Abstract
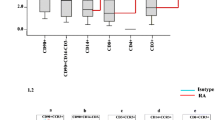
The inflamed synovial tissue of rheumatoid arthritis (RA) is characterized by an infiltration with Th1 cells that predominantly express the chemokine receptors CXCR3 and CCR5. In this study, we investigated the production of the CXCR3-agonistic chemokines CXCL9, CXCL10, and CXCL11 by synovial tissue cells and synovial fibroblast-cell lines (fourth or fifth passage) from RA patients. Concentrations of all CXCR3 ligands in synovial fluids were markedly higher in RA patients than in osteoarthritis (OA) patients. Synovial tissue cells from RA patients more strongly expressed mRNAs for CXCR3 ligands and spontaneously secreted larger amounts of these chemokine proteins than the cells from OA patients. The mRNA expression of all CXCR3 ligands was induced in synovial fibroblasts from RA patients after stimulation with interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), or interleukin-1 beta (IL-1β). However, synovial fibroblasts significantly secreted CXCL9 and CXCL10 proteins, but not CXCL11 protein, after IFN-γ stimulation and secreted only CXCL10 protein after TNF-α or IL-1β stimulation. When stimulated with a combination of IFN-γ and TNF-α, these cells were able to secrete large amounts of all three chemokines. These results indicate that synovial fibroblasts may be involved in perpetuating the Th1 immune response by producing the Th1-associated CXCR3 ligands, and the synergistic effect of IFN-γ and TNF-α may be important for their chemokine production in RA joints.






Similar content being viewed by others
References
Feldmann M, Brennan FM, Maini RN (1996) Role of cytokines in rheumatoid arthritis. Annu Rev Immunol 14:397–440
Feldmann M, Maini RN (2001) Anti-TNFα therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol 19:163-196
Miossec P, van den Berg W (1997) Th1/Th2 cytokine balance in arthritis. Arthritis Rheum 40:2105-2115
Morita Y, Yamamura M, Kawashima M, Harada S, Tsuji K, Shibuya K, Maruyama K, Makino H (1998) Flow cytometric single-cell analysis of cytokine production byCD4+ T cells in synovial tissue and peripheral blood from patients with rheumatoid arthritis. Arthritis Rheum 41:1669-1676
Yamamura M, Kawashima M, Taniai M, Yamauchi H, Tanimoto T, Kurimoto M, Morita Y, Ohmoto Y, Makino H (2001) Interferon-γ-inducing activity of interleukin-18 in the joint with rheumatoid arthritis. Arthritis Rheum 44:275-285
Morita Y, Yamamura M, Nishida K, Harada S, Okamoto H, Inoue H, Ohmoto Y, Modlin RL, Makino H (1998) Expression of interleukin-12 in synovial tissue from patients with rheumatoid arthritis. Arthritis Rheum 41:306-314
Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, Dayer JM (1998) CCR5 is characteristic of Th1 lymphocytes. Nature 391:344-345
Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu L, Butcher EC (2001) Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest 108:1331-1339
Ruth JH, Rottman JB, Katschke KJ Jr, Qin S, Wu L, LaRosa G, Ponath P, Pope RM, Koch AE (2001) Selective lymphocyte chemokine receptor expression in the rheumatoid joint. Arthritis Rheum 44:2750-2760
Zlotnik A, Yoshie O (2000) Chemokines: a new classification system and their role in immunity. Immunity 12:121–127
Sallusto F, Mackay CR, Lanzavecchia A. (2000) The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol 18:593–620
Abbas AK, Murphy KM, Sher A (1996) Functional diversity of helper T lymphocytes. Nature 383:787–793
Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F (1998) Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1 s) and Th2 s. J Exp Med 187:129–134
Sallusto F, Lenig D, Mackay CR, Lanzavecchia A (1998) Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med 187:875–883
Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, Hamid Q, Luster AD (1999) The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol162:3549–3558
Sorensen TL, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik VA, Qin S, Rottman J, Sellebjerg F, Strieter RM, Frederiksen JL, Ransohoff RM (1999) Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest103:807–815
Agostini C, Cassatella M, Zambello R, Trentin L, Gasperini S, Perin A, Piazza F, Siviero M, Facco M, Dziejman M, Chilosi M, Qin S, Luster AD, Semenzato G (1998) Involvement of the IP-10 chemokine in sarcoid granulomatous reactions. J Immunol 161:6413–6420
Patel DD, Zachariah JP, Whichard LP (2001) CXCR3 and CCR5 ligands in rheumatoid arthritis synovium. Clin Immunol 98:39–45
Mohan K, Ding Z, Hanly J, Issekutz TB (2002) IFN-γ-inducible T cell α chemoattractant is a potent stimulator of normal human blood T lymphocyte transendothelial migration: differential regulation by IFN-γ and TNF-α. J Immunol168:6420–6428
Harada S, Yamamura M, Okamoto H, Morita Y, Kawashima M, Aita T, Makino H (1999) Production of interleukin-7 and interleukin-15 by fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum 42:1508–1516
Hosaka S, Akahoshi T, Wada C, Kondo H (1994) Expression of the chemokine superfamily in rheumatoid arthritis. Clin Exp Immunol 97:451–457
Rathanaswami P, Hachicha M, Sadick M, Schall TJ, McColl SR (1993) Expression of the cytokine RANTES in human rheumatoid synovial fibroblasts. Differential regulation of RANTES and interleukin-8 genes by inflammatory cytokines. J Biol Chem 268:5834–5839
Arnett FC, Edworthy SM, Bloch A, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA Jr, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Okamoto H, Yamamura M, Morita Y, Harada S, Makino H, Ota Z. (1997) The synovial expression and serum levels of interleukin-6, interleukin-11, leukemia inhibitory factor, and oncostatin M in rheumatoid arthritis. Arthritis Rheum 40:1096–1105
Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, Modlin RL (1991) Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254:277–279
Luster AD, Unkeless JC, Ravetch JV (1985) γ-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature 315:672–676
Liao F, Rabin RL, Yannelli JR, Koniaris LG, Vanguri P, Farber JM (1995) Human Mig chemokine: biochemical and functional characterization. J Exp Med 182:1301–1314
Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, Sahagan BG, Neote K (1998) Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med 187:2009–2021
Mach F, Sauty A, Iarossi AS, Sukhova GK, Neote K, Libby P, Luster AD (1999) Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J Clin Invest. 104:1041–1050
Bedard PA, Golds EE (1993) Cytokine-induced expression of mRNAs for chemotactic factors in human synovial cells and fibroblasts. J Cell Physiol 154:433–441
Nanki T, Hayashida K, El-Gabalawy HS, Suson S, Shi K, Girschick HJ, Yavuz S, Lipsky PE (2000) Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. J Immunol 165:6590–6598
Zimmermann T, Kunisch E, Pfeiffer R, Hirth A, Stahl HD, Sack U, Laube A, Liesaus E, Roth A, Palombo-Kinne E, Emmrich F, Kinne RW (2001) Isolation and characterization of rheumatoid arthritis synovial fibroblasts from primary culture—primary culture cells markedly differ from fourth-passage cells. Arthritis Res 3:72–76
Hirth A, Skapenko A, Kinne RW, Emmrich F, Schulze-Koops H, Sack U (2002) Cytokine mRNA and protein expression in primary-culture and repeated-passage synovial fibroblasts from patients with rheumatoid arthritis. Arthritis Res 4:117–125
Aaronson DS, Horvath CM (2002) A road map for those who know JAK-STAT. Science 296:1653–1655
Ghosh S, May MJ, Kopp EB (1998) NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 16:225–260
Ohmori Y, Hamilton TA (1993) Cooperative interaction between interferon (IFN) stimulus response element and κB sequence motifs controls IFNγ- and lipopolysaccharide-stimulated transcription from the murine IP-10 promoter. J Biol Chem 268:6677–6688
Farber JM (1997) Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol 61:246–257
Acknowledgements
We thank Dr. H. Inoue, Dr. K. Nishida, and Ms A. Yoshida (Okayama University) for providing clinical samples and technical assistance. This work was supported in part by grants-in-aid (10670411/1270426) from the Ministry of Education, Science, and Culture of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ueno, A., Yamamura, M., Iwahashi, M. et al. The production of CXCR3-agonistic chemokines by synovial fibroblasts from patients with rheumatoid arthritis. Rheumatol Int 25, 361–367 (2005). https://doi.org/10.1007/s00296-004-0449-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-004-0449-x