white light reducing the probability of external photo-excitation of intrinsic chromophores or photosensitive biological processes. Typical kinetics of the measurements is shown in Fig. 2 displaying a decay curve and low intensity signal that stabilizes in minutes. It can be seen from Fig. 2 that after 3 to 5 min in our experimental setup, the effects of DL vanished and steady-state measurements could be recorded. All further results analyzed here were performed after DL vanished and the emission reached the steady-state phase. The collecting time for each steady measurement was 200 s (i.e., the last 200 s in the 500 s measurement displayed in Fig. 2).
The preincubation with AgNP leads to increase steady-state luminescence as shown in Fig. 3. It can be seen that the presence of AgNP enhances the BPE in an almost linear fashion at lower concentration values while reaching a plateau at higher concentration. This behavior is usually seen in most luminescence experiments due to multiple factors including concentration quenching, scattering, and absorption. Nevertheless, AgNPs act as a stimulator of luminescent processes, effect that may be useful in imaging or in light therapies. In order to better understand the process by which BPE is enhanced in the presence of AgNP, further investigations were conducted.
The mechanisms of light emission are a subject of continuous debate in the biophotonic field (Popp et al. 1988; Cohen and Popp 1997; Kobayashi and Inaba 2000; Chang 2008; Brizhik 2008; Bajpai 2003). Chemiluminescence as a mechanism of producing excited species that emit light by themselves or transfer their energy to intrinsic fluorophores is a widespread model. Although spectral analysis is not feasible due to very low emission intensities some data suggest a broad emission in the entire visible spectrum (Popp et al. 1988, 2003, 1994) therefore the identification of emitting species is not an easy task. Among suggested excited species one of major importance for biological studies that can trigger a cascade of other excited species is singlet oxygen (1O2) (Villablanca and Cilento 1985; Cilento 1988; Rastogi and Pospíšil 2010). Detecting 1O2 can be done by direct measurements by its infrared emission at 1,270 nm or by indirect measurements using inhibitors or sensors selectively sensitive to 1O2. In our experiments, we employed the latter method using MVP as a 1O2 sensor and NaN3 as a 1O2 scavenger. The effects of the addition of the singlet oxygen luminescent sensor (MVP) or scavenger (NaN3) are presented in Fig. 4 for the normal cell culture to establish reference values. The presence of the 1O2 scavenger decreased the intensity of luminescence while the presence of the 1O2 sensor increases it confirming the presence of 1O2 in normal cells. The effect of AgNP (at 10−10 M) on the BPE recorded in the presence of NaN3 and MVP is shown in Fig. 5. The results show that the addition of NaN3 decreases BPE whereas MVP addition increases the BPE in similar manner as with cells cultivated in normal media. It can be seen that the presence of AgNP also increases BPE in the presence of NaN3, the 1O2 scavenger, however, at a less extent than in the control cells (1.2 versus 1.4 cps). Calculating relative change in luminescence as [L(test) − L(control)] / L(control) where L is the average luminescence after subtracting the background we obtain a 33 % decrease in luminescence with addition of NaN3. This reduction is decreased to 11 % in the presence of AgNP.
The results presented in Fig. 4 confirm that 1O2 is generated during normal cell metabolism as sensed by MVP and that reactions initiated by 1O2 contributes at least one third of the average cell’s luminescence as shown by inhibition with NaN3. This complements previous results of Van Wijk (2008) and of Rastogi and Pospíšil (2010) for normal cells, by demonstrating that for cancer cells there also is continuous 1O2 production and that 1O2 is one but not the only source of BPE. On the other hand, AgNP increases the BPE in all cases, including in the presence of MVP and NaN3 (Fig. 5). As a control, a direct test of MVP luminescence generated by direct interaction with AgNp was performed and no MVP emission was detected for concentrations used above. Both the increase of the luminescence in the presence of MVP and its quenching by NaN3 confirms that AgNP themselves contribute to generation of singlet oxygen, hypothesis suggested before (Hossu et al. 2010; Ahamed et al. 2008; Carlson et al. 2008). The phenomenon is believed to be initiated by superoxide radicals that are produced directly at AgNP interface, released into the media where they initiate the cascade of reactions common to ROS. These reactions are already part of the cellular metabolism leading to singlet oxygen and excited carbonyl groups (Kobayashi and Inaba 2000; Chang 2008) that emits light themselves or further trigger chemical reactions involving secondary excited species. Nevertheless, the presence of AgNPs in the concentration used in our experiments confirms that they enhance the BPE due to the contribution of these oxidative reactions initiated by 1O2.
The second mechanism of BPE that can be affected by AgNP is the intrinsic cellular florescence (Hossu et al. 2010; Popp 2008). In this case, high energetic chemical reactions instead of leading directly to luminescent species, transfer the energy of the intermediate excited complex to vicinity fluorophores. On the other hand, it was demonstrated that metal nanoparticles enhances the surrounding electromagnetic field through a plasmon resonance mechanism (Aslan et al. 2005; Chen and Sun 2008; Biju et al. 2008; Lakowicz et al. 2008), leading to enhance excitation and to stimulated fluorescent emission. For this process to happen, the NPs need to be located in the vicinity of luminescent molecules at very short distances and for duration longer than relaxation time. The absorption of AgNPs inside various compartments of cells has already been documented (Carlson et al. 2008; Davda and Labhasetwar 2002). The process is dependent of many variables like: cell type, exposure surface, AgNP size, and surface coating. In time, due to internal cellular traffic NPs will be segregated and they may also agglomerate. Taking into account that the cellular content is an actual gel like structure and that there are plenty of exposed –SH and –NH2 groups that interact strongly with AgNP, it can be inferred that the average distance between AgNP and optic active molecules inside cells is short enough and fluctuates slow enough to allow for energy transfer to these other luminescent species to take place. Corroborating these facts with our results that 1O2 scavenger could not prevent increase of BPE by AgNPs, we consider then that AgNPs also increase bioluminescence by plasmon resonance enhancement of energy transfer to vicinity chromophores and of their fluorescence.
Finally, some of the BPE reactions were suggested to be the result of reactions involved in cell death and apoptosis [Cohen and Popp 1997; Sławinski 2003], mostly due to unchecked oxidative reactions. Since this imply that AgNP toxicity in addition to altering BPE will also induce cell death, we verified if cell destruction by AgNP happens in our case by using MTT cell viability assay. Due to the fact that the initial concentration of cells used for the assay was smaller than the one used in BPE emission, we could study the effects of AgNP in concentration up to ten times higher than initial relative concentration. The results of the assay are shown in Fig. 6. The results demonstrate that even at concentration of AgNP ten times higher than those used in BPE measurements the viability of the cells was statistically similar. Therefore, we can conclude that at used concentration, AgNP does not enhance apoptotic cellular mechanisms of PC3 cells. On the other hand, the fact that ROS are generated via 1O2 mechanism, it opens the door for AgNP to be used as possible sensitizer in therapies that implies the presence of 1O2 and ROS as a mechanism of action.
4 Conclusions
The effect of AgNP on human tissue is of real interest due to increase presence of AgNP in household and therapeutic available products. We demonstrated that AgNP enhanced BPE in PC3 cells, that the emission increases with AgNP concentration, and that AgNP do not affect cell viability in concentrations used. One proposed mechanism is the enhanced production of 1O2 and the results supporting this theory were presented. The luminescence was not completely prevented by 1O2 scavengers suggesting that other oxygen independent mechanisms also contribute to BPE. Reasons to support plasmon resonant enhancement of intrinsic fluorescence as a second BPE enhancing mechanism were also discussed.
Abbreviations
- BPE:
-
Biophoton emission
- NP:
-
Nanoparticles
- PMT:
-
Photonultiplier tube
- CPS:
-
Counts per second
- ROS:
-
Reactive oxygen species
References
Ahamed M, Karns M, Goodson M, Rowe J, Hussain SM, Schlager JJ, Hong Y (2008) DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol Appl Pharm 233:404–410
Amano T, Kobayashi M, Devaraj B, Usa M, Inaba H (1995) Ultraweak biophoton emission imaging of transplanted bladder cancer. Urol Res 23(5):315–318
AshaRani PV, Mun GL, Prakash Hande M, Valiyaveettil S (2009) Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3(2):279–290
Aslan K, Lakowicz JR, Geddes CD (2005) Plasmon light scattering in biology and medicine new sensing approaches, visions and perspectives. Curr Opin Chem Biol 9(5):538–544
Bajpai RP (2003) Quantum coherence of biophotons and living systems. Indian J Exp Biol 41(5):514–527
Biju V, Itoh T, Anas A, Sujith A, Ishikawa M (2008) Semiconductor quantum dots and metal nanoparticles syntheses, optical properties, and biological applications. Anal Bioanal Chem 391(7):2469–2495
Brizhik L (2008) Nonlinear mechanism for weak photon emission from biosystems. Indian J Exp Biol 46(5):353–357
Brizhik L, Scordino A, Triglia A, Musumeci F (2001) Delayed luminescence of biological systems arising from correlated many soliton states. Phys Rev E 64:031902
Carlson C, Hussain SM, Schrand A, Braydich-Stolle L, Hess K, Jones R, Schlager J (2008) Unique cellular interaction of silver nanoparticles size dependent generation of reactive oxygen species. J Phys Chem B 112:13608–13619
Chang JJ (2008) Physical properties of biophotons and their biological functions. Indian J Exp Biol 46(5):371–377
Chen X, Schluesener HJ (2008) Nanosilver a nanoproduct in medical application. Toxicol Lett 176(1):1–12
Chen W, Sun XK (2008) Luminescent nanoparticles for biological applications Imaging, therapy, and targeting strategies. In: Chen X (ed) Recent advances of bioconjugate chemistry in molecular imaging. Research Signpost, Kerala, India, pp 399–449
Cilento G (1988) Photochemistry without light. Experientia 44:572–576
Cohen S, Popp FA (1997) Biophoton emission of the human body. J Photochem Photobiol B 40(2):187–189
Davda J, Labhasetwar V (2002) Characterization of nanoparticle uptake by endothelial cells. Int J Pharm 233:51–59
Devaraj B, Usa M, Inaba H (1997) Biophotons ultraweak light emission from living systems. Curr Opin Solid State Mat Sci 2:188–193
El Badawy AM, Silva RG, Morris B, Scheckel KG, Suidan MT, Tolaymat TM (2011) Surface charge-dependent toxicity of silver nanoparticles. Environ Sci Technol 45(1):283–287
Grasso F, Grillo C, Musumeci F, Triglia A, Rodolico G, Cammisuli F, Rinzivillo C, Fragati G, Santuccio A, Rodolico M (1992) Photon emission from normal and tumor human tissues. Experientia 48:10–13
Hackenberg S, Scherzed A, Kessler M, Hummel S, Technau A, Froelich K, Ginzkey C, Koehler C, Hagen R, Kleinsasser N (2011) Silver nanoparticles evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol Lett 201:27–33
Hossu M, Rupert R (2006) Quantum events of biophoton emission associated with complementary and alternative medicine therapies a descriptive pilot study. J Altern Complement Med 12(2):119–124
Hossu M, Ma L, Chen W (2010) Nonlinear enhancement of spontaneous biophoton emission of sweet potato by silver nanoparticles. J Photochem Photobiol B 99(1):44–48
Huang T, Nallathamby PD, Gillet D, Xu XH (2007) Design and synthesis of single-nanoparticle optical biosensors for imaging and characterization of single receptor molecules on single living cells. Anal Chem 79(20):7708–7718
Kim HW, Sim SB, Kim CK, Kim J, Choi C, You H, Soh KS (2005a) Spontaneous photon emission and delayed luminescence of two types of human lung cancer tissues adenocarcinoma and squamous cell carcinoma. Cancer Lett 229(2):283–289
Kim J, Choi C, Lim J, You H, Sim SB, Yom YK, Kim EH, Soh KS (2005b) Measurements of spontaneous ultraweak photon emission and delayed luminescence from human cancer tissues. J Altern Complement Med 11(5):879–884
Kim J, Lim J, Kim H, Ahn S, Sim SB, Soh KS (2006) Scanning spontaneous photon emission from transplanted ovarian tumor of mice using a photomultiplier tube. Electromagn Biol Med 25(2):97–102
Kobayashi M, Inaba H (2000) Photon statistics and correlation analysis of ultraweak light originating from living organisms for extraction of biological information. Appl Opt 39(1):183–192
Lakowicz JR, Ray K, Chowdhury M, Szmacinski H, Fu Y, Zhang J, Nowaczyk K (2008) Plasmon-controlled fluorescence a new paradigm in fluorescence spectroscopy. Analyst 133(10):1308–1346
Lansdown AB (2006) Silver in health care antimicrobial effects and safety in use. Curr Probl Dermatol 33:17–34
Nowack B (2010) Chemistry nanosilver revisited downstream science 330(6007):1054–1055
Popp FA (2003) Properties of biophotons and their theoretical implications. Indian J Exp Biol 41(5):391–402
Popp FA (2008) Principles of complementary medicine in terms of a suggested scientific basis. Indian J Exp Biol 46(5):378–383
Popp FA (2009) Cancer growth and its inhibition in terms of coherence. Electromagn Biol Med 28(1):53–60
Popp FA, Nagl W, Li KH, Scholz W, Weingärtner O, Wolf R (1984) Biophoton emission. New evidence for coherence and DNA as source. Cell Biophys 6(1):33–52
Popp FA, Li KH, Mei WP, Galle MR, Neurohr R (1988) Physical aspects of biophotons. Experientia 44(7):576–585
Popp FA, Gu Q, Li KH (1994) Biophoton emission experimental background and theoretical approaches. Mod Phys Lett B 8:1269–1296
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27(1):76–83
Rastogi A, Pospíšil P (2010) Ultra-weak photon emission as a non-invasive tool for monitoring of oxidative processes in the epidermal cells of human skin: comparative study on the dorsal and the palm side of the hand. Skin Res Tech 16(3):365–370
Sławinski J (2003) Biophotons from stressed and dying organisms toxicological aspects. Indian J Exp Biol 41(5):483–493
Takeda M, Kobayashi M, Takayama M, Suzuki S, Ishida T, Ohnuki K, Moriya T, Ohuchi N (2004) Biophoton detection as a novel technique for cancer imaging. Cancer Sci 95(8):656–661
Van Wijk R, Van Wijk EP, Wiegant FA, Ives J (2008) Free radicals and low-level photon emission in human pathogenesis state of the art. Indian J Exp Biol 46(5):273–309
Villablanca M, Cilento G (1985) Enzymatic generation of electronically excited states by electron transfer. Photochem Photobiol 42:591–597
Zhang Y, Peng H, Huang W, Zhou Y, Yan D (2008) Facile preparation and characterization of highly antimicrobial colloid Ag or Au nanoparticles. J Colloid Interface Sci 325(2):371–376
Acknowledgments
We would like to acknowledge the support from the startup funds from UTA, the NSF and DHS joint ARI program (2011-DN-077-ARI053-03, CBET-1039068), DOD DTRA08-005, and the U.S. Army Medical Research Acquisition Activity (USAMRAA) under Contracts of W81XWH-10-1-0279 and W81XWH-10-1-0234. We are also grateful to Dr. Ronald Rupert from Parker University, Dallas, TX, for providing the Hamamatsu Photomultiplier.
Highlights
Enhanced biophoton emission of PC3 cancer cells was detected in the presence of Ag nanoparticles (NP); cells survival is not affected by the presence of AgNP; singlet oxygen contribution to bioluminescence was demonstrated; plasmon resonance enhancement of intrinsic fluorescence by AgNP is discussed as a secondary bioluminescence enhancer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Hossu, M., Ma, L., Zou, X. et al. Enhancement of biophoton emission of prostate cancer cells by Ag nanoparticles. Cancer Nano 4, 21–26 (2013). https://doi.org/10.1007/s12645-013-0034-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12645-013-0034-7