Abstract
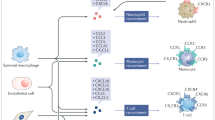
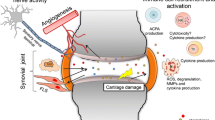
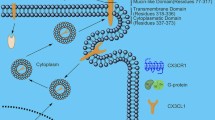
Chemokines are pivotal players in instigation and perpetuation of synovitis through leukocytes egress from the blood circulation into the inflamed articulation. Multitudinous literature addressing the involvement of the dual-function interferon (IFN)-inducible chemokines CXCL9, CXCL10 and CXCL11 in diseases characterized by chronic inflammatory arthritis emphasizes the need for detangling their etiopathological relevance. Through interaction with their mutual receptor CXC chemokine receptor 3 (CXCR3), the chemokines CXCL9, CXCL10 and CXCL11 exert their hallmark function of coordinating directional trafficking of CD4+ TH1 cells, CD8+ T cells, NK cells and NKT cells towards inflammatory niches. Among other (patho)physiological processes including infection, cancer, and angiostasis, IFN-inducible CXCR3 ligands have been implicated in autoinflammatory and autoimmune diseases. This review presents a comprehensive overview of the abundant presence of IFN-induced CXCR3 ligands in bodily fluids of patients with inflammatory arthritis, the outcomes of their selective depletion in rodent models, and the attempts at developing candidate drugs targeting the CXCR3 chemokine system. We further propose that the involvement of the CXCR3 binding chemokines in synovitis and joint remodeling encompasses more than solely the directional ingress of CXCR3-expressing leukocytes. The pleotropic actions of the IFN-inducible CXCR3 ligands in the synovial niche reiteratively illustrate the extensive complexity of the CXCR3 chemokine network, which is based on the intercommunion of IFN-inducible CXCR3 ligands with distinct CXCR3 isoforms, enzymes, cytokines, and infiltrated and resident cells present in the inflamed joints.






Similar content being viewed by others
Data availability
Information supporting the manuscript can be found in the Supplementary Material.
Abbreviations
- AA:
-
Rat adjuvant arthritis
- ACKR:
-
Atypical chemokine receptor
- ACR:
-
American College of Rheumatology
- AOSD:
-
Adult-onset Still’s disease
- AS:
-
Ankylosing spondylitis
- BMMs:
-
Bone marrow-derived macrophages
- CAIA:
-
Type II collagen antibody-induced arthritis
- cAMP:
-
Cyclic adenosine monophosphate
- CDAI:
-
Clinical disease activity index
- CFA:
-
Complete Freund’s adjuvant
- CIA:
-
Type II collagen-induced arthritis
- CRP:
-
C-reactive proteins
- DARC:
-
Duffy antigen receptor for chemokines
- DAS28:
-
Disease activity score in 28 joints
- DC:
-
Dendritic cell
- DMARDs:
-
Disease-modifying anti-rheumatic drugs
- DPP:
-
Dipeptidyl peptidase
- ERK:
-
Extracellular signal-regulated kinase
- ESR:
-
Erythrocyte sedimentation rate
- FLS:
-
Fibroblast-like synoviocytes
- GAG:
-
Glycosaminoglycan
- GATA3:
-
GATA-binding protein 3
- GPCR:
-
G protein-coupled receptor
- HC:
-
Healthy control
- HEK:
-
Human embryonal kidney
- HLH:
-
Hemophagocytic lymphohistiocytosis
- IFN:
-
Interferon
- IL-:
-
Interleukin
- IP-10/CXCL10:
-
Interferon-\(\gamma\) inducible protein of 10 kDa
- IRSE:
-
Interferon response element
- I-TAC/CXCL11:
-
Interferon-inducible T-cell α chemoattractant
- JIA:
-
Juvenile idiopathic arthritis
- MAS:
-
Macrophage activation syndrome
- Mig/CXCL9:
-
Monokine induced by interferon-\(\gamma\)
- MMPs:
-
Matrix metalloproteinases
- NK:
-
Natural killer
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- PBMC:
-
Peripheral blood mononuclear cells
- pDC:
-
Plasmacytoid dendritic cells
- PF-4/CXCL4:
-
Platelet factor-4
- PF-4var1/CXCL4L1:
-
Platelet factor-4 gene variant
- PLC:
-
Phospholipase C
- RA:
-
Rheumatoid arthritis
- RANKL:
-
Receptor activator of nuclear factor kappa-Β ligand
- RF:
-
Rheumatoid factor
- SF:
-
Synovial fluid
- sJIA:
-
Systemic JIA
- SJC:
-
Swollen joint counts
- STAT:
-
Signal transducer and activator
- TJC:
-
Tender joint counts
- TJI:
-
Traumatic joint injury
- TJR:
-
Total joint replacement
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor α
- TRAP:
-
Tartrate-resistant acid phosphatase
References
Zlotnik A, Yoshie O (2012) The chemokine superfamily revisited. Immunity 36(5):705–716. https://doi.org/10.1016/j.immuni.2012.05.008
Luster AD (1998) Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med 338(7):436–445. https://doi.org/10.1056/NEJM199802123380706
Thelen M, Stein JV (2008) How chemokines invite leukocytes to dance. Nat Immunol 9(9):953–959. https://doi.org/10.1038/ni.f.207/1
Proost P, Struyf S, Van Damme J, Fiten P, Ugarte-Berzal E, Opdenakker G (2017) Chemokine isoforms and processing in inflammation and immunity. J Autoimmun 85:45–57. https://doi.org/10.1016/j.jaut.2017.06.009
Opdenakker G, Proost P, Van Damme J (2016) Microbiomic and posttranslational modifications as preludes to autoimmune diseases. Trends Mol Med 22(9):746–757. https://doi.org/10.1016/j.molmed.2016.07.002
Lee EY, Lee ZH, Song YW (2013) The interaction between CXCL10 and cytokines in chronic inflammatory arthritis. Autoimmun Rev 12(5):554–557. https://doi.org/10.1016/j.autrev.2012.10.001
Abron JD, Singh NP, Murphy AE, Mishra MK, Price RL, Nagarkatti M et al (2018) Differential role of CXCR3 in inflammation and colorectal cancer. Oncotarget 9(25):17928–17936. https://doi.org/10.18632/oncotarget.24730
Kuo PT, Zeng Z, Salim N, Mattarollo S, Wells JW, Leggatt GR (2018) The role of CXCR3 and its chemokine ligands in skin disease and cancer. Front Med 5:271. https://doi.org/10.3389/fmed.2018.00271
Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP (2005) CXC chemokines in angiogenesis. Cytokine Growth Factor Rev 16(6):593–609. https://doi.org/10.1016/j.cytogfr.2005.04.007
Keeley EC, Mehrad B, Strieter RM (2011) Chemokines as mediators of tumor angiogenesis and neovascularization. Exp Cell Res 317(5):685–690. https://doi.org/10.1016/j.yexcr.2010.10.020
Wang J, Knaut H (2014) Chemokine signaling in development and disease. Dev 141(22):4199–4205. https://doi.org/10.1242/dev.101071
Stein JV, Nombela-Arrieta C (2005) Chemokine control of lymphocyte trafficking: a general overview. Immunology 116(1):1–12. https://doi.org/10.1111/j.1365-2567.2005.02183.x
Chen K, Bao Z, Tang P, Gong W, Yoshimura T, Wang JM (2018) Chemokines in homeostasis and diseases. Cell Mol Immunol 15(4):324–334. https://doi.org/10.1038/cmi.2017.134
Moser B, Wolf M, Walz A, Loetscher P (2004) Chemokines: multiple levels of leukocyte migration control. Trends Immunol 25(2):75–84. https://doi.org/10.1016/j.it.2003.12.005
Gouwy M, Struyf S, Proost P, Van Damme J (2005) Synergy in cytokine and chemokine networks amplifies the inflammatory response. Cytokine Growth Factor Rev 16(6):561–580. https://doi.org/10.1016/j.cytogfr.2005.03.005
Zlotnik A, Yoshie O (2000) Chemokines: a new classification system and their role in immunity. J Cult Herit 1(2):121–127. https://doi.org/10.1016/s1074-7613(00)80165-x
Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ et al (2014) International union of pharmacology. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev 66(1):1–79. https://doi.org/10.1124/pr.113.007724
Bacon K, Baggiolini M, Broxmeyer H, Horuk R, Lindley I, Mantovani A et al (2003) Chemokine/chemokine receptor nomenclature. Cytokine 21(1):48–49. https://doi.org/10.1016/S1043-4666(02)00493-3
Moelants EAV, Mortier A, Van Damme J, Proost P (2013) In vivo regulation of chemokine activity by post-translational modification. Immunol Cell Biol 91(6):402–407. https://doi.org/10.1038/icb.2013.16
Mortier A, Gouwy M, Van Damme J, Proost P (2011) Effect of posttranslational processing on the in vitro and in vivo activity of chemokines. Exp Cell Res 317(5):642–654. https://doi.org/10.1016/j.yexcr.2010.11.016
Mortier A, Van DJ, Proost P (2008) Regulation of chemokine activity by posttranslational modification. Pharmacol Ther 120(2):197–217. https://doi.org/10.1016/j.pharmthera.2008.08.006
Groom JR, Luster AD (2011) CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol 89(2):1–8. https://doi.org/10.1038/icb.2010.158.CXCR3
Van Raemdonck K, Van den Steen PE, Liekens S, Van Damme J, Struyf S (2015) CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev 26(3):311–327. https://doi.org/10.1016/j.cytogfr.2014.11.009
Luster AD, Unkeless JC, Ravetch JV (1985) Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature 315(6021):672–676. https://doi.org/10.1038/315672a0
Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP et al (1998) Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med 187(12):2009–2021. https://doi.org/10.1084/jem.187.12.2009
Lee HH, Farber JM (1996) Localization of the gene for the human MIG cytokine on chromosome 4q21 adjacent to IP10 reveals a chemokine “mini-cluster.” Cytogenet Genome Res 74(4):255–258. https://doi.org/10.1159/000134428
Tensen CP, Flier J, Van Der Raaij-Helmer EMH, Sampat-Sardjoepersad S, Van Der Schors RC, Leurs R et al (1999) Human IP-9: a keratinocyte-derived high affinity CXC-chemokine ligand for the IP-10/Mig receptor (CXCR3). J Invest Dermatol 112(5):716–722. https://doi.org/10.1046/j.1523-1747.1999.00581.x
Farber JM (1990) A macrophage mRNA selectively induced by γ-interferon encodes a member of the platelet factor 4 family of cytokines. Proc Natl Acad Sci USA 87(14):5238–5242. https://doi.org/10.1073/pnas.87.14.5238
Struyf S, Salogni L, Burdick MD, Vandercappellen J, Gouwy M, Noppen S et al (2011) Angiostatic and chemotactic activities of the CXC chemokine CXCL4L1 (platelet factor-4 variant) are mediated by CXCR3. Blood 117(2):480–488. https://doi.org/10.1182/blood-2009-11-253591
Mueller A, Meiser A, McDonagh EM, Fox JM, Petit SJ, Xanthou G et al (2008) CXCL4-induced migration of activated T lymphocytes is mediated by the chemokine receptor CXCR3. J Leukoc Biol 83(4):875–882. https://doi.org/10.1189/jlb.1006645
Farber JM (1997) Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol 61(3):246–257. https://doi.org/10.1002/jlb.61.3.246
Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I et al (1996) Chemokine Receptor Specific for IP10 and Mig: structure, function, and expression in activated T-lymphocytes. J Exp Med 184(3):963–969. https://doi.org/10.1084/jem.184.3.963
Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR et al (2001) Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol 166(11):6477–6482. https://doi.org/10.4049/jimmunol.166.11.6477
Robertson MJ (2002) Role of chemokines in the biology of natural killer cells. J Leukoc Biol 71(2):173–183. https://doi.org/10.1189/jlb.71.2.173
Taub DD, Sayers TJ, Carter CRD, Ortaldo JR (1995) Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol 155:3877–3888
Inngjerdingen M, Damaj B, Maghazachi AA (2001) Expression and regulation of chemokine receptors in human natural killer cells. Blood 97(2):367–375. https://doi.org/10.1182/blood.v97.2.367
Maghazaciu AA, Skålhegg BS, Rolstad B, Al-Aoukaty A (1997) Interferon-inducible protein-10 and lymphotactin induce the chemotaxis and mobilization of intracellular calcium in natural killer cells through pertussis toxin-sensitive and -insensitive heterotrimeric G-proteins. FASEB J 11(10):765–774. https://doi.org/10.1096/fasebj.11.10.9271361
Thapa M, Welner RS, Pelayo R, Carr D (2008) CXCL9 and CXCL10 expression are critical for control of genital herpes simplex virus type 2 infection through mobilization of HSV-specific CTL and NK cells to the nervous system. J Immunol 180(2):1098–1106. https://doi.org/10.4049/jimmunol.180.2.1098
Wennerberg E, Kremer V, Childs R, Lundqvist A (2015) CXCL10-induced migration of adoptively transferred human natural killer cells toward solid tumors causes regression of tumor growth in vivo. Cancer Immunol Immunother 64(2):225–235. https://doi.org/10.1007/s00262-014-1629-5
Xie JH, Nomura N, Lu M, Chen SL, Koch GE, Weng Y et al (2003) Antibody-mediated blockade of the CXCR3 chemokine receptor results in diminished recruitment of T helper 1 cells into sites of inflammation. J Leukoc Biol 73(6):771–780. https://doi.org/10.1189/jlb.1102573
Strieter RM, Kunkel SL, Arenberg DA, Burdick MD, Polverini PJ (1995) Interferon γ-inducible protein-10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem Biophys Res Commun 210(1):51–57. https://doi.org/10.1006/bbrc.1995.1626
Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S et al (1995) Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med 182(1):155–162. https://doi.org/10.1084/jem.182.1.155
Huang B, Wang W, Li Q, Wang Z, Yan B, Zhang Z et al (2016) Osteoblasts secrete Cxcl9 to regulate angiogenesis in bone. Nat Commun 7:13885. https://doi.org/10.1038/ncomms13885
Romagnani P, Annunziato F, Lazzeri E, Cosmi L, Beltrame C, Lasagni L et al (2001) Interferon-inducible protein 10, monokine induced by interferon gamma, and interferon-inducible T-cell alpha chemoattractant are produced by thymic epithelial cells and attract T-cell receptor (TCR) αβ+ CD8+ single-positive T cells, TCRγδ+ T cells. Blood 97(3):601–607. https://doi.org/10.1182/blood.V97.3.601
Proost P, Struyf S, Loos T, Gouwy M, Schutyser E, Conings R et al (2006) Coexpression and interaction of CXCL10 and CD26 in mesenchymal cells by synergising inflammatory cytokines: CXCL8 and CXCL10 are discriminative markers for autoimmune arthropathies. Arthritis Res Ther 8(4):R107. https://doi.org/10.1186/ar1997
Metzemaekers M, Vanheule V, Janssens R, Struyf S, Proost P (2018) Overview of the mechanisms that may contribute to the non-redundant activities of interferon-inducible CXC chemokine receptor 3 ligands. Front Immunol 8:1970. https://doi.org/10.3389/fimmu.2017.01970
Proost P, Verpoest S, De Borne K, Van SE, Struyf S, Put W et al (2004) Synergistic induction of CXCL9 and CXCL11 by Toll-like receptor ligands and interferon-γ in fibroblasts correlates with elevated levels of CXCR3 ligands in septic arthritis synovial fluids. J Leukoc Biol 75(5):777–784. https://doi.org/10.1189/jlb.1003524
Loos T, Dekeyzer L, Struyf S, Schutyser E, Gijsbers K, Gouwy M et al (2006) TLR ligands and cytokines induce CXCR3 ligands in endothelial cells: enhanced CXCL9 in autoimmune arthritis. Lab Investig 86(9):902–916. https://doi.org/10.1038/labinvest.3700453
Hensbergen PJ, Verzijl D, Balog CIA, Dijkman R, Van Der Schors RC, Van Der Raaij-Helmer EMH et al (2004) Furin is a chemokine-modifying enzyme: In vitro and in vivo processing of CXCL10 generates a C-terminally truncated chemokine retaining full activity. J Biol Chem 279(14):13402–13411. https://doi.org/10.1074/jbc.M312814200
Hensbergen PJ, Van Der Raaij-Helmer EMH, Dijkman R, Van Der Schors RC, Werner-Felmayer G, Boorsma DM et al (2001) Processing of natural and recombinant CXCR3-targeting chemokines and implications for biological activity. Eur J Biochem 268(18):4992–4999. https://doi.org/10.1046/j.0014-2956.2001.02433.x
Erdel M, Theurl M, Meyer M, Duba HC, Utermann G, Gabriele WF (2001) High-resolution mapping of the human 4q21 and the mouse 5E3 SCYB chemokine cluster by fiber-fluorescence in situ hybridization. Immunogenetics 53(7):611–615. https://doi.org/10.1007/s002510100363
Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L et al (2003) An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med 197(11):1537–1549. https://doi.org/10.1084/jem.20021897
Proost P, Schutyser E, Menten P, Struyf S, Wuyts A, Opdenakker G et al (2001) Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood 98(13):3554–3561. https://doi.org/10.1182/blood.V98.13.3554
Thompzson BD, Jin Y, Wu KH, Colvin RA, Luster AD, Birnbaumer L et al (2007) Inhibition of Gai2 activation by Gai3 in CXCR3-mediated signaling. J Biol Chem 282(13):9547–9555. https://doi.org/10.1074/jbc.M610931200
Smit MJ, Verdijk P, Van der Raaij-Helmer EMH, Navis M, Hensbergen PJ, Leurs R et al (2003) CXCR3-mediated chemotaxis of human T cells is regulated by a Gi-and phospholipase C-dependent pathway and not via activation of MEK/p44/p42 MAPK nor Akt/PI-3 kinase. Blood 102(6):1959–1965. https://doi.org/10.1182/blood-2002-12-3945
Loos T, Mortier A, Gouwy M, Ronsse I, Put W, Lenaerts JP et al (2008) Citrullination of CXCL10 and CXCL11 by peptidylarginine deiminase: a naturally occurring posttranslational modification of chemokines and new dimension of immunoregulation. Blood 112(7):2648–2656. https://doi.org/10.1182/blood-2008-04-149039
Berchiche YA, Sakmar TP (2016) CXC chemokine receptor 3 alternative splice variants selectively activate different signaling pathways. Mol Pharmacol 90(4):483–495. https://doi.org/10.1124/mol.116.105502
Smith JS, Alagesan P, Desai NK, Pack TF, Wu JH, Inoue A et al (2017) C-X-C motif chemokine receptor 3 splice variants differentially activate beta-arrestins to regulate downstream signaling pathways. Mol Pharmacol 92(2):136–150. https://doi.org/10.1124/mol.117.108522
Korniejewska A, Mcknight AJ, Johnson Z, Watson ML, Ward SG (2011) Expression and agonist responsiveness of CXCR3 variants in human T lymphocytes. Immunology 132(4):503–515. https://doi.org/10.1111/j.1365-2567.2010.03384.x
Bonacchi A, Romagnani P, Romanelli RG, Efsen E, Annunziato F, Lasagni L et al (2001) Signal transduction by the chemokine receptor CXCR3: Activation of Ras/ERK, Src, and phosphatidylinositol 3-kinase/Akt controls cell migration and proliferation in human vascular pericytes. J Biol Chem 276(13):9945–9954. https://doi.org/10.1074/jbc.M010303200
Ehlert JE, Addison CA, Burdick MD, Kunkel SL, Strieter RM (2004) Identification and partial characterization of a variant of human CXCR3 generated by posttranscriptional exon skipping. J Immunol 173(10):6234–6240. https://doi.org/10.4049/jimmunol.173.10.6234
Reynders N, Abboud D, Baragli A, Noman MZ, Rogister B, Niclou SP et al (2019) The distinct roles of CXCR3 variants and their ligands in the tumor microenvironment. Cells 8(6):613. https://doi.org/10.3390/cells8060613
Ranjbaran H, Wang Y, Manes TD, Yakimov AO, Akhtar S, Kluger MS et al (2006) Heparin displaces interferon-γ–inducible chemokines (IP-10, I-TAC, and Mig) sequestered in the vasculature and and inhibits the transendothelial migration and arterial recruitment of T cells. Circulation 114:1293–1300. https://doi.org/10.1161/CIRCULATIONAHA.106.631457
Kohrgruber N, Gröger M, Meraner P, Petzelbauer P, Brandt S, Stingl G et al (2004) Plasmacytoid dendritic cell recruitment by immobilized CXCR3 ligands. J Immunol 173(11):6592–6602. https://doi.org/10.4049/jimmunol.173.11.6592
Luster AD, Greenberg SM, Leder P (1995) The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J Exp Med 182(1):219–231. https://doi.org/10.1084/jem.182.1.219
Saxena A, Bujak M, Frunza O, Dobaczewski M, Gonzalez-quesada C, Lu B et al (2014) CXCR3-independent actions of the CXC chemokine CXCL10 in the infarcted myocardium and in isolated cardiac fibroblasts are mediated through proteoglycans. Cardiovasc Res 103(2):217–227. https://doi.org/10.1093/cvr/cvu138
Jiang D, Liang J, Campanella GS, Guo R, Yu S, Xie T et al (2010) Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest 120(6):2049–2057. https://doi.org/10.1172/JCI38644
Chen JP, Lu HL, Lai SL, Gabriele S, Sung JM, Lu MY et al (2006) Dengue virus induces expression of CXC chemokine Ligand 10/IFN-γ-Inducible Protein 10, which competitively inhibits viral binding to cell surface heparan sulfate. J Immunol 177(5):3185–3195. https://doi.org/10.4049/jimmunol.177.5.3185
Campanella GSV, Colvin RA, Luster AD (2010) CXCL10 can inhibit endothelial cell proliferation independently of CXCR3. PLoS ONE 5(9):e12700. https://doi.org/10.1371/journal.pone.0012700
Metzemaekers M, Mortier A, Janssens R, Boff D, Vanbrabant L, Lamoen N et al (2017) Glycosaminoglycans regulate CXCR3 ligands at distinct levels: protection against processing by dipeptidyl peptidase IV/CD26 and interference with receptor signaling. Int J Mol Sci 18(7):1513. https://doi.org/10.3390/ijms18071513
Gardner L, Patterson AM, Ashton BA, Stone MA, Middleton J (2004) The human Duffy antigen binds selected inflammatory but not homeostatic chemokines. Biochem Biophys Res Commun 321(2):306–312. https://doi.org/10.1016/j.bbrc.2004.06.146
Pruenster M, Mudde L, Bombosi P, Dimitrova S, Zsak M, Middleton J et al (2009) The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat Immunol 10(1):101–108. https://doi.org/10.1038/ni.1675
Kashiwazaki M, Tanaka T, Kanda H, Ebisuno Y, Izawa D, Fukuma N et al (2003) A high endothelial venule-expressing promiscuous chemokine receptor DARC can bind inflammatory, but not lymphoid, chemokines and is dispensable for lymphocyte homing under physiological conditions. Int Immunol 15(10):1219–1227. https://doi.org/10.1093/intimm/dxg121
Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z et al (2006) A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med 203(9):2201–2213. https://doi.org/10.1084/jem.20052144
Chevigné A, Janji B, Meyrath M, Reynders N, D’uonnolo G, Uchański T et al (2021) CXCL10 is an agonist of the CC family chemokine scavenger receptor ACKR2/D6. Cancers (Basel). 13(5):1054. https://doi.org/10.3390/cancers13051054
Xanthou G, Duchesnes CE, Williams TJ, Pease JE (2003) CCR3 functional responses are regulated by both CXCR3 and its ligands CXCL9, CXCL10 and CXCL11. Eur J Immunol 33(8):2241–2250. https://doi.org/10.1002/eji.200323787
Loetscher P, Pellegrino A, Gong JH, Mattioli I, Loetscher M, Bardi G et al (2001) The ligands of CXC chemokine receptor 3, I-TAC, mig, and IP10, are natural antagonists for CCR3. J Biol Chem 276(5):2986–2991. https://doi.org/10.1074/jbc.M005652200
Petkovic V, Moghini C, Paoletti S, Uguccioni M, Gerber B (2004) I-TAC/CXCL11 is a natural antagonist for CCR5. J Leukoc Biol 76(3):701–708. https://doi.org/10.1189/jlb.1103570
Crijns H, Vanheule V, Proost P (2020) Targeting chemokine—glycosaminoglycan interactions to inhibit inflammation. Front Immunol 11:483. https://doi.org/10.3389/fimmu.2020.00483
Lande R, Giacomini E, Serafini B, Rosicarelli B, Sebastiani GD, Minisola G et al (2004) Characterization and recruitment of plasmacytoid dendritic cells in synovial fluid and tissue of patients with chronic inflammatory arthritis. J Immunol 173(4):2815–2824. https://doi.org/10.4049/jimmunol.173.4.2815
Hartl D, Krauss-Etschmann S, Koller B, Hordijk PL, Kuijpers TW, Hoffmann F et al (2008) Infiltrated neutrophils acquire novel chemokine receptor expression and chemokine responsiveness in chronic inflammatory lung diseases. J Immunol 181(11):8053–8067. https://doi.org/10.4049/jimmunol.181.11.8053
Ichikawa A, Kuba K, Morita M, Chida S, Tezuka H, Hara H et al (2013) CXCL10-CXCR3 enhances the development of neutrophil-mediated fulminant lung injury of viral and nonviral origin. Am J Respir Crit Care Med 187(1):65–77. https://doi.org/10.1164/rccm.201203-0508OC
Floudas A, Neto N, Marzaioli V, Murray K, Moran B, Monaghan MG et al (2020) Pathogenic, glycolytic PD-1+ B cells accumulate in the hypoxic RA joint. JCI Insight. https://doi.org/10.1172/jci.insight.139032
Zohar Y, Wildbaum G, Novak R, Salzman AL, Thelen M, Alon R et al (2014) CXCL11-dependent induction of FOXP3-negative regulatory T cells suppresses autoimmune encephalomyelitis. J Clin Invest 124(5):2009–2022. https://doi.org/10.1172/JCI71951
Schulthess FT, Paroni F, Sauter NS, Shu L, Ribaux P, Haataja L et al (2009) CXCL10 impairs β cell function and viability in diabetes through TLR4 signaling. Cell Metab 9(2):125–139. https://doi.org/10.1016/j.cmet.2009.01.003
Lee JH, Kim B, Jin WJ, Kim HH, Ha H, Lee ZH (2017) Pathogenic roles of CXCL10 signaling through CXCR3 and TLR4 in macrophages and T cells: relevance for arthritis. Arthritis Res Ther 19(1):163. https://doi.org/10.1186/s13075-017-1353-6
Soejima K, Rollins BJ (2001) A functional IFN-γ-inducible protein-10/CXCL10-specific receptor expressed by epithelial and endothelial cells that is neither CXCR3 nor glycosaminoglycan. J Immunol 167(11):6576–6582. https://doi.org/10.4049/jimmunol.167.11.6576
Campanella GSV, Grimm J, Manice LA, Colvin RA, Medoff BD, Gregory R et al (2006) Oligomerization of CXCL10 is necessary for endothelial cell presentation and in vivo activity. J Immunol 177(10):6991–6998. https://doi.org/10.4049/jimmunol.177.10.6991
Dyer DP, Migliorini E, Salanga CL, Thakar D, Handel TM, Richter RP (2017) Differential structural remodelling of heparan sulfate by chemokines: the role of chemokine oligomerization. Open Biol 7(1):160286. https://doi.org/10.1098/rsob.160286
Dyer DP, Salanga CL, Volkman BF, Kawamura T, Handel TM (2015) The dependence of chemokine-glycosaminoglycan interactions on chemokine oligomerization. Glycobiology 26(3):312–326. https://doi.org/10.1093/glycob/cwv100
Severin IC, Gaudry JP, Johnson Z, Kungl A, Jansma A, Gesslbauer B et al (2010) Characterization of the chemokine CXCL11-heparin interaction suggests two different affinities for glycosaminoglycans. J Biol Chem 285(23):17713–17724. https://doi.org/10.1074/jbc.M109.082552
Firestein GS (2003) Evolving concepts of rheumatoid arthritis. Nature 423(6937):356–361. https://doi.org/10.1038/nature01661
Smolen JS, Aletaha D, McInnes IB (2016) Rheumatoid arthritis. Lancet 388(10055):2023–2038. https://doi.org/10.1016/S0140-6736(16)30173-8
Miyabe Y, Miyabe C, Iwai Y, Luster AD (2020) Targeting the chemokine system in rheumatoid arthritis and vasculitis. JMA J 3(3):182–192. https://doi.org/10.31662/jmaj.2020-0019
Szekanecz Z, Koch AE (2016) Successes and failures of chemokine-pathway targeting in rheumatoid arthritis. Nat Rev Rheumatol 12(1):5–13. https://doi.org/10.1038/nrrheum.2015.157
Elemam NM, Hannawi S, Maghazachi AA (2020) Role of chemokines and chemokine receptors in Rheumatoid arthritis. ImmunoTarg Ther 9:43–56. https://doi.org/10.2147/ITT.S243636
Yap HY, Tee S, Wong M, Chow SK, Peh SC, Teow SY (2018) Pathogenic role of immune cells in rheumatoid arthritis: implications in clinical treatment and biomarker development. Cells 7(10):161. https://doi.org/10.3390/cells7100161
Groom JR, Luster AD (2011) CXCR3 in T cell function. Exp Cell Res 317(5):620–631. https://doi.org/10.1016/j.yexcr.2010.12.017.CXCR3
Campanella GSV, Tager AM, El Khoury JK, Thomas SY, Abrazinski TA, Manice LA et al (2008) Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria. Proc Natl Acad Sci U S A 105(12):4814–4819. https://doi.org/10.1073/pnas.0801544105
Mohan K, Issekutz TB (2007) Blockade of chemokine receptor CXCR3 inhibits T Cell recruitment to inflamed joints and decreases the severity of adjuvant arthritis. J Immunol 179(12):8463–8469. https://doi.org/10.4049/jimmunol.179.12.8463
Steinmetz OM, Turner JE, Paust HJ, Lindner M, Peters A, Heiss K et al (2009) CXCR3 mediates renal Th1 and Th17 immune response in murine lupus nephritis. J Immunol 183(7):4693–4704. https://doi.org/10.4049/jimmunol.0802626
Patel DD, Zachariah JP, Whichard LP (2001) CXCR3 and CCR5 ligands in rheumatoid arthritis synovium. Clin Immunol 98(1):39–45. https://doi.org/10.1006/clim.2000.4957
Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M et al (1998) The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest 101(4):746–754. https://doi.org/10.1172/JCI1422
Loetscher P, Uguccioni M, Lorenza B, Baggiolini M, Moser B (1997) CCR5 is characteristic of Th1 lymphocytes. Nature 391(6665):344–345. https://doi.org/10.1038/34814
Ruth JH, Rottman JB, Katschke KJ, Qin S, Wu L, Larosa G et al (2001) Selective lymphocyte chemokine receptor expression in the rheumatoid joint. Arthritis Rheum 44(12):2750–2760
Ruschpler P, Lorenz P, Eichler W, Koczan D, Hänel C, Scholz R et al (2003) High CXCR3 expression in synovial mast cells associated with CXCL9 and CXCL10 expression in inflammatory synovial tissues of patients with rheumatoid arthritis. Arthritis Res Ther 5(5):241–252. https://doi.org/10.1186/ar783
Aldridge J, Ekwall AKH, Mark L, Bergström B, Andersson K, Gjertsson I et al (2020) T helper cells in synovial fluid of patients with rheumatoid arthritis primarily have a Th1 and a CXCR3+ Th2 phenotype. Arthritis Res Ther 22(1):245. https://doi.org/10.1186/s13075-020-02349-y
Aeberli D, Seitz M, Jüni P, Villiger PM (2005) Increase of peripheral CXCR3 positive T lymphocytes upon treatment of RA patients with TNF-α inhibitors. Rheumatology 44(2):172–175. https://doi.org/10.1093/rheumatology/keh437
Dalbeth N, Callan MFC (2002) A subset of natural killer cells is greatly expanded within inflamed joints. Arthritis Rheum 46(7):1763–1772. https://doi.org/10.1002/art.10410
Tsubaki T, Takegawa S, Hanamoto H, Arita N, Kamogawa J, Yamamoto H et al (2005) Accumulation of plasma cells expressing CXCR3 in the synovial sublining regions of early rheumatoid arthritis in association with production of Mig/CXCL9 by synovial fibroblasts. Clin Exp Immunol 141(2):363–371. https://doi.org/10.1111/j.1365-2249.2005.02850.x
Katschke KJ, Rottman JB, Ruth JH, Qin S, Wu L, LaRosa G et al (2001) Differential expression of chemokine receptors on peripheral blood, synovial fluid, and synovial tissue monocytes/macrophages in rheumatoid arthritis. Arthritis Rheum 44(5):1022–1032. https://doi.org/10.1002/1529-0131(200105)44:5%3c1022::AID-ANR181%3e3.0.CO;2-N
Nanki T, Takada K, Komano Y, Morio T, Kanegane H, Nakajima A et al (2009) Chemokine receptor expression and functional effects of chemokines on B cells: implication in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther 11(5):R149. https://doi.org/10.1186/ar2823
Ota Y, Niiro H, Ota S, Ueki N, Tsuzuki H, Nakayama T et al (2016) Generation mechanism of RANKL(+) effector memory B cells: relevance to the pathogenesis of rheumatoid arthritis. Arthritis Res Ther 18:67. https://doi.org/10.1186/s13075-016-0957-6
Ravelli A, Schiappapietra B, Verazza S, Martini A (2017) Chapter 7—Juvenile idiopathic arthritis. In: The heart in rheumatic, autoimmune and inflammatory diseases, pp 167–87. https://doi.org/10.1016/B978-0-12-803267-1.00007-7
Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C (2021) The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int 41(5):863–877. https://doi.org/10.1007/s00296-020-04731-0
Gerfaud-Valentin M, Jamilloux Y, Iwaz J, Sève P (2014) Adult-onset Still’s disease. Autoimmun Rev 13(7):708–722. https://doi.org/10.1016/j.autrev.2014.01.058
Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H (2020) Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 29–30:100587. https://doi.org/10.1016/j.eclinm.2020.100587
Runsheng W, Ward MM (2018) Epidemiology of axial spondyloarthritis: an update. Curr Opin Pharmacol 30(2):137–143. https://doi.org/10.1097/BOR.0000000000000475
Scotti L, Franchi M, Marchesoni A, Corrao G (2018) Prevalence and incidence of psoriatic arthritis: a systematic review and meta-analysis. Semin Arthritis Rheum 48(1):28–34. https://doi.org/10.1016/j.semarthrit.2018.01.003
McBride S, Mowbray J, Caughey W, Wong E, Luey C, Siddiqui A et al (2020) Epidemiology, management, and outcomes of large and small native joint septic arthritis in adults. Clin Infect Dis 70(2):271–279. https://doi.org/10.1093/cid/ciz265
Tamer TM (2013) Hyaluronan and synovial joint: Function, distribution and healing. Interdiscip Toxicol 6(3):111–125. https://doi.org/10.2478/intox-2013-0019
Balazs EA (1974) The physical properties of synovial fluid and the special role of hyaluronic acid. In: Disorders of the knee. Philadelphia, pp 63–75
Kokkonen H, Söderström I, Rocklöv J, Hallmans G, Lejon K, Dahlqvist SR (2010) Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum 62(2):383–391. https://doi.org/10.1002/art.27186
Ueno A, Yamamura M, Iwahashi M, Okamoto A, Aita T, Ogawa N et al (2005) The production of CXCR3-agonistic chemokines by synovial fibroblasts from patients with rheumatoid arthritis. Rheumatol Int 25(5):361–367. https://doi.org/10.1007/s00296-004-0449-x
Imam AM, Hamed AM, Nasef SI, Hassan AM, Omar HH (2019) Biochemical analysis of C-X-C motif chemokine ligand 10 (CXCL10) as a biomarker in patients with rheumatoid arthritis. Egypt J Immunol 26(2):79–86
Han BK, Kuzin I, Gaughan JP, Olsen NJ, Bottaro A (2016) Baseline CXCL10 and CXCL13 levels are predictive biomarkers for tumor necrosis factor inhibitor therapy in patients with moderate to severe rheumatoid arthritis: a pilot, prospective study. Arthritis Res Ther. https://doi.org/10.1186/s13075-016-0995-0
Jude C, Dejica D, Samasca G, Balacescu L, Balacescu O (2013) Soluble CD163 serum levels are elevated and correlated with IL-12 and CXCL10 in patients with long-standing rheumatoid arthritis. Rheumatol Int 33(4):1031–1037. https://doi.org/10.1007/s00296-012-2459-4
Hueber W, Tomooka BH, Zhao X, Kidd BA, Drijfhout JW, Fries JF et al (2007) Proteomic analysis of secreted proteins in early rheumatoid arthritis: anti-citrulline autoreactivity is associated with upregulation of proinflammatory cytokines. Ann Rheum Dis 66(6):712–719. https://doi.org/10.1136/ard.2006.054924
Han JH, Suh C, Jung J, Nam J, Kwon JE, Yim H et al (2015) Association of CXCL10 and CXCL13 levels with disease activity and cutaneous manifestation in active adult-onset Still’s disease. Arthritis Res Ther 17(1):260. https://doi.org/10.1186/s13075-015-0773-4
Han JH, Suh C, Jung J, Ahn M, Han MH, Kwon JE (2017) Elevated circulating levels of the interferon-γ-induced chemokines are associated with disease activity and cutaneous manifestations in adult-onset Still’s disease. Sci Rep. https://doi.org/10.1038/srep46652
Hanaoka R, Kasama T, Muramatsu M, Yajima N, Shiozawa F, Miwa Y et al (2003) A novel mechanism for the regulation of IFN- γ inducible protein-10 expression in rheumatoid arthritis. Arthritis Res Ther 5(2):74–81. https://doi.org/10.1186/ar616
Kuan WP, Tam L, Wong C, Ko FWS, Li T, Zhu T et al (2010) CXCL9 and CXCL10 as sensitive markers of disease activity in patients with rheumatoid arthritis. J Rheumatol 37(2):257–264. https://doi.org/10.3899/jrheum.090769
Pandya JM, Lundell AC, Andersson K, Nordström I, Theander E, Rudin A (2017) Blood chemokine profile in untreated early rheumatoid arthritis: CXCL10 as a disease activity marker. Arthritis Res Ther 19(1):20. https://doi.org/10.1186/s13075-017-1224-1
Muhsin HY, Kadri ZHM, Ad’hiah AH, Mayouf KZ (2020) Predictive significance of CXCL8, CXCL10 and CXCL16 in juvenile idiopathic and rheumatoid arthritis Iraqi patients. Egypt Rheumatol 42(2):153–157. https://doi.org/10.1016/j.ejr.2019.06.002
Ichikawa T, Kageyama Y, Kobayashi H, Kato N, Tsujimura K, Koide Y (2010) Etanercept treatment reduces the serum levels of interleukin-15 and interferon-gamma inducible protein-10 in patients with rheumatoid arthritis. Rheumatol Int 30(6):725–730. https://doi.org/10.1007/s00296-009-1356-y
Sucur A, Jajic Z, Artukovic M, Matijasevic MI, Anic B, Flegar D et al (2017) Chemokine signals are crucial for enhanced homing and differentiation of circulating osteoclast progenitor cells. Arthritis Res Ther 19(1):142. https://doi.org/10.1186/s13075-017-1337-6
Lee EY, Seo MR, Juhnn YS, Kim JY, Hong YJ, Lee YJ et al (2011) Potential role and mechanism of IFN-gamma inducible protein-10 on receptor activator of nuclear factor kappa-B ligand (RANKL) expression in rheumatoid arthritis. Arthritis Res Ther 13(3):R104. https://doi.org/10.1186/ar3385
Yu K, Proost P (2022) Insights into peptidylarginine deiminase expression and citrullination pathways. Trends Cell Biol. https://doi.org/10.1016/j.tcb.2022.01.014
Meeuwisse CM, Van Der LMP, Rullmann TA, Allaart CF, Nelissen R, Huizinga TW et al (2011) Identification of CXCL13 as a marker for rheumatoid arthritis outcome using an in silico model of the rheumatic joint. Arthritis Rheumatol 63(5):1265–1273. https://doi.org/10.1002/art.30273
Dennis Jr G, Holweg CTJ, Kummerfeld SK, Choy DF, Setiadi AF, Hackney JA et al (2014) Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res Ther 16(2):R90. https://doi.org/10.1186/ar4555
Greisen SR, Schelde KK, Rasmussen TK, Kragstrup TW, Stengaard-pedersen K, Hetland ML et al (2014) CXCL13 predicts disease activity in early rheumatoid arthritis and could be an indicator of the therapeutic ‘window of opportunity.’ Arthritis Res Ther 16(5):434. https://doi.org/10.1186/s13075-014-0434-z
Bechman K, Dalrymple A, Southey-bassols C, Cope AP, Galloway JB (2020) A systematic review of CXCL13 as a biomarker of disease and treatment response in rheumatoid arthritis. BMC Rheumatol 4(1):70. https://doi.org/10.1186/s41927-020-00154-3
García-López MÁ, Sánchez-Madrid F, Rodríguez-Frade JM, Mellado M, Acevedo A, García MI et al (2001) CXCR3 chemokine receptor distribution in normal and inflamed tissues: expression on activated lymphocytes, endothelial cells, and dendritic cells. Lab Investig 81(3):409–418. https://doi.org/10.1038/labinvest.3780248
Erdem H, Pay S, Musabak U, Simsek I, Dinc A, Pekel A et al (2007) Synovial angiostatic non-ELR CXC chemokines in inflammatory arthritides: does CXCL4 designate chronicity of synovitis? Rheumatol Int 27(10):969–973. https://doi.org/10.1007/s00296-007-0317-6
Schmutz C, Hulme A, Burman A, Salmon M, Ashton B, Buckley C et al (2005) Chemokine receptors in the rheumatoid synovium: upregulation of CXCR5. Arthritis Res Ther 7(2):217–229. https://doi.org/10.1186/ar1475
Yoshida S, Arakawa F, Higuchi F, Ishibashi Y, Goto M, Sugita Y et al (2012) Gene expression analysis of rheumatoid arthritis synovial lining regions by cDNA microarray combined with laser microdissection: Up-regulation of inflammation-associated STAT1, IRF1, CXCL9, CXCL10, and CCL5. Scand J Rheumatol 41(3):170–179. https://doi.org/10.3109/03009742.2011.623137
Lee EJ, Lilja S, Li X, Schäfer S, Zhang H, Benson M (2020) Bulk and single cell transcriptomic data indicate that a dichotomy between inflammatory pathways in peripheral blood and arthritic joints complicates biomarker discovery. Cytokine 127:154960. https://doi.org/10.1016/j.cyto.2019.154960
Kang SE, Park JK, Yoo HJ, Kang H, Park YW, Park BC et al (2021) Efficacy of novel bispecific antibody targeting TNF-α/CXCL10 in the treatment of experimental arthritis. Transl Res 232:75–87. https://doi.org/10.1016/j.trsl.2021.01.004
O’Boyle G, Fox CRJ, Walden HR, Willet JDP, Mavin ER, Hine DW et al (2012) Chemokine receptor CXCR3 agonist prevents human T-cell migration in a humanized model of arthritic inflammation. Proc Natl Acad Sci USA 109(12):4598–4603. https://doi.org/10.1073/pnas.1118104109
König A, Krenn V, Toksoy A, Gerhard N, Gillitzer R (2000) Mig, GROα and RANTES messenger RNA expression in lining layer, infiltrates and different leucocyte populations of synovial tissue from patients with rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Virchows Arch 436(5):449–458. https://doi.org/10.1007/s004280050472
Gloghini A, Volpe R, Carbone A (1990) Ki-M6 immunostaining in routinely processed sections of reactive and neoplastic human lymphoid tissue. Am J Clin Pathol 94(6):734–741. https://doi.org/10.1093/ajcp/94.6.734
Nakayama T, Yoshimura M, Higashioka K, Miyawaki K, Ota Y, Ayano M et al (2021) Type 1 helper T cells generate CXCL9/10-producing T-bet+ effector B cells potentially involved in the pathogenesis of rheumatoid arthritis. Cell Immunol 360:104263. https://doi.org/10.1016/j.cellimm.2020.104263
Proost P, Vynckier AK, Mahieu F, Put W, Grillet B, Struyf S et al (2003) Microbial Toll-like receptor ligands differentially regulate CXCL10/IP-10 expression in fibroblasts and mononuclear leukocytes in synergy with IFN-γ and provide a mechanism for enhanced synovial chemokine levels in septic arthritis. Eur J Immunol 33(11):3146–3153. https://doi.org/10.1002/eji.200324136
Sauty A, Colvin RA, Wagner L, Rochat S, Spertini F, Luster AD (2001) CXCR3 internalization following T cell-endothelial cell contact: preferential role of IFN-inducible T cell α chemoattractant (CXCL11). J Immunol 167(12):7084–7093. https://doi.org/10.4049/jimmunol.167.12.7084
Karin N, Wildbaum G, Thelen M (2016) Biased signaling pathways via CXCR3 control the development and function of CD4+ T cell subsets. J Leukoc Biol 99(6):857–862. https://doi.org/10.1189/jlb.2MR0915-441R
Bédard PA, Golds EE (1993) Cytokine-induced expression of mRNAs for chemotactic factors in human synovial cells and fibroblasts. J Cell Physiol 154(2):433–441. https://doi.org/10.1002/jcp.1041540227
Yoshitomi H (2019) Regulation of immune responses and chronic inflammation by fibroblast-like synoviocytes. Front Immunol 10:1395. https://doi.org/10.3389/fimmu.2019.01395
Nanki T, Shimaoka T, Hayashida K, Taniguchi K, Yonehara S, Miyasaka N (2005) Pathogenic role of the CXCL16-CXCR6 pathway in rheumatoid arthritis. Arthritis Rheum 52(10):3004–3014. https://doi.org/10.1002/art.21301
Shi K, Hayashida K, Kaneko M, Hashimoto J, Tomita T, Lipsky PE et al (2001) Lymphoid chemokine B cell-attracting chemokine-1 (CXCL13) is expressed in germinal center of ectopic lymphoid follicles within the synovium of chronic arthritis patients. J Immunol 166(1):650–655. https://doi.org/10.4049/jimmunol.166.1.650
Rump L, Mattey DL, Kehoe O, Middleton J (2017) An initial investigation into endothelial CC chemokine expression in the human rheumatoid synovium. Cytokine 97:133–140. https://doi.org/10.1016/j.cyto.2017.05.023
Bartok B, Firestein GS (2010) Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev 233(1):233–255. https://doi.org/10.1111/j.0105-2896.2009.00859.x
Jay GD, Britt DE, Cha CJ (2000) Lubricin is a product of megakaryocyte stimulating factor gene expression by human synovial fibroblasts. J Rheumatol 27(3):594–600
Bottini N, Firestein GS (2013) Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol 9(1):24–33. https://doi.org/10.1038/nrrheum.2012.190
Nygaard G, Firestein GS (2020) Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat Rev Rheumatol 16(6):316–333. https://doi.org/10.1038/s41584-020-0413-5
Kuranobu T, Mokuda S, Oi K, Tokunaga T, Yukawa K, Kohno H et al (2020) Activin A expressed in rheumatoid synovial cells downregulates TNFα-induced CXCL10 expression and osteoclastogenesis. Pathobiology 87(3):198–207. https://doi.org/10.1159/000506260
Chicheportiche Y, Chicheportiche R, Sizing I, Thompson J, Benjamin CB, Ambrose C et al (2002) Proinflammatory activity of TWEAK on human dermal fibroblasts and synoviocytes: Blocking and enhancing effects of anti-TWEAK monoclonal antibodies. Arthritis Res 4(2):126–133. https://doi.org/10.1186/ar388
Moret FM, Hack CE, Van Der Wurff-Jacobs KMG, De Jager W, Radstake TRDJ, Lafeber FPJG et al (2013) Intra-articular CD1c-expressing myeloid dendritic cells from rheumatoid arthritis patients express a unique set of T cell-attracting chemokines and spontaneously induce Th1, Th17 and Th2 cell activity. Arthritis Res Ther 15(5):R155. https://doi.org/10.1186/ar4338
Fong KY, Boey ML, Koh WH, Feng PH (1994) Cytokine concentrations in the synovial fluid and plasma of rheumatoid arthritis patients: correlation with bony erosions. Clin Exp Rheumatol 12(1):55–58
Ohta K, Naruse T, Kato H, Ishida Y, Nakagawa T, Ono S et al (2017) Differential regulation by IFN-γ on TNF-α-induced chemokine expression in synovial fibroblasts from temporomandibular joint. Mol Med Rep 16(5):6850–6857. https://doi.org/10.3892/mmr.2017.7432
Qi XF, Kim DH, Yoon YS, Jin D, Huang XZ, Li JH et al (2009) Essential involvement of cross-talk between IFN-γ and TNF-α in CXCL10 production in human THP-1 monocytes. J Cell Physiol 220(3):690–697. https://doi.org/10.1002/jcp.21815
Van Kuijk AWR, Wijbrandts CA, Vinkenoog M, Zheng TS, Reedquist KA, Tak PP (2010) TWEAK and its receptor Fn14 in the synovium of patients with rheumatoid arthritis compared to psoriatic arthritis and its response to tumour necrosis factor blockade. Ann Rheum Dis 69(1):301–304. https://doi.org/10.1136/ard.2008.090548
Al-Banna NA, Vaci M, Slauenwhite D, Johnston B, Issekutz TB (2014) CCR4 and CXCR3 play different roles in the migration of T cells to inflammation in skin, arthritic joints, and lymph nodes. Eur J Immunol 44(6):1633–1643. https://doi.org/10.1002/eji.201343995
Jacobs JP, Ortiz-Lopez A, Campbell JJ, Gerard CJ, Mathis D, Christophe B (2010) Deficiency of CXCR2, but not of other chemokine receptors attenuates a murine model of autoantibody-mediated arthritis. Arthritis Rheum 62(7):1921–1932. https://doi.org/10.1002/art.27470
Bakheet SA, Alrwashied BS, Ansari MA, Nadeem A, Attia SM, Alanazi MM et al (2020) CXC chemokine receptor 3 antagonist AMG487 shows potent anti-arthritic effects on collagen-induced arthritis by modifying B cell inflammatory profile. Immunol Lett 225:74–81. https://doi.org/10.1016/j.imlet.2020.06.014
Jenh CH, Cox MA, Cui L, Reich EP, Sullivan L, Chen SC et al (2012) A selective and potent CXCR3 antagonist SCH 546738 attenuates the development of autoimmune diseases and delays graft rejection. BMC Immunol 13(1):2. https://doi.org/10.1186/1471-2172-13-2
Kim B, Lee JH, Jin WJ, Kim HH, Ha H, Lee ZH (2018) JN-2, a C-X-C motif chemokine receptor 3 antagonist, ameliorates arthritis progression in an animal model. Eur J Pharmacol 823:1–10. https://doi.org/10.1016/j.ejphar.2018.01.037
Bakheet SA, Alrwashied BS, Ansari MA, Nadeem A, Attia SM, Assiri MA et al (2020) CXCR3 antagonist AMG487 inhibits glucocorticoid-induced tumor necrosis factor-receptor-related protein and inflammatory mediators in CD45 expressing cells in collagen-induced arthritis mouse model. Int Immunopharmacol 84:106494. https://doi.org/10.1016/j.intimp.2020.106494
Bakheet SA, Ansari MA, Nadeem A, Attia SM, Alhoshani AR, Gul G et al (2019) CXCR3 antagonist AMG487 suppresses rheumatoid arthritis pathogenesis and progression by shifting the Th17/Treg cell balance. Cell Signal 64:109395. https://doi.org/10.1016/j.cellsig.2019.109395
Yang YF, Mukai T, Gao P, Yamaguchi N, Ono S, Iwaki H et al (2002) A non-peptide CCR5 antagonist inhibits collagen-induced arthritis by modulating T cell migration without affecting anti-collagen T cell responses. Eur J Immunol 32(8):2124–2132. https://doi.org/10.1002/1521-4141(200208)32:8%3c2124::AID-IMMU2124%3e3.0.CO;2-S
Pickens SR, Chamberlain ND, Volin MV, Mandelin AM, Agrawal H, Matsui M et al (2011) Local expression of interleukin-27 ameliorates collagen-induced arthritis. Arthritis Rheum 63(8):2289–2298. https://doi.org/10.1002/art.30324
Jin WJ, Kim B, Kim D, Choo HYP, Kim HH, Ha H et al (2017) NF-κB signaling regulates cell-autonomous regulation of CXCL10 in breast cancer 4T1 cells. Exp Mol Med 49(2):e295. https://doi.org/10.1038/emm.2016.148
Nedjai B, Li H, Stroke IL, Wise EL, Webb ML, Merritt JR et al (2012) Small molecule chemokine mimetics suggest a molecular basis for the observation that CXCL10 and CXCL11 are allosteric ligands of CXCR3. Br J Pharmacol 166(3):912–923. https://doi.org/10.1111/j.1476-5381.2011.01660.x
Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD (2002) IFN-γ-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol 168(7):3195–3204. https://doi.org/10.4049/jimmunol.168.7.3195
Salomon I, Netzer N, Wildbaum G, Schif-Zuck S, Maor G, Karin N (2002) Targeting the function of IFN-γ-inducible protein 10 suppresses ongoing adjuvant arthritis. J Immunol 169(5):2685–2693. https://doi.org/10.4049/jimmunol.169.5.2685
Kwak HB, Ha H, Kim HN, Lee JH, Hun SK, Lee S et al (2008) Reciprocal cross-talk between RANKL and interferon-γ-inducible protein 10 is responsible for bone-erosive experimental arthritis. Arthritis Rheum 58(5):1332–1342. https://doi.org/10.1002/art.23372
Furman BD, Kent CL, Huebner JL, Kraus VB, McNulty AL, Farshid G et al (2018) CXCL10 is upregulated in synovium and cartilage following articular fracture. J orthop 36(4):1220–1227. https://doi.org/10.1002/jor.23735.CXCL10
Collins KH, Reimer RA, Seerattan RA, Leonard TR, Herzog W (2015) Using diet-induced obesity to understand a metabolic subtype of osteoarthritis in rats. Osteoarthr Cartil 23(6):957–965. https://doi.org/10.1016/j.joca.2015.01.015
Sierro F, Biben C, Martínez-Muñoz L, Mellado M, Ransohoff RM, Li M et al (2007) Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci USA 104(37):14759–14764. https://doi.org/10.1073/pnas.0702229104
Andrews SP, Cox RJ (2016) Small molecule CXCR3 antagonists. J Med Chem 59(7):2894–2917. https://doi.org/10.1021/acs.jmedchem.5b01337
Pease JE (2017) Designing small molecule CXCR3 antagonists. Expert Opin Drug Discov 12(2):159–168. https://doi.org/10.1080/17460441.2017.1268597
Schall TJ, Proudfoot AEI (2011) Overcoming hurdles in developing successful drugs targeting chemokine receptors. Nat Rev Immunol 11(5):355–363. https://doi.org/10.1038/nri2972
Christen U, McGavern DB, Luster AD, von Herrath MG, Oldstone MBA (2003) Among CXCR3 chemokines, IFN-γ-inducible protein of 10 kDa (CXC chemokine ligand (CXCL) 10) but not monokine induced by IFN-γ (CXCL9) imprints a pattern for the subsequent development of autoimmune disease. J Immunol 171(12):6838–6845. https://doi.org/10.4049/jimmunol.171.12.6838
Narumi S, Kaburaki T, Yoneyama H, Iwamura H, Kobayashi Y, Matsushima K (2002) Neutralization of IFN-inducible protein 10/CXCL10 exacerbates experimental autoimmune encephalomyelitis. Eur J Immunol 32(6):1784–1791. https://doi.org/10.1002/1521-4141(200206)32:6%3c1784::AID-IMMU1784%3e3.0.CO;2-R
Crosignani S, Missotten M, Cleva C, Dondi R, Ratinaud Y, Humbert Y et al (2010) Discovery of a novel series of CXCR3 antagonists. Bioorgan Med Chem Lett 20(12):3614–3617. https://doi.org/10.1016/j.bmcl.2010.04.113
Liu J, Fu Z, Li AR, Johnson M, Zhu L, Marcus A et al (2009) Optimization of a series of quinazolinone-derived antagonists of CXCR3. Bioorgan Med Chem Lett 19(17):5114–5118. https://doi.org/10.1016/j.bmcl.2009.07.032
Du X, Gustin DJ, Chen X, Duquette J, McGee LR, Wang Z et al (2009) Imidazo-pyrazine derivatives as potent CXCR3 antagonists. Bioorgan Med Chem Lett 19(17):5200–5204. https://doi.org/10.1016/j.bmcl.2009.07.021
Hayes ME, Wallace GA, Grongsaard P, Bischoff A, George DM, Miao W et al (2008) Discovery of small molecule benzimidazole antagonists of the chemokine receptor CXCR3. Bioorgan Med Chem Lett 18(5):1573–1576. https://doi.org/10.1016/j.bmcl.2008.01.074
Watson RJ, Allen DR, Birch HL, Chapman GA, Hannah DR, Knight RL et al (2007) Development of CXCR3 antagonists. Part 2: Identification of 2-amino(4-piperidinyl)azoles as potent CXCR3 antagonists. Bioorgan Med Chem Lett. 17(24):6806–6810. https://doi.org/10.1016/j.bmcl.2007.10.029
Allen DR, Bolt A, Chapman GA, Knight RL, Meissner JWG, Owen DA et al (2007) Identification and structure-activity relationships of 1-aryl-3-piperidin-4-yl-urea derivatives as CXCR3 receptor antagonists. Bioorgan Med Chem Lett 17(3):697–701. https://doi.org/10.1016/j.bmcl.2006.10.088
Storelli S, Verdijk P, Verzijl D, Timmerman H, Van De Stolpe AC, Tensen CP et al (2005) Synthesis and structure-activity relationship of 3-phenyl-3H-quinazolin-4-one derivatives as CXCR3 chemokine receptor antagonists. Bioorgan Med Chem Lett 15(11):2910–2913. https://doi.org/10.1016/j.bmcl.2005.03.070
Heise CE, Pahuja A, Hudson SC, Mistry MS, Putnam AL, Gross MM et al (2005) Pharmacological characterization of CXC chemokine receptor 3 ligands and a small molecule antagonist. J Pharmacol Exp Ther 313(3):1263–1271. https://doi.org/10.1124/jpet.105.083683
Johnson M, Li AR, Liu J, Fu Z, Zhu L, Miao S et al (2007) Discovery and optimization of a series of quinazolinone-derived antagonists of CXCR3. Bioorgan Med Chem Lett 17(12):3339–3343. https://doi.org/10.1016/j.bmcl.2007.03.106
Wijtmans M, Verzijl D, Leurs R, De Esch IJP, Smit MJ (2008) Towards small-molecule CXCR3 ligands with clinical potential. ChemMedChem 3(6):861–872. https://doi.org/10.1002/cmdc.200700365
Wijtmans M, Scholten D, Mooij W, Smit MJ, de Esch IJP, de Graaf C et al (2014) Exploring the CXCR3 chemokine receptor with small-molecule antagonists and agonist. Top Med Chem 10(October):169–244. https://doi.org/10.1007/7355
Medina J, Johnson M, Li A, Liu Z, Huang A, Zhu L et al (2002) CXCR3 antagonists. WO02083143
Schall T, Dairaghi D, McMaster B (2001) Compounds and methods for modulating CXCR3 function. WO0116114
Henne KR, Tran TB, VandenBrink BM, Rock DA, Aidasani DK, Subramanian R et al (2012) Sequential metabolism of AMG 487, a novel CXCR3 antagonist, results in formation of quinone reactive metabolites that covalently modify CYP3A4 Cys239 and cause time-dependent inhibition of the enzyme. Drug Metab Dispos 40(7):1429–1440. https://doi.org/10.1124/dmd.112.045708
Berry K, Friedrich M, Kersey K, Stempien MJ, Wagner F, van Lier JJ et al (2004) Evaluation of T0906487, a CXCR3 antagonist, in a phase 2a psoriasis trial. Inflamm Res 53(Suppl. 3):S222
Rottman JB, Smith TL, Ganley KG, Kikuchi T, Krueger JG (2001) Potential role of the chemokine receptors CXCR3, CCR4, and the integrin αEβ7 in the pathogenesis of psoriasis vulgaris. Lab Investig 81(3):335–347. https://doi.org/10.1038/labinvest.3780242
Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P et al (1999) The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 400(6746):776–780. https://doi.org/10.1038/23495
Homey B, Dieu-Nosjean MC, Wiesenborn A, Massacrier C, Pin JJ, Oldham E et al (2000) Up-regulation of macrophage inflammatory protein-3α/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol 164(12):6621–6632. https://doi.org/10.4049/jimmunol.164.12.6621
Goeddel DV (2004) Tularik expects to begin a Phase 2 study of T487 in patients with rheumatoid arthritis in the first quarter of this year. In: Presented at the 22nd annual JP Morgan healthcare conference, San Fransisco
Laragione T, Brenner M, Li W, Gulko PS (2008) Cia5d regulates a new fibroblast-like synoviocyte invasion-associated gene expression signature. Arthritis Res Ther 10(4):R92. https://doi.org/10.1186/ar2476
Laragione T, Brenner M, Sherry B, Gulko PS (2011) CXCL10 and its receptor CXCR3 regulate synovial fibroblast invasion. Arthritis Rheum 63(11):3274–3283. https://doi.org/10.1002/art.30573.CXCL10
García-Vicuña R, Gómez-Gaviro MV, Domínguez-Luis MJ, Pec MK, González-Alvaro I, Alvaro-Gracia JM et al (2004) CC and CXC chemokine receptors mediate migration, proliferation, and matrix metalloproteinase production by fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Rheum 50(12):3866–3877. https://doi.org/10.1002/art.20615
Walser TC, Rifat S, Ma X, Kundu N, Ward C, Goloubeva O et al (2006) Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer. Cancer Res 66(15):7701–7707. https://doi.org/10.1158/0008-5472.CAN-06-0709
Ha Y, Liu H, Zhu S, Yi P, Liu W, Nathanson J et al (2017) Critical role of the CXCL10/C-X-C chemokine receptor 3 axis in promoting leukocyte recruitment and neuronal injury during traumatic optic neuropathy induced by optic nerve crush. Am J Pathol 187(2):352–365. https://doi.org/10.1016/j.ajpath.2016.10.009
Zhang X, Han J, Man K, Li X, Du J, Chu ESH et al (2016) CXC chemokine receptor 3 promotes steatohepatitis in mice through mediating inflammatory cytokines, macrophages and autophagy. J Hepatol 64(1):160–170. https://doi.org/10.1016/j.jhep.2015.09.005
Hall SE, Mao A, Nicolaidou V, Finelli M, Wise EL, Nedjai B et al (2009) Elucidation of binding sites of dual antagonists in the human chemokine receptors CCR2 and CCR5. Mol Pharmacol 75(6):1325–1336. https://doi.org/10.1124/mol.108.053470
Nedjai B, Viney JM, Li H, Hull C, Anderson CA, Horie T et al (2015) CXCR3 antagonist VUF10085 binds to an intrahelical site distinct from that of the broad spectrum antagonist TAK-779. Br J Pharmacol 172(7):1822–1833. https://doi.org/10.1111/bph.13027
Baba M, Nishimura O, Kanzaki N, Okamoto M, Sawada H, Iizawa Y et al (1999) A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci U S A 96(10):5698–5703. https://doi.org/10.1073/pnas.96.10.5698
Ni J, Zhu YN, Zhong XG, Ding Y, Hou LF, Tong XK et al (2009) The chemokine receptor antagonist, TAK-779, decreased experimental autoimmune encephalomyelitis by reducing inflammatory cell migration into the central nervous system, without affecting T cell function. Br J Pharmacol 158(8):2046–2056. https://doi.org/10.1111/j.1476-5381.2009.00528.x
Tokuyama H, Ueha S, Kurachi M, Matsushima K, Moriyasu F, Blumberg RS et al (2005) The simultaneous blockade of chemokine receptors CCR2, CCR5 and CXCR3 by a non-peptide chemokine receptor antagonist protects mice from dextran sodium sulfate-mediated colitis. Int Immunol 17(8):1023–1034. https://doi.org/10.1093/intimm/dxh284
Akahori T, Sho M, Kashizuka H, Nomi T, Kanehiro H, Nakajima Y (2006) A novel CCR5/CXCR3 antagonist protects intestinal ischemia/reperfusion injury. Transplant Proc 38(10):3366–3368. https://doi.org/10.1016/j.transproceed.2006.10.115
Bastani S, Sherman W, Schnickel GT, Hsieh GR, Bhatia R, Fishbein MC et al (2009) Chemokine receptor blockade with a synthetic nonpeptide compound attenuates cardiac allograft vasculopathy. Transplantation 88(8):995–1001. https://doi.org/10.1097/TP.0b013e3181b9ccd5
Baba M, Takashima K, Miyake H, Kanzaki N, Teshima K, Wang X et al (2005) TAK-652 inhibits CCR5-mediated human immunodeficiency virus type 1 infection in vitro and has favorable pharmacokinetics in humans. Antimicrob Agents Chemother 49(11):4584–4591. https://doi.org/10.1128/AAC.49.11.4584-4591.2005
Nair AG, Wong MKC, Shu Y, Jiang Y, Jenh CH, Kim SH et al (2014) IV. Discovery of CXCR3 antagonists substituted with heterocycles as amide surrogates: Improved PK, hERG and metabolic profiles. Bioorgan Med Chem Lett 24(4):1085–1088. https://doi.org/10.1016/j.bmcl.2014.01.009
Kim SH, Anilkumar GN, Zawacki LG, Zeng Q, Yang DY, Shao Y et al (2011) III. Identification of novel CXCR3 chemokine receptor antagonists with a pyrazinyl-piperazinyl-piperidine scaffold. Bioorgan Med Chem Lett 21(23):6982–6986. https://doi.org/10.1016/j.bmcl.2011.09.120
Stroke IL, Cole AG, Simhadri S, Brescia MR, Desai M, Zhang JJ et al (2006) Identification of CXCR3 receptor agonists in combinatorial small-molecule libraries. Biochem Biophys Res Commun 349(1):221–228. https://doi.org/10.1016/j.bbrc.2006.08.019
Yellin M, Paliienko I, Balanescu A, Ter-vartanian S, Tseluyko V, Xu L et al (2012) A phase II, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of MDX-1100, a fully human anti-CXCL10 monoclonal antibody, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheum 64(6):1730–1739. https://doi.org/10.1002/art.34330
Kuhne M, Preston B, Wallace S, Chen S, Geetha Vasudevan AW, Cardarelli P (2007) MDX-1100, a fully human anti-CXCL10 (IP-10) antibody, is a high affinity, neutralizing antibody that has entered Phase I clinical trials for the treatment of Ulcerative Colitis. J Immunol 178:S241
Medarex Inc (2009) Medarex announces primary endpoint achieved in MDX-1100 anti-IP-10 antibody phase 2 trial for rheumatoid arthritis. https://www.fiercebiotech.com/biotech/medarex-announces-primary-endpoint-achieved-mdx-1100-anti-ip-10-antibody-phase, p 3
Senolt L (2019) Emerging therapies in rheumatoid arthritis: focus on monoclonal antibodies. F1000Res 8:F1000 Faculty Rev-1549. https://doi.org/10.1268/f1000research.18688.1
Mayer L, Sandborn WJ, Stepanov Y, Maccarone J, Tao X, Lu LA et al (2010) A randomized, placebo-controlled trial of MDX-1100, an anti-IP-10 antibody, for moderately to severely active ulcerative colitis. Gastroenterology 139(1):e17–e18. https://doi.org/10.1053/j.gastro.2010.05.065
Smolen JS, Aletaha D (2015) Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol 11(5):276–289. https://doi.org/10.1038/nrrheum.2015.8
Nastase MV, Zeng-Brouwers J, Beckmann J, Tredup C, Christen U, Radeke HH et al (2018) Biglycan, a novel trigger of Th1 and Th17 cell recruitment into the kidney. Matrix Biol 68–69:293–317. https://doi.org/10.1016/j.matbio.2017.12.002
Smith JS, Nicholson LT, Suwanpradid J, Glenn RA, Knape NM, Alagesan P et al (2019) Biased agonists of the chemokine receptor CXCR3 differentially control chemotaxis and inflammation. Sci Signal 11(555):eaaq1075. https://doi.org/10.1126/scisignal.aaq1075
Bernat V, Admas TH, Brox R, Heinemann FW, Tschammer N (2014) Boronic acids as probes for investigation of allosteric modulation of the chemokine receptor CXCR3. ACS Chem Biol 9(11):2664–2677. https://doi.org/10.1021/cb500678c
Bernat V, Brox R, Heinrich MR, Auberson YP, Tschammer N (2015) Ligand-biased and probe-dependent modulation of chemokine receptor CXCR3 signaling by negative allosteric modulators. ChemMedChem 10(3):566–574. https://doi.org/10.1002/cmdc.201402507
Fleishaker DL, Garcia Meijide JA, Petrov A, Kohen MD, Wang X, Menon S et al (2012) Maraviroc, a chemokine receptor-5 antagonist, fails to demonstrate efficacy in the treatment of patients with rheumatoid arthritis in a randomized, double-blind placebo-controlled trial. Arthritis Res Ther 14(1):R11. https://doi.org/10.1186/ar3685
Lee J, Ajani JA, Chung HC, Kang YK, Iqbal S, Allen S et a1 (2021) Phase Ib/II open-label, randomised evaluation of second-line atezolizumab + linagliptin vs ramucirumab + paclitaxel in MORPHEUS-Gastric cancer. In: 2021 EMSO congress
Prakken B, Albani S, Martini A (2011) Juvenile idiopathic arthritis. Lancet 377(9783):2138–2149. https://doi.org/10.1016/S0140-6736(11)60244-4
Petty RE, Taunton R, Southwood PM, Baum J, Glass DN, Goldenberg J, He X et al (2004) International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 31(2):390–392
Minden K, Niewerth M, Listing J, Biedermann T, Bollow M, Schöntube M et al (2002) Long-term outcome in patients with juvenile idiopathic arthritis. Arthritis Rheum 46(9):2392–2401. https://doi.org/10.1002/art.10444
Packham JC, Hall MA (2002) Long-term follow-up of 246 adults with juvenile idiopathic arthritis: education and employement. Rheumatology 41(12):1428–1435. https://doi.org/10.1093/rheumatology/41.12.1428
Zak M, Pedersen FK (2000) Juvenile chronic arthritis into adulthood: a long-term follow-up study. Rheumatology 39(2):198–204. https://doi.org/10.1093/rheumatology/39.2.198
Peterson LS, Mason T, Nelson AM, O’Fallon WM, Gabriel SE (1997) Psychosocial outcomes and health status of adults who have had juvenile rheumatoid arthritis: a controlled population-based study. Arthritis Rheum 40(12):2235–2240. https://doi.org/10.1002/art.1780401219
De Jager W, Hoppenreijs EPAH, Wulffraat NM, Wedderburn LR, Kuis W, Prakken BJ (2007) Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross-sectional study. Ann Rheum Dis 66(5):589–598. https://doi.org/10.1136/ard.2006.061853
Pharoah DS, Varsani H, Tatham RW, Newton KR, De JW, Prakken BJ et al (2006) Expression of the inflammatory chemokines CCL5, CCL3 and CXCL10 in juvenile idiopathic arthritis, and demonstration of CCL5 production by an atypical subset of CD8+ T cells. Arthritis Res Ther 8(2):R50. https://doi.org/10.1186/ar1913
Hunter PJ, Nistala K, Jina N, Eddaoudi A, Thomson W, Hubank M et al (2010) Biologic predictors of extension of oligoarticular juvenile idiopathic arthritis as determined from synovial fluid cellular composition and gene expression. Arthritis Rheum 62(3):896–907. https://doi.org/10.1002/art.27284
Gattorno M, Prigione I, Morandi F, Gregorio A, Chiesa S, Ferlito F et al (2004) Phenotypic and functional characterisation of CCR7+ and CCR7- CD4+ memory T cells homing to the joints in juvenile idiopathic arthritis. Arthritis Res Ther 7(2):256–267. https://doi.org/10.1186/ar1485
Brescia AMC, Simonds MM, Sullivan KE, Rose CD (2017) Secretion of pro-inflammatory cytokines and chemokines and loss of regulatory signals by fibroblast-like synoviocytes in juvenile idiopathic arthritis. Proteom Clin Appl 11(5–6):1600088. https://doi.org/10.1002/prca.201600088
Martini G, Zulian F, Calabrese F, Bortoli M, Facco M, Cabrelle A et al (2005) CXCR3/CXCL10 expression in the synovium of children with juvenile idiopathic arthritis. Arthritis Res Ther 7(2):241–249. https://doi.org/10.1186/ar1481
Issekutz AC, Quinn PJ, Lang B, Ramsey S, Huber AM, Rowter D et al (2011) Coexpression of chemokine receptors CCR5, CXCR3, and CCR4 and ligands for P- and E-selectin on T lymphocytes of patients with juvenile idiopathic arthritis. Arthritis Rheum 63(11):3467–3476. https://doi.org/10.1002/art.30521
Petty P, Cassidy J (2011) Oligoarthritis. Textbook for pediatric rheumatology, 6th edn. Elsevier, Oxford, pp 262–271
Wedderburn LR, Robinson N, Patel A, Varsani H, Woo P (2000) Selective recruitment of polarized T cells expressing CCR5 and CXCR3 to the inflamed joints of children with juvenile idiopathic arthritis. Arthritis Rheum 43(4):765–774. https://doi.org/10.1002/1529-0131(200004)43:4%3c765::AID-ANR7%3e3.0.CO;2-B
Black APB, Bhayani H, Ryder CAJ, Pugh MT, Gardner-Medwin JMM, Southwood TR (2003) An association between the acute phase response and patterns of antigen induced T cell proliferation in juvenile idiopathic arthritis. Arthritis Res Ther 5(5):277–284. https://doi.org/10.1186/ar791
Silverman ED, Isacovics B, Petsche D, Laxer RM (1993) Synovial fluid cells in juvenile arthritis: Evidence of selective T cell migration to inflamed tissue. Clin Exp Immunol 91(1):90–95. https://doi.org/10.1111/j.1365-2249.1993.tb03360.x
Murray KJ, Luyrink L, Grom AA, Passo MH, Emery H, Witte D et al (1996) Immunohistological characteristics of T cell infiltrates in different forms of childhood onset chronic arthritis. J Rheumatol 23(12):2116–2124
Gregorio A, Gambini C, Gerloni V, Parafioriti A, Sormani MP, Gregorio S et al (2007) Lymphoid neogenesis in juvenile idiopathic arthritis correlates with ANA positivity and plasma cells infiltration. Rheumatology 46(2):308–313. https://doi.org/10.1093/rheumatology/kel225
Corcione A, Ferlito F, Gattorno M, Gregorio A, Pistorio A, Gastaldi R et al (2009) Phenotypic and functional characterization of switch memory B cells from patients with oligoarticular juvenile idiopathic arthritis. Arthritis Res Ther 11(5):R150. https://doi.org/10.1186/ar2824
Hardison JL, Wrightsman RA, Carpenter PM, Lane TE, Manning JE (2006) The chemokines CXCL9 and CXCL10 promote a protective immune response but do not contribute to cardiac inflammation following infection with Trypanosoma cruzi. Infect Immun 74(1):125–134. https://doi.org/10.1128/IAI.74.1.125-134.2006
Jarvis JN, Jiang K, Petty HR, Centola M (2007) Neutrophils: the forgotten cell in JIA disease pathogenesis. Pediatr Rheumatol 5:13. https://doi.org/10.1186/1546-0096-5-13
Arve-Butler S, Schmidt T, Mossberg A, Berthold E, Gullstrand B, Bengtsson AA et al (2021) Synovial fluid neutrophils in oligoarticular juvenile idiopathic arthritis have an altered phenotype and impaired effector functions. Arthritis Res Ther 23(1):109. https://doi.org/10.1186/s13075-021-02483-1
Cassatella MA, Gasperini S, Calzetti F, Bertagnin A, Luster AD, McDonald PP (1997) Regulated production of the interferon-γ-inducible protein-10 (IP-10) chemokine by human neutrophils. Eur J Immunol 27(1):111–115. https://doi.org/10.1002/eji.1830270117
Gasperini S, Marchi M, Calzetti F, Laudanna C, Vicentini L, Olsen H et al (1999) gene expression and production of the monokine induced by IFN-γ (MIG), IFN-inducible T cell α chemoattractant (I-TAC), and IFN-γ-inducible protein-10 (IP-10) chemokines by human neutrophils. J Immunol 162(8):4928–4937
Metzemaekers M, Malengier-Devlies B, Yu K, Vandendriessche S, Yserbyt J, Matthys P et al (2021) Synovial fluid neutrophils from patients with juvenile idiopathic arthritis display a hyperactivated phenotype. Arthritis Rheumatol 73(5):875–884. https://doi.org/10.1002/art.41605
Schmidt T, Berthold E, Arve-Butler S, Gullstrand B, Mossberg A, Kahn F et al (2020) Children with oligoarticular juvenile idiopathic arthritis have skewed synovial monocyte polarization pattern with functional impairment - a distinct inflammatory pattern for oligoarticular juvenile arthritis. Arthritis Res Ther 22(1):186. https://doi.org/10.1186/s13075-020-02279-9
Mellins ED, MacAubas C, Grom AA (2011) Pathogenesis of systemic juvenile idiopathic arthritis: some answers, more questions. Nat Rev Rheumatol 7(7):416–426. https://doi.org/10.1038/nrrheum.2011.68
Still GF (1897) On a form of chronic joint disease in children. Med-Chir Trans 80:47-60.9
Vastert SJ, Kuis W, Grom AA (2009) Systemic JIA: new developments in the understanding of the pathophysiology and therapy. Best Pract Res Clin Rheumatol 23(5):655–664. https://doi.org/10.1016/j.berh.2009.08.003
Sawhney S, Magalhães CS (2006) Paediatric rheumatology—a global perspective. Best Pract Res Clin Rheumatol 20(2):201–221. https://doi.org/10.1016/j.berh.2005.11.007
Grom AA, Passo M (1996) Macrophage activation syndrome in systemic juvenile idiopathic arthritis. J Pediatr 129(5):630–632. https://doi.org/10.1016/s0022-3476(96)70140-3
Sawhney S, Woo P, Murray KJ (2001) Macrophage activation syndrome: a potentially fatal complication of rheumatic disorders. Arch Dis Child 85(5):421–426. https://doi.org/10.1136/adc.85.5.421
Behrens EM, Beukelman T, Paessler M, Cron RQ (2007) Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J Rheumatol 34(5):1133–1138
Bleesing J, Prada A, Siegel DM, Villanueva J, Olson J, Ilowite NT et al (2007) The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor α-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum 56(3):965–971. https://doi.org/10.1002/art.22416
Ravelli A, Grom AA, Behrens EM, Cron RQ (2012) Macrophage activation syndrome as part of systemic juvenile idiopathic arthritis: diagnosis, genetics, pathophysiology and treatment. Genes Immun 13(4):289–298. https://doi.org/10.1038/gene.2012.3
Janka GE (2012) Familial and acquired hemophagocytic lymphohistiocytosis. Annu Rev Med 63:233–246. https://doi.org/10.1146/annurev-med-041610-134208
Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL (2011) How I treat hemophagocytic lymphohistiocytosis. Blood 118(15):4041–4052. https://doi.org/10.1182/blood-2011-03-278127
Billiau AD, Roskams T, Van Damme-Lombaerts R, Matthys P, Wouters C (2005) Macrophage activation syndrome: characteristic findings on liver biopsy illustrating the key role of activated, IFN-γ-producing lymphocytes and IL-6- and TNF-α-producing macrophages. Blood 105(4):1648–1651. https://doi.org/10.1182/blood-2004-08-2997
Jordan MB, Hildeman D, Kappler J, Marrack P (2004) An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood 104(3):735–743. https://doi.org/10.1182/blood-2003-10-3413
Osugi Y, Hara J, Tagawa S, Takai K, Hosoi G, Matsuda Y et al (1997) Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood 89(11):4100–4103. https://doi.org/10.1182/blood.v89.11.4100
Henter J, Elinder G, Soder O, Hansson M, Andersson B, Andersson U (1991) Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood 78(11):2918–2922. https://doi.org/10.1182/blood.v78.11.2918.2918
Xu XJ, Tang YM, Song H, Yang SL, Xu WQ, Zhao N et al (2012) Diagnostic accuracy of a specific cytokine pattern in hemophagocytic lymphohistiocytosis in children. J Pediatr 160(6):984-990.e1. https://doi.org/10.1016/j.jpeds.2011.11.046
Schmid JP, Ho CH, Chrétien F, Lefebure JM, Pivert G, Kosco-Vilbois M et al (2009) Neutralization of IFNγ defeats haemophagocytosis in LCMV-infected perforin- and Rab27a-deficient mice. EMBO Mol Med 1(2):112–124. https://doi.org/10.1002/emmm.200900009
Canna SW (2014) Interferon-γ: friend or foe in systemic juvenile idiopathic arthritis and adult still’s disease. Arthritis Rheumatol 66(5):1072–1076. https://doi.org/10.1002/art.38362
Sikora KA, Fall N, Thornton S, Grom AA (2012) The limited role of interferon-γ in systemic juvenile idiopathic arthritis cannot be explained by cellular hyporesponsiveness. Arthritis Rheum 64(11):3799–3808. https://doi.org/10.1002/art.34604
Bracaglia C, De GK, Marafon DP, Guilhot F, Ferlin W, Prencipe G et al (2017) Elevated circulating levels of interferon-γ and interferon-γ-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann Rheum Dis 76(1):166–172. https://doi.org/10.1136/annrheumdis-2015-209020
Fall N, Barnes M, Thornton S, Luyrink L, Olson J, Ilowite NT et al (2007) Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum 56(11):3793–3804. https://doi.org/10.1002/art.22981
Put K, Avau A, Brisse E, Mitera T, Proost P, Bader-meunier B et al (2015) Cytokines in systemic juvenile idiopathic arthritis and haemophagocytic lymphohistiocytosis: tipping the balance between interleukin-18 and interferon-γ. Rheumatology 54(8):1507–1517. https://doi.org/10.1093/rheumatology/keu524
De Benedetti F, Prencipe G, Bracaglia C, Marasco E, Grom AA (2021) Targeting interferon-γ in hyperinflammation: opportunities and challenges. Nat Rev Rheumatol 17(11):678–691. https://doi.org/10.1038/s41584-021-00694-z
Qu H, Sundberg E, Aulin C, Neog M, Palmblad K, Horne AC et al (2021) Immunoprofiling of active and inactive systemic juvenile idiopathic arthritis reveals distinct biomarkers: a single-center study. Pediatr Rheumatol 19(1):173. https://doi.org/10.1186/s12969-021-00660-9
Gattorno M, Piccini A, Lasigliè D, Tassi S, Brisca G, Carta S et al (2008) The pattern of response to anti-interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum 58(5):1505–1515. https://doi.org/10.1002/art.23437
Ogilvie EM, Khan A, Hubank M, Kellam P, Woo P (2007) Specific gene expression profiles in systemic juvenile idiopathic arthritis. Arthritis Rheum 56(6):1954–1965. https://doi.org/10.1002/art.22644
Saha B, Jyothi Prasanna S, Chandrasekar B, Nandi D (2010) Gene modulation and immunoregulatory roles of Interferon gamma. Cytokine 50(1):1–14. https://doi.org/10.1016/j.cyto.2009.11.021
Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J (2005) Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med 201(9):1479–1486. https://doi.org/10.1084/jem.20050473
Quartier P, Allantaz F, Cimaz R, Pillet P, Messiaen C, Bardin C et al (2011) A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial). Ann Rheum Dis 70(5):747–754. https://doi.org/10.1136/ard.2010.134254
Hinze T, Kessel C, Hinze CH, Seibert J, Gram H, Foell D (2021) A dysregulated interleukin-18-interferon-γ-CXCL9 axis impacts treatment response to canakinumab in systemic juvenile idiopathic arthritis. Rheumatol 60(11):5165–5174. https://doi.org/10.1093/rheumatology/keab113
De Jager W, Vastert SJ, Beekman JM, Wulffraat NM, Kuis W, Coffer PJ et al (2009) Defective phosphorylation of interleukin-18 receptor β causes impaired natural killer cell function in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum 60(9):2782–2793. https://doi.org/10.1002/art.24750
Weiss ES, Girard-Guyonvarc’h C, Holzinger D, De Jesus AA, Tariq Z, Picarsic J et al (2018) Interleukin-18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome. Blood 131(13):1442–1455. https://doi.org/10.1182/blood-2017-12-820852
Avau A, Mitera T, Put S, Put K, Brisse E, Filtjens J et al (2014) Systemic juvenile idiopathic arthritis-like syndrome in mice following stimulation of the immune system with freund’s complete adjuvant: regulation by interferon-γ. Arthritis Rheumatol 66(5):1340–1351. https://doi.org/10.1002/art.38359
Behrens EM, Canna SW, Slade K, Rao S, Kreiger PA, Paessler M et al (2011) Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J Clin Invest 121(6):2264–2277. https://doi.org/10.1172/JCI43157
Prencipe G, Caiello I, Pascarella A, Grom AA, Bracaglia C, Chatel L et al (2018) Neutralization of IFN-γ reverts clinical and laboratory features in a mouse model of macrophage activation syndrome. J Allergy Clin Immunol 141(4):1439–1449. https://doi.org/10.1016/j.jaci.2017.07.021
Strippoli R, Carvello F, Scianaro R, De Pasquale L, Vivarelli M, Petrini S et al (2012) Amplification of the response to toll-like receptor ligands by prolonged exposure to interleukin-6 in mice: implication for the pathogenesis of macrophage activation syndrome. Arthritis Rheum 64(5):1680–1688. https://doi.org/10.1002/art.33496
Bracaglia C, Marafon DP, Caiello I, de Graaf K, Guilhot F, Ferlin W et al (2015) High levels of interferon-gamma (IFNγ) in macrophage activation syndrome (MAS) and CXCL9 levels as a biomarker for IFNγ production in MAS. In: Pediatric rheumatology, p O84. https://doi.org/10.1186/1546-0096-13-S1-O84
Takada H, Takahata Y, Nomura A, Ohga S, Mizuno Y, Hara T (2003) Increased serum levels of interferon-γ-inducible protein 10 and monokine induced by gamma interferon in patients with haemophagocytic lymphohistiocytosis. Clin Exp Immunol 133(3):448–453. https://doi.org/10.1046/j.1365-2249.2003.02237.x
Jordan M, Prof FL, Allen C, De Benedetti F, Grom AA, Ballabio M et al (2015) A novel targeted approach to the treatment of hemophagocytic lymphohistiocytosis (HLH) with an anti-interferon gamma (IFNγ) monoclonal antibody (mAb), NI-0501: first results from a pilot phase 2 study in children with primary HLH. Blood 126(23):3. https://doi.org/10.1182/blood.V126.23.LBA-3.LBA-3
Alfaleh MA, Alsaab HO, Mahmoud AB, Alkayyal AA, Jones ML, Mahler SM et al (2020) Phage display derived monoclonal antibodies: from bench to bedside. Front Immunol 11:1986. https://doi.org/10.3389/fimmu.2020.01986
De Benedetti F, Grom AA, Brogan P, Bracaglia C, Pardeo M, Marucci G et al (2021) Macrophage Activation Syndrome (MAS) in Systemic Juvenile Idiopathic Arthritis (sJIA): treatment with emapalumab, an Anti-Interferon Gamma (IFNγ) monoclonal antibody. Blood 138(Supplement 1):2058–2058. https://doi.org/10.1182/blood-2021-147596
Novick D, Kim S, Kaplanski G, Dinarello CA (2013) Interleukin-18, more than a Th1 cytokine. Semin Immunol 25(6):439–448. https://doi.org/10.1016/j.smim.2013.10.014
Shimizu M, Nakagishi Y, Inoue N, Mizuta M, Ko G, Saikawa Y et al (2015) Interleukin-18 for predicting the development of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Clin Immunol 160(2):277–281. https://doi.org/10.1016/j.clim.2015.06.005
Ohmori Y, Hamilton TA (1997) IL-4-induced STAT6 suppresses IFN-gamma-stimulated STAT1-dependent transcription in mouse macrophages. J Immunol 159(11):5474–5482
Wong P, Severns CW, Guyer NB, Wright TM (1994) A unique palindromic element mediates gamma interferon induction of mig gene expression. Mol Cell Biol 14(2):914–922. https://doi.org/10.1128/mcb.14.2.914-922.1994
Wright TM, Farber JM (1991) 5’ Regulatory region of a novel cytokine gene mediates selective activation by interferon γ. J Exp Med 173(2):417–422. https://doi.org/10.1084/jem.173.2.417
Ohmori Y, Hamilton TA (1993) Cooperative interaction between interferon (IFN) stimulus response element and κB sequence motifs controls IFNγ- and lipopolysaccharide-stimulated transcription from the murine IP-10 promoter. J Biol Chem 268(9):6677–6688. https://doi.org/10.1016/s0021-9258(18)53303-2
Majumder S, Zhou LZ, Chaturvedi P, Babcock G, Aras S, Ransohoff RM (1998) p48/STAT-1alpha-containing complexes play a predominant role in induction of IFN-gamma-inducible protein 10 kDa (IP-10) by IFN-gamma alone or in synergy with TNF-alpha. J Immunol 161(9):4736–4744
Tensen CP, Flier J, Rampersad SS, Sampat-Sardjoepersad S, Scheper RJ, Boorsma DM et al (1999) Genomic organization, sequence and transcriptional regulation of the human CXCL 11 gene. Biochim Biophys Acta Gene Struct Expr 1446(1–2):167–172. https://doi.org/10.1016/S0167-4781(99)00084-6
Sandhya Rani MR, Foster GR, Leung S, Leaman D, Stark GR, Ransohoff RM (1996) Characterization of β-R1, a gene that is selectively induced by interferon β (IFN-β) compared with IFN-α. J Biol Chem 271(37):22878–22884. https://doi.org/10.1074/jbc.271.37.22878
Wang Q, Nagarkar DR, Bowman ER, Schneider D, Gosangi B, Lei J et al (2009) Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J Immunol 183(11):6989–6997. https://doi.org/10.4049/jimmunol.0901386
Kadavath S, Efthimiou P (2015) Adult-onset Still’s disease-pathogenesis, clinical manifestations, and new treatment options. Ann Med 47(1):6–14. https://doi.org/10.3109/07853890.2014.971052
Giacomelli R, Ruscitti P, Shoenfeld Y (2018) A comprehensive review on adult onset Still’s disease. J Autoimmun 93:24–36. https://doi.org/10.1016/j.jaut.2018.07.018
Bywaters EG (1971) Still’s disease in the adult. Ann Rheum Dis Rheum Dis 30(2):121–133. https://doi.org/10.1136/ard.30.2.121
Nirmala N, Brachat A, Feist E, Blank N, Specker C, Witt M et al (2015) Gene-expression analysis of adult-onset Still’s disease and systemic juvenile idiopathic arthritis is consistent with a continuum of a single disease entity. Pediatr Rheumatol. https://doi.org/10.1186/s12969-015-0047-3
Vastert SJ, Jamilloux Y, Quartier P, Ohlman S, Osterling Koskinen L, Kullenberg T et al (2019) Anakinra in children and adults with Still’s disease. Rheumatol (United Kingdom) 58(Suppl 6):vi9–vi22. https://doi.org/10.1093/rheumatology/kez350
Pay S, Türkçapar N, Kalyoncu M, Şimşek I, Beyan E, Ertenli I et al (2006) A multicenter study of patients with adult-onset Still’s disease compared with systemic juvenile idiopathic arthritis. Clin Rheumatol 25(5):639–644. https://doi.org/10.1007/s10067-005-0138-5
Ruscitti P, Cipriani P, Di Benedetto P, Liakouli V, Carubbi F, Berardicurti O et al (2017) Advances in immunopathogenesis of macrophage activation syndrome during rheumatic inflammatory diseases: toward new therapeutic targets? Expert Rev Clin Immunol 13(11):1041–1047. https://doi.org/10.1080/1744666X.2017.1372194
Sfriso P, Bindoli S, Doria A, Feist E, Galozzi P (2020) Canakinumab for the treatment of adult-onset Still’s disease. Expert Rev Clin Immunol 16(2):129–138. https://doi.org/10.1080/1744666X.2019.1707664
Kasama T, Furuya H, Yanai R (2012) Correlation of serum CX3CL1 level with disease activity in adult-onset Still’s disease and significant involvement in hemophagocytic syndrome. Clin Rheumatol 31(5):853–860. https://doi.org/10.1007/s10067-012-1952-1
Kim HA, Kim YH, Jeon YK, Yang WI, Kwon JE, Han JH (2019) Histopathology and expression of the chemokines CXCL10, CXCL13, and CXCR3 and the endogenous TLR-4 ligand S100A8/A9 in lymph nodes of patients with adult-onset Still’s disease. Sci Rep 9(1):7517. https://doi.org/10.1038/s41598-019-44032-6
Han JH, Ahn MH, Jung JY, Suh CH, Kwon JE, Yim H et al (2019) The levels of CXCL12 and its receptor, CXCR4, as a biomarker of disease activity and cutaneous manifestation in adult-onset Still’s disease. Clin Exp Rheumatol 37(6):67–73
Mitrovic S, Fautrel B (2018) New markers for adult-onset still’s disease. Jt Bone Spine 85(3):285–293. https://doi.org/10.1016/j.jbspin.2017.05.011
Soy M, Ergin M, Paydas S (2004) Lymphadenopathy in adult-onset still’s disease mimicking peripheral T-cell lymphoma. Clin Rheumatol 23(1):81–82. https://doi.org/10.1007/s10067-003-0826-y
Jeon YK, Paik JH, Park SS, Park SO, Kim YA, Kim JE et al (2004) Spectrum of lymph node pathology in adult onset Still’s disease; analysis of 12 patients with one follow up biopsy. J Clin Pathol 57(10):1052–1056. https://doi.org/10.1136/jcp.2004.018010
Valente RM, Banks PM, Conn DL (1989) Characterization of lymph node histology in adult onset Still’s disease. J Rheumatol 16(3):349–354
Chen DY, Lan JL, Lin FJ, Hsieh TY, Wen MC (2004) Predominance of Th1 cytokine in peripheral blood and pathological tissues of patients with active untreated adult onset Still’s disease. Ann Rheum Dis 63(10):1300–1306. https://doi.org/10.1136/ard.2003.013680
Kim HA, An JM, Nam JY, Jeon JAY, Suh CH (2012) Serum S100A8/A9, but not follistatin-like protein 1 and interleukin 18, may be a useful biomarker of disease activity in adult-onset still’s disease. J Rheumatol 39(7):1399–1406. https://doi.org/10.3899/jrheum.120079
Holzinger D, Frosch M, Kastrup A, Prince FHM, Otten MH, Van Suijlekom-Smit LWA et al (2012) The toll-like receptor 4 agonist MRP8/14 protein complex is a sensitive indicator for disease activity and predicts relapses in systemic-onset juvenile idiopathic arthritis. Ann Rheum Dis 71(6):974–980. https://doi.org/10.1136/annrheumdis-2011-200598
Wang J, Vodovotz Y, Fan L, Li Y, Liu Z, Namas R et al (2015) Injury-induced MRP8/MRP14 stimulates IP-10/CXCL10 in monocytes/macrophages. FASEB J 29(1):250–262. https://doi.org/10.1096/fj.14-255992
Maranini B, Ciancio G, Govoni M (2021) Adult-onset still’s disease: novel biomarkers of specific subsets, disease activity, and relapsing forms. Int J Mol Sci 22(24):13320. https://doi.org/10.3390/ijms222413320
Walsh JA, Magrey M (2021) Clinical manifestations and diagnosis of axial spondyloarthritis. JCR J Clin Rheumatol 27(8):e547–e560. https://doi.org/10.1097/rhu.0000000000001575
Mauro D, Thomas R, Guggino G, Lories R, Brown MA, Ciccia F (2021) Ankylosing spondylitis: an autoimmune or autoinflammatory disease? Nat Rev Rheumatol 17(7):387–404. https://doi.org/10.1038/s41584-021-00625-y
Rudwaleit M, Van Der Heijde D, Landewé R, Akkoc N, Brandt J, Chou CT et al (2011) The Assessment of SpondyloArthritis international Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 70(1):25–31. https://doi.org/10.1136/ard.2010.133645
Robinson PC, van der Linden S, Khan MA, Taylor WJ (2021) Axial spondyloarthritis: concept, construct, classification and implications for therapy. Nat Rev Rheumatol 17(2):109–118. https://doi.org/10.1038/s41584-020-00552-4
van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27(4):361–368. https://doi.org/10.1002/art.1780270401
Goie The HS, Steven MM, van der Linden SM, Cats A (1985) Evaluation of diagnostic criteria for ankylosing spondylitis: a comparison of the Rome, New York and modified New York criteria in patients with a positive clinical history screening test for ankylosing spondylitis. Rheumatology 24(3):242–249. https://doi.org/10.1093/rheumatology/24.3.242
Carron P, De Craemer AS, Van Den Bosch F (2020) Peripheral spondyloarthritis: a neglected entity—state of the art. RMD Open 6(1):e001136. https://doi.org/10.1136/rmdopen-2019-001136
Parida JR, Kumar S, Ahmed S, Chaurasia S, Mukherjee R, Singh R et al (2021) Reactive arthritis and undifferentiated peripheral spondyloarthritis share human leucocyte antigen B27 subtypes and serum and synovial fluid cytokine profiles. Rheumatol (United Kingdom) 60(6):3004–3011. https://doi.org/10.1093/rheumatology/keaa746
Abji F, Pollock RA, Liang K, Chandran V, Gladman DD (2016) Brief report: CXCL10 is a possible biomarker for the development of psoriatic arthritis among patients with psoriasis. Arthritis Rheumatol 68(12):2911–2916. https://doi.org/10.1002/art.39800
Muntyanu A, Abji F, Liang K, Pollock RA, Chandran V, Gladman DD (2016) Differential gene and protein expression of chemokines and cytokines in synovial fluid of patients with arthritis. Arthritis Res Ther 18(1):296. https://doi.org/10.1186/s13075-016-1196-6
Veale DJ, Fearon U (2018) The pathogenesis of psoriatic arthritis. Lancet 391(10136):2273–2284. https://doi.org/10.1016/S0140-6736(18)30830-4
Coates LC, Helliwell PS (2017) Psoriatic arthritis: state of the art review. Clin Med 17(1):65–70. https://doi.org/10.7861/clinmedicine.17-1-65
Olivieri I, D’Angelo S, Palazzi C, Padula A (2014) Advances in the management of psoriatic arthritis. Nat Rev Rheumatol 10(9):531–542. https://doi.org/10.1038/nrrheum.2014.106
Touma Z, Thavaneswaran A, Chandran V, Pellett F, Cook RJ, Gladman DD (2016) Clinical and demographic characteristics of erosion-free and erosion-present status in psoriatic arthritis in a cohort study. J Rheumatol 43(6):1057–1062. https://doi.org/10.3899/jrheum.150466
Haroon M, Gallagher P, FitzGerald O (2015) Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis 74(6):1045–1050. https://doi.org/10.1136/annrheumdis-2013-204858
Antonelli A, Fallahi P, Sedie AD, Ferrari SM, Maccheroni M, Bombardieri S et al (2009) High values of Th1 (CXCL10) and Th2 (CCL2) chemokines in patients with psoriatic arthtritis. Clin Exp Rheumatol 27(1):22–27
Pollock RA, Abji F, Liang K, Chandran V, Pellett FJ, Virtanen C et al (2015) Gene expression differences between psoriasis patients with and without inflammatory arthritis. J Invest Dermatol 135(2):620–623. https://doi.org/10.1038/jid.2014.414
Antonelli A, Fallahi P, Sedie AD, Ferrari SM, Maccheroni M, Bombardieri S et al (2008) High values of alpha (CXCL10) and beta (CCL2) circulating chemokines in patients with psoriatic arthritis, in presence or absence of autoimmune thyroiditis. Autoimmunity 41(7):537–542. https://doi.org/10.1080/08916930802170401
Devito A (2014) Interferon γ-induced chemokines in psoriatic arthritis. Clin Ter 165(6):e442–e446. https://doi.org/10.7417/CT.2014.1790
Lima XT, Oliveira RTD, Braga FG, Magalhães RF, Mamoni RL, Blotta MHSL (2015) Circulating levels of chemokines in psoriasis. Autoimmunity 48(1):57–60. https://doi.org/10.3109/08916934.2014.947476
Schafer PH, Parton A, Gandhi AK, Capone L, Adams M, Wu L et al (2010) Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol 159(4):842–855. https://doi.org/10.1111/j.1476-5381.2009.00559.x
Gottlieb AB, Chamian F, Masud S, Cardinale I, Abello MV, Lowes MA et al (2005) TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J Immunol 175(4):2721–2729. https://doi.org/10.4049/jimmunol.175.4.2721
Elia G (2018) MIG in psoriatic arthritis. Clin Ter 169(6):E297-302. https://doi.org/10.7417/CT.2018.2097
Gottlieb AB, Luster AD, Posnett DN, Martin CD (1988) Detection of a γ interferon-induced protein IP-10 in psoriatic plaques. J Exp Med 168(3):941–948. https://doi.org/10.1084/jem.168.3.941
Ottaviani C, Nasorri F, Bedini C, de Pità O, Girolomoni G, Cavani A (2006) CD56brightCD16(-) NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol 36(1):118–128. https://doi.org/10.1002/eji.200535243
Zhu W, He X, Cheng K, Zhang L, Chen D, Wang X et al (2019) Ankylosing spondylitis: etiology, pathogenesis, and treatments. Bone Res 7:22. https://doi.org/10.1038/s41413-019-0057-8
Generali E, Bose T, Selmi C, Voncken JW, Damoiseaux JGMC (2018) Nature versus nurture in the spectrum of rheumatic diseases: Classification of spondyloarthritis as autoimmune or autoinflammatory. Autoimmun Rev 17(9):935–941. https://doi.org/10.1016/j.autrev.2018.04.002
Wang J, Zhao Q, Wang G, Yang C, Xu Y, Li Y et al (2016) Circulating levels of Th1 and Th2 chemokines in patients with ankylosing spondylitis. Cytokine 81:10–14. https://doi.org/10.1016/j.cyto.2016.01.012
Duftner C, Dejaco C, Kullich W, Klauser A, Goldberger C, Falkenbach A et al (2006) Preferential type 1 chemokine receptors and cytokine production of CD28-T cells in ankylosing spondylitis. Ann Rheum Dis 65(5):647–653. https://doi.org/10.1136/ard.2005.042085
Aggarwal A, Agarwal S, Misra R (2007) Chemokine and chemokine receptor analysis reveals elevated interferon-inducible protein-10 (IP)-10/CXCL10 levels and increased number of CCR5+ and CXCR3+ CD4 T cells in synovial fluid of patients with enthesitis-related arthritis (ERA). Clin Exp Immunol 148(3):515–519. https://doi.org/10.1111/j.1365-2249.2007.03377.x
Shirtliff ME, Mader JT (2002) Acute septic arthritis. Clin Microbiol Rev 15(4):527–544. https://doi.org/10.1128/CMR.15.4.527-544.2002
Ross JJ (2017) Septic arthritis of native joints. Infect Dis Clin North Am 31(2):203–218. https://doi.org/10.1016/j.idc.2017.01.001
Long B, Koyfman A, Gottlieb M (2019) Evaluation and management of septic arthritis and its mimics in the emergency department. West J Emerg Med 20(2):331–341. https://doi.org/10.5811/westjem.2018.10.40974
Goldenberg DL, Reed JI (1985) Bacterial arthritis. N Engl J Med 312(12):764–771. https://doi.org/10.1016/S0140-6736(86)90235-7
Schutyser E, Struyf S, Wuyts A, Put W, Geboes K, Grillet B et al (2001) Selective induction of CCL18/PARC by staphylococcal enterotoxins in mononuclear cells and enhanced levels in septic and rheumatoid arthritis. Eur J Immunol 31(12):3755–62. https://doi.org/10.1002/1521-4141(200112)31:12<3755::aid-immu3755>3.0.co;2-o
Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM (2001) Cutting edge: IFN-inducible ELR-CXC chemokines display defensin-like antimicrobial activity. J Immunol 167(2):623–627. https://doi.org/10.4049/jimmunol.167.2.623
Antonia AL, Gibbs KD, Trahair ED, Pittman KJ, Martin AT, Schott BH et al (2019) Pathogen evasion of chemokine response through suppression of CXCL10. Front Cell Infect Microbiol 9:280. https://doi.org/10.3389/fcimb.2019.00280
Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB et al (2016) Osteoarthritis. Nat Rev Dis Prim 2:16072. https://doi.org/10.1038/nrdp.2016.72
Katz JN, Arant KR, Loeser RF (2021) Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA J Am Med Assoc 325(6):568–578. https://doi.org/10.1001/jama.2020.22171
Sohn DH, Sokolove J, Sharpe O, Erhart JC, Chandra PE, Lahey LJ et al (2012) Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther 14(1):R7. https://doi.org/10.1186/ar3555
Manferdini C, Paolella F, Gabusi E, Cattini L, Rojewski M, Schrezenmeier H et al (2020) Osteoarthritic milieu affects adipose-derived mesenchymal stromal cells. J Orthop Res 38(2):336–347. https://doi.org/10.1002/jor.24446
Beekhuizen M, Gierman LM, van Spil WE, Van Osch GJVM, Huizinga TWJ, Saris DBF et al (2013) An explorative study comparing levels of soluble mediators in control and osteoarthritic synovial fluid. Osteoarthr Cartil 21(7):918–922. https://doi.org/10.1016/j.joca.2013.04.002
Thomas Vangsness J, Burke WS, Narvy SJ, MacPhee RD, Fedenko AN (2011) Human knee synovial fluid cytokines correlated with grade of knee osteoarthritis—a pilot study. Bull NYU Hosp Jt Dis 69(2):122–127
Huss RS, Huddleston JI, Goodman SB, Butcher EC, Zabel BA (2010) Synovial tissue-infiltrating natural killer cells in osteoarthritis and periprosthetic inflammation. Arthritis Rheum 62(12):3799–3805. https://doi.org/10.1002/art.27751
Gobezie R, Kho A, Krastins B, Sarracino DA, Thornhill TS, Chase M et al (2007) High abundance synovial fluid proteome: distinct profiles in health and osteoarthritis. Arthritis Res Ther 9(2):R36. https://doi.org/10.1186/ar2172
Metzemaekers M, Abouelasrar Salama S, Vandooren J, Mortier A, Janssens R, Vandendriessche S et al (2021) From ELISA to immunosorbent tandem mass spectrometry proteoform analysis: the example of CXCL8/interleukin-8. Front Immunol 12:644725. https://doi.org/10.3389/fimmu.2021.644725
Endres M, Andreas K, Kalwitz G, Freymann U, Neumann K, Ringe J et al (2010) Chemokine profile of synovial fluid from normal, osteoarthritis and rheumatoid arthritis patients: CCL25, CXCL10 and XCL1 recruit human subchondral mesenchymal progenitor cells. Osteoarthr Cartil 18(11):1458–1466. https://doi.org/10.1016/j.joca.2010.08.003
Bondeson J, Blom AB, Wainwright S, Hughes C, Caterson B, Van Den Berg WB (2010) The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum 62(3):647–657. https://doi.org/10.1002/art.27290
Hsueh MF, Zhang X, Wellman SS, Bolognesi MP, Kraus VB (2021) Synergistic roles of macrophages and neutrophils in osteoarthritis progression. Arthritis Rheumatol 73(1):89–99. https://doi.org/10.1002/art.41486
Benigni G, Dimitrova P, Antonangeli F, Sanseviero E, Milanova V, Blom A et al (2017) CXCR3/CXCL10 axis regulates neutrophil–NK cell cross-talk determining the severity of experimental osteoarthritis. J Immunol 198(5):2115–2124. https://doi.org/10.4049/jimmunol.1601359
Park JH, Lee NK, Lee SY (2017) Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol Cells 40(10):706–713. https://doi.org/10.14348/molcells.2017.0225
Clem K, Galdzicka M, Faryna DM, Soto E, Ostroff GR, Ginns EI (2008) Development of a novel non-viral osteoprotegerin gene therapy for treating low bone density. Mol Ther 16(Supplement 1):S49-50. https://doi.org/10.1016/s1525-0016(16)39531-4
Lisignoli G, Toneguzzi S, Piacentini A, Cattini L, Lenti A, Tschon M et al (2003) Human osteoblasts express functional CXC chemokine receptors 3 and 5: activation by their ligands, CXCL10 and CXCL13, significantly induces alkaline phosphatase and β-N-acetylhexosaminidase release. J Cell Physiol 194(1):71–79. https://doi.org/10.1002/jcp.10188
De Ceuninck F, Dassencourt L, Anract P (2004) The inflammatory side of human chondrocytes unveiled by antibody microarrays. Biochem Biophys Res Commun 323(3):960–969. https://doi.org/10.1016/j.bbrc.2004.08.184
Szulc P, Bauer DC (2013) Biochemical markers of bone turnover in osteoporosis. In: Osteoporosis, 4th edn, pp 1573–1610. https://doi.org/10.1016/B978-0-12-415853-5.00067-4
Marks SC, Seifert MF (1985) The lifespan of osteoclasts: experimental studies using the giant granule cytoplasmic marker characteristic of beige mice. Bone 6(6):451–455. https://doi.org/10.1016/8756-3282(85)90223-6
Šućur A, Katavić V, Kelava T, Jajić Z, Kovačić N, Grčević D (2014) Induction of osteoclast progenitors in inflammatory conditions: key to bone destruction in arthritis. Int Orthop 38(9):1893–1903. https://doi.org/10.1007/s00264-014-2386-y
Deal C (2012) Bone loss in rheumatoid arthritis: systemic, periarticular, and focal. Curr Rheumatol Rep 14(3):231–237. https://doi.org/10.1007/s11926-012-0253-7
Ikić M, Jajić Z, Lazić E, Ivčević S, Grubišić F, Marušić A et al (2014) Association of systemic and intra-articular osteoclastogenic potential, pro-inflammatory mediators and disease activity with the form of inflammatory arthritis. Int Orthop 38(1):183–192. https://doi.org/10.1007/s00264-013-2121-0
Laragione T, Brenner M, Mello A, Symons M, Gulko PS (2008) The arthritis severity locus Cia5d is a novel genetic regulator of the invasive properties of synovial fibroblasts. Arthritis Rheum 58(8):2296–2306. https://doi.org/10.1002/art.23610
Izquierdo E, Cañete JD, Celis R, Del Rey MJ, Usategui A, Marsal S et al (2011) Synovial fibroblast hyperplasia in rheumatoid arthritis: clinicopathologic correlations and partial reversal by anti-tumor necrosis factor therapy. Arthritis Rheum 63(9):2575–2583. https://doi.org/10.1002/art.30433
Qu Z, Garcia CH, Orourke LM, Planck SR, Kohli M, Rosenbaum JT (1994) Local proliferation of fibroblast-like synoviocytes contributes to synovial hyperplasia. Results of proliferating cell nuclear antigen/cyclin, c-myc, and nucleolar organizer region staining. Arthritis Rheum 37(2):212–220. https://doi.org/10.1002/art.1780370210
Korsunsky I, Wei K, Pohin M, Kim EY, Barone F, Major T et al (2022) Cross-tissue, single-cell stromal atlas identifies shared pathological fibroblast phenotypes in four chronic inflammatory diseases. Med 3(7):481-518.e14. https://doi.org/10.1016/j.medj.2022.05.002
Zhang F, Kevin W, Slowikowski K, Fonseka CY, Rao DA, Kelly S et al (2019) Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat Immunol 20(7):928–942. https://doi.org/10.1038/s41590-019-0378-1
Burrage PS, Mix KS, Constance E (2006) Brinckerhoff. Matrix metalloproteinases: role in arthritis. Front Biosci 11:529–543. https://doi.org/10.2741/1817
Bin SH, Yokota H (2002) Reduction of cytokine-induced expression and activity of MMP-1 and MMP-13 by mechanical strain in MH7A rheumatoid synovial cells. Matrix Biol 21(3):263–270. https://doi.org/10.1016/S0945-053X(02)00003-3
Smolian H, Aurer A, Sittinger M, Zacher J, Bernimoulin JP, Burmester GR et al (2001) Secretion of gelatinases and activation of gelatinase A (MMP-2) by human rheumatoid synovial fibroblasts. Biol Chem 382(10):1491–1499. https://doi.org/10.1515/BC.2001.183
Kumkumian GK, Lafyatis R, Remmers EF, Case JP, Kim SJ, Wilder RL (1989) Platelet-derived growth factor and IL-1 interactions in rheumatoid arthritis. Regulation of synoviocyte proliferation, prostaglandin production, and collagenase transcription. J Immunol 143(3):833–837
Giegold O, Ogrissek N, Richter C, Schröder M, Herrero San Juan M, Pfeilschifter JM et al (2013) CXCL9 causes heterologous desensitization of CXCL12-mediated memory T lymphocyte activation. J Immunol 190(7):3696–3705. https://doi.org/10.4049/jimmunol.1101293
Buljevic S, Detel D, Pucar LB, Mihelic R, Madarevic T, Sestan B et al (2013) Levels of dipeptidyl peptidase IV/CD26 substrates neuropeptide y and vasoactive intestinal peptide in rheumatoid arthritis patients. Rheumatol Int 33(11):2867–2874. https://doi.org/10.1007/s00296-013-2823-z
Cordero OJ, Varela-Calviño R, López-González T, Calviño-Sampedro C, Viñuela JE, Mouriño C et al (2015) CD26 Expression on T helper populations and sCD26 serum levels in patients with rheumatoid arthritis. PLoS ONE 10(7):e0131992. https://doi.org/10.1371/journal.pone.0131992
Morgan R, Endres J, Behbahani-Nejad N, Phillips K, Ruth JH, Friday SC et al (2015) Expression and function of aminopeptidase N/CD13 produced by fibroblast-like synoviocytes in rheumatoid arthritis: role of CD13 in chemotaxis of cytokine-activated t cells independent of enzymatic activity. Arthritis Rheumatol 67(1):74–85. https://doi.org/10.1002/art.38878
Tchetverikov I, Lohmander LS, Verzijl N, Huizinga TWJ, TeKoppele JM, Hanemaaijer R et al (2005) MMP protein and activity levels in synovial fluid from patients with joint injury, inflammatory arthritis, and osteoarthritis. Ann Rheum Dis 64(5):694–698. https://doi.org/10.1136/ard.2004.022434
Kazantseva MG, Hung NA, Highton J, Hessian PA (2013) MMP expression in rheumatoid inflammation: the rs11568818 polymorphism is associated with MMP-7 expression at an extra-articular site. Genes Immun 14(3):162–169. https://doi.org/10.1038/gene.2012.65
Cao R, Zhang Y, Du J, Chen S, Wang N, Ying H et al (2020) Increased FURIN expression in rheumatoid arthritis patients and its anti-inflammatory effect. J Clin Lab Anal 34(12):e23530. https://doi.org/10.1002/jcla.23530
Valli A, Ranta N, Grönholm A, Silvennoinen O, Pesu M, Isomäki P (2019) Increased expression of the proprotein convertase enzyme FURIN in rheumatoid arthritis. Scand J Rheumatol 48(3):173–177. https://doi.org/10.1080/03009742.2018.1520294
Repnik U, Starr AE, Overall CM, Turk B (2015) Cysteine cathepsins activate ELR chemokines and inactivate non-ELR chemokines. J Biol Chem 290(22):13800–13811. https://doi.org/10.1074/jbc.M115.638395
Lambeir AM, Proost P, Durinx C, Bal G, Senten K, Augustyns K et al (2001) Kinetic investigation of chemokine truncation by CD26/dipeptidyl peptidase IV reveals a striking selectivity within the chemokine family. J Biol Chem 276(32):29839–29845. https://doi.org/10.1074/jbc.M103106200
Ludwig A, Schiemann F, Mentlein R, Lindner B, Brandt E (2002) Dipeptidyl peptidase IV (CD26) on T cells cleaves the CXC chemokine CXCL11 (I-TAC) and abolishes the stimulating but not the desensitizing potential of the chemokine. J Leukoc Biol 72:183–191. https://doi.org/10.1189/jlb.72.1.183
Ajami K, Pitman MR, Wilson CH, Park J, Menz RI, Starr AE et al (2008) Stromal cell-derived factors 1α and 1β, inflammatory protein-10 and interferon-inducible T cell chemo-attractant are novel substrates of dipeptidyl peptidase 8. FEBS Lett 582(5):819–825. https://doi.org/10.1016/j.febslet.2008.02.005
Proost P, Mortier A, Loos T, Vandercappellen J, Gouwy M, Ronsse I et al (2007) Proteolytic processing of CXCL11 by CD13/aminopeptidase N impairs CXCR3 and CXCR7 binding and signaling and reduces lymphocyte and endothelial cell migration. Blood 110(1):37–44. https://doi.org/10.1182/blood-2006-10-049072
Patterson AM, Siddall H, Chamberlain G, Gardner L, Middleton J (2002) Expression of the Duffy antigen/receptor for chemokines (DARC) by the inflamed synovial endothelium. J Pathol 197(1):108–116. https://doi.org/10.1002/path.1100
Watanabe K, Penfold MET, Matsuda A, Ohyanagi N, Kaneko K, Miyabe Y et al (2010) Pathogenic role of CXCR7 in rheumatoid arthritis. Arthritis Rheum 62(11):3211–3220. https://doi.org/10.1002/art.27650
Baldwin HM, Singh MD, Codullo V, King V, Wilson H, McInnes I et al (2017) Elevated ACKR2 expression is a common feature of inflammatory arthropathies. Rheumatol (United Kingdom) 56(9):1607–1617. https://doi.org/10.1093/rheumatology/kex176
Miyabe Y, Miyabe C, Mani V, Mempel TR, Luster AD (2019) Atypical complement receptor C5aR2 transports C5a to initiate neutrophil adhesion and inflammation. Sci Immunol 4(35):eaav5951. https://doi.org/10.1126/sciimmunol.aav5951
Smith E, McGettrick HM, Stone MA, Shaw JS, Middleton J, Nash GB et al (2008) Duffy antigen receptor for chemokines and CXCL5 are essential for the recruitment of neutrophils in a multicellular model of rheumatoid arthritis synovium. Arthritis Rheum 58(7):1968–1973. https://doi.org/10.1002/art.23545
Świdrowska-Jaros J, Smolewska E (2018) A fresh look at angiogenesis in juvenile idiopathic arthritis. Cent Eur J Immunol 43(3):325–330. https://doi.org/10.5114/ceji.2018.80052
Mease PJ (2011) Inflammatory musculoskeletal disease: Identification and assessment. J Rheumatol 38(3):557–561. https://doi.org/10.3899/jrheum.101121
Linkov F, Gu Y, Arslan AA, Liu M, Shore RE, Koenig KL et al (2009) Reliability of tumor markers, chemokines, and metastasis-related molecules in serum. Eur Cytokine Netw 20(1):21–26. https://doi.org/10.1684/ecn.2009.0146
Vanheule V, Metzemaekers M, Janssens R, Struyf S, Proost P (2018) How post-translational modifications influence the biological activity of chemokines. Cytokine 109:29–51. https://doi.org/10.1016/j.cyto.2018.02.026
Barreira R, Laird ME, Yatim N, Fiette L, Ingersoll MA, Albert ML (2015) Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat Immunol 16(8):850–858. https://doi.org/10.1038/ni.3201
Casrouge A, Bisiaux A, Stephen L, Schmolz M, Mapes J, Pfister C et al (2011) Discrimination of agonist and antagonist forms of CXCL10 in biological samples. Clin Exp Immunol 167(1):137–148. https://doi.org/10.1111/j.1365-2249.2011.04488.x
Petrone L, Bondet V, Vanini V, Cuzzi G, Palmieri F, Palucci I et al (2019) First description of agonist and antagonist IP-10 in urine of patients with active TB. Int J Infect Dis 78:15–21. https://doi.org/10.1016/j.ijid.2018.09.001
Meissner EG, Decalf J, Casrouge A, Masur H (2015) Dynamic changes of post-translationally modified forms of CXCL10 and soluble DPP4 in HCV subjects receiving interferon-free therapy. PLoS ONE 10(7):0133236. https://doi.org/10.1371/journal.pone.0133236
Casrouge A, Decalf J, Ahloulay M, Lababidi C, Mansour H, Vallet-Pichard A et al (2011) Evidence for an antagonist form of the chemokine CXCL10 in patients chronically infected with HCV. J Clin Invest 121(1):308–317. https://doi.org/10.1172/JCI40594DS1
Riva A, Laird M, Casrouge A, Ambrozaitis A, Williams R, Naoumov NV et al (2014) Truncated CXCL10 is associated with failure to achieve spontaneous clearance of acute hepatitis C infection. Hepatology 60(2):487–496. https://doi.org/10.1002/hep.27139
Acknowledgements
Figures were created with Biorender.com and ACD/ChemSketch.
Funding
This work was funded by a C1 grant (C16/17/010) from KU Leuven, project G067123N of FWO Flanders, and by the Rega Foundation. LD obtained a predoctoral fellowship Fundamental Research of FWO Flanders (11L3122N).
Author information
Authors and Affiliations
Contributions
LD wrote the initial manuscript. All authors contributed to the study conception and design, provided their comments on former versions of the manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dillemans, L., De Somer, L., Neerinckx, B. et al. A review of the pleiotropic actions of the IFN-inducible CXC chemokine receptor 3 ligands in the synovial microenvironment. Cell. Mol. Life Sci. 80, 78 (2023). https://doi.org/10.1007/s00018-023-04715-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04715-w