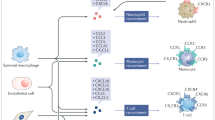
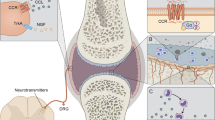
Abstract
Purpose of Review
The involvement of chemokines and their receptors in the genesis and perpetuation of rheumatoid arthritis, spondyloarthritis, and osteoarthritis has been clearly recognized for a long time. Nevertheless, the complexity of their contribution to these diseases is now becoming evident and this review focuses on published evidence on their mechanism of action.
Recent Findings
Studies performed on patients and in vivo models have identified a number of chemokine-mediated pathways involved in various aspects of arthritogenic processes. Chemokines promote leukocyte infiltration and activation, angiogenesis, osteoclast differentiation, and synoviocyte proliferation and activation and participate to the generation of pain by regulating the release of neurotransmitters.
Summary
A number of chemokines are expressed in a timely controlled fashion in the joint during arthropathies, regulating all the aspects of inflammation as well as the equilibrium between damage and repair and between relief and pain. Thus, the targeting of specific chemokine/chemokine receptor interactions is considered a promising tool for therapeutic intervention.

Similar content being viewed by others
Abbreviations
- RA:
-
Rheumatoid arthritis
- SpA:
-
Spondyloarthritis
- OA:
-
Osteoarthritis
- ACPAs:
-
Anti-citrullinated protein antibodies
- NK:
-
Natural killer
- ST:
-
Synovial tissue
- SF:
-
Synovial fluid
- ECM:
-
Extracellular matrix
- ROS:
-
Reactive oxygen species
- FLS:
-
Fibroblast-like synoviocytes
- CNS:
-
Central nervous system
- MMPs:
-
Metalloproteases
- TRPV1:
-
Transient receptor potential cation channel subfamily V member 1
- CatS:
-
Cathepsin S
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–61. doi:10.1038/nature01661.
McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–19. doi:10.1056/NEJMra1004965.
Pratesi F, Dioni I, Tommasi C, Alcaro MC, Paolini I, Barbetti F, et al. Antibodies from patients with rheumatoid arthritis target citrullinated histone 4 contained in neutrophils extracellular traps. Ann Rheum Dis. 2014;73(7):1414–22. doi:10.1136/annrheumdis-2012-202765.
Lebre MC, Jongbloed SL, Tas SW, Smeets TJ, McInnes IB, Tak PP. Rheumatoid arthritis synovium contains two subsets of CD83-DC-LAMP-dendritic cells with distinct cytokine profiles. Am J Pathol. 2008;172(4):940–50. doi:10.2353/ajpath.2008.070703.
Chabaud M, Fossiez F, Taupin JL, Miossec P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol. 1998;161(1):409–14.
Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888–98. doi:10.1056/NEJMra0707449.
Ciccia F, Guggino G, Rizzo A, Manzo A, Vitolo B, La Manna MP, et al. Potential involvement of IL-9 and Th9 cells in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford). 2015;54(12):2264–72. doi:10.1093/rheumatology/kev252.
Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350(25):2572–81. doi:10.1056/NEJMoa032534.
Glatigny S, Fert I, Blaton MA, Lories RJ, Araujo LM, Chiocchia G, et al. Proinflammatory Th17 cells are expanded and induced by dendritic cells in spondylarthritis-prone HLA-B27-transgenic rats. Arthritis Rheum. 2012;64(1):110–20. doi:10.1002/art.33321.
Baeten D, Van den Bosch F, Elewaut D, Stuer A, Veys EM, De Keyser F. Needle arthroscopy of the knee with synovial biopsy sampling: technical experience in 150 patients. Clin Rheumatol. 1999;18(6):434–41.
Reece RJ, Canete JD, Parsons WJ, Emery P, Veale DJ. Distinct vascular patterns of early synovitis in psoriatic, reactive, and rheumatoid arthritis. Arthritis Rheum. 1999;42(7):1481–4. doi:10.1002/1529-0131(199907)42:7<1481::AID-ANR23>3.0.CO;2-E.
Baeten D, Demetter P, Cuvelier C, Van Den Bosch F, Kruithof E, Van Damme N, et al. Comparative study of the synovial histology in rheumatoid arthritis, spondyloarthropathy, and osteoarthritis: influence of disease duration and activity. Ann Rheum Dis. 2000;59(12):945–53.
Baeten D, Kruithof E, De Rycke L, Boots AM, Mielants H, Veys EM, et al. Infiltration of the synovial membrane with macrophage subsets and polymorphonuclear cells reflects global disease activity in spondyloarthropathy. Arthritis Res Ther. 2005;7(2):R359–69. doi:10.1186/ar1501.
Noordenbos T, Yeremenko N, Gofita I, van de Sande M, Tak PP, Canete JD, et al. Interleukin-17-positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum. 2012;64(1):99–109. doi:10.1002/art.33396.
Al-Mossawi MH, Ridley A, Kiedel S, Bowness P. The role of natural killer cells, gamma delta T-cells and other innate immune cells in spondyloarthritis. Curr Opin Rheumatol. 2013;25(4):434–9. doi:10.1097/BOR.0b013e3283620163.
Scrivo R, Morrone S, Spadaro A, Santoni A, Valesini G. Evaluation of degranulation and cytokine production in natural killer cells from spondyloarthritis patients at single-cell level. Cytometry B Clin Cytom. 2011;80(1):22–7. doi:10.1002/cyto.b.20549.
Smith MD, Triantafillou S, Parker A, Youssef PP, Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997;24(2):365–71.
Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44(6):1237–47. doi:10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F.
Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis—results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13(5):361–7. doi:10.1016/j.joca.2005.01.005.
Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6(11):625–35. doi:10.1038/nrrheum.2010.159.
Huss RS, Huddleston JI, Goodman SB, Butcher EC, Zabel BA. Synovial tissue-infiltrating natural killer cells in osteoarthritis and periprosthetic inflammation. Arthritis Rheum. 2010;62(12):3799–805. doi:10.1002/art.27751.
Klein-Wieringa IR, de Lange-Brokaar BJ, Yusuf E, Andersen SN, Kwekkeboom JC, Kroon HM, et al. Inflammatory cells in patients with endstage knee osteoarthritis: a comparison between the synovium and the infrapatellar fat pad. J Rheumatol. 2016;43(4):771–8. doi:10.3899/jrheum.151068.
Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, et al. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. 2014;66(1):1–79. doi:10.1124/pr.113.007724.
Thelen M, Stein JV. How chemokines invite leukocytes to dance. Nat Immunol. 2008;9(9):953–9. doi:10.1038/ni.f.207.
Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36(5):705–16. doi:10.1016/j.immuni.2012.05.008.
Zingoni A, Rocchi M, Storlazzi CT, Bernardini G, Santoni A, Napolitano M. Isolation and chromosomal localization of GPR31, a human gene encoding a putative G protein-coupled receptor. Genomics. 1997;42(3):519–23. doi:10.1006/geno.1997.4754.
Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354(6):610–21. doi:10.1056/NEJMra052723.
Bernardini G, Sciume G, Santoni A. Differential chemotactic receptor requirements for NK cell subset trafficking into bone marrow. Front Immunol. 2013;4:12. doi:10.3389/fimmu.2013.00012.
Zabel BA, Rott A, Butcher EC. Leukocyte chemoattractant receptors in human disease pathogenesis. Annu Rev Pathol. 2015;10:51–81. doi:10.1146/annurev-pathol-012513-104640.
Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford). 2010;49(9):1618–31. doi:10.1093/rheumatology/keq045.
Thieblemont N, Wright HL, Edwards SW, Witko-Sarsat V. Human neutrophils in auto-immunity. Semin Immunol. 2016;28(2):159–73. doi:10.1016/j.smim.2016.03.004.
Cedergren J, Forslund T, Sundqvist T, Skogh T. Intracellular oxidative activation in synovial fluid neutrophils from patients with rheumatoid arthritis but not from other arthritis patients. J Rheumatol. 2007;34(11):2162–70.
Fujishima S, Hoffman AR, Vu T, Kim KJ, Zheng H, Daniel D, et al. Regulation of neutrophil interleukin 8 gene expression and protein secretion by LPS, TNF-alpha, and IL-1 beta. J Cell Physiol. 1993;154(3):478–85. doi:10.1002/jcp.1041540305.
Christensen AD, Haase C, Cook AD, Hamilton JA. K/BxN serum-transfer arthritis as a model for human inflammatory arthritis. Front Immunol. 2016;7:213. doi:10.3389/fimmu.2016.00213.
Chou RC, Kim ND, Sadik CD, Seung E, Lan Y, Byrne MH, et al. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity. 2010;33(2):266–78. doi:10.1016/j.immuni.2010.07.018.
Greisen SR, Schelde KK, Rasmussen TK, Kragstrup TW, Stengaard-Pedersen K, Hetland ML, et al. CXCL13 predicts disease activity in early rheumatoid arthritis and could be an indicator of the therapeutic ‘window of opportunity’. Arthritis Res Ther. 2014;16(5):434. doi:10.1186/s13075-014-0434-z.
Yeo L, Adlard N, Biehl M, Juarez M, Smallie T, Snow M, et al. Expression of chemokines CXCL4 and CXCL7 by synovial macrophages defines an early stage of rheumatoid arthritis. Ann Rheum Dis. 2016;75(4):763–71. doi:10.1136/annrheumdis-2014-206921.
Bugatti S, Manzo A, Benaglio F, Klersy C, Vitolo B, Todoerti M, et al. Serum levels of CXCL13 are associated with ultrasonographic synovitis and predict power Doppler persistence in early rheumatoid arthritis treated with non-biological disease-modifying anti-rheumatic drugs. Arthritis Res Ther. 2012;14(1):R34. doi:10.1186/ar3742.
Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O’Fallon WM, et al. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 2001;167(2):1072–80.
Manzo A, Paoletti S, Carulli M, Blades MC, Barone F, Yanni G, et al. Systematic microanatomical analysis of CXCL13 and CCL21 in situ production and progressive lymphoid organization in rheumatoid synovitis. Eur J Immunol. 2005;35(5):1347–59. doi:10.1002/eji.200425830.
Koch AE, Kunkel SL, Harlow LA, Johnson B, Evanoff HL, Haines GK, et al. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J Clin Invest. 1992;90(3):772–9. doi:10.1172/JCI115950.
Volin MV, Shah MR, Tokuhira M, Haines GK, Woods JM, Koch AE. RANTES expression and contribution to monocyte chemotaxis in arthritis. Clin Immunol Immunopathol. 1998;89(1):44–53.
Ruth JH, Shahrara S, Park CC, Morel JC, Kumar P, Qin S, et al. Role of macrophage inflammatory protein-3 alpha and its ligand CCR6 in rheumatoid arthritis. Lab Invest. 2003;83(4):579–88.
Yoshida K, Korchynskyi O, Tak PP, Isozaki T, Ruth JH, Campbell PL, et al. Citrullination of epithelial neutrophil-activating peptide 78/CXCL5 results in conversion from a non-monocyte-recruiting chemokine to a monocyte-recruiting chemokine. Arthritis Rheumatol. 2014;66(10):2716–27. doi:10.1002/art.38750.
Weber C, Weber KS, Klier C, Gu S, Wank R, Horuk R, et al. Specialized roles of the chemokine receptors CCR1 and CCR5 in the recruitment of monocytes and T(H)1-like/CD45RO(+) T cells. Blood. 2001;97(4):1144–6.
Haringman JJ, Kraan MC, Smeets TJ, Zwinderman KH, Tak PP. Chemokine blockade and chronic inflammatory disease: proof of concept in patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62(8):715–21.
Zapico I, Coto E, Rodriguez A, Alvarez C, Torre JC, Alvarez V. CCR5 (chemokine receptor-5) DNA-polymorphism influences the severity of rheumatoid arthritis. Genes Immun. 2000;1(4):288–9. doi:10.1038/sj.gene.6363673.
Buckley CD, Amft N, Bradfield PF, Pilling D, Ross E, Arenzana-Seisdedos F, et al. Persistent induction of the chemokine receptor CXCR4 by TGF-beta 1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J Immunol. 2000;165(6):3423–9.
Matsui T, Akahoshi T, Namai R, Hashimoto A, Kurihara Y, Rana M, et al. Selective recruitment of CCR6-expressing cells by increased production of MIP-3 alpha in rheumatoid arthritis. Clin Exp Immunol. 2001;125(1):155–61.
Haudenschild DR, Nguyen B, Chen J, D’Lima DD, Lotz MK. Rho kinase-dependent CCL20 induced by dynamic compression of human chondrocytes. Arthritis Rheum. 2008;58(9):2735–42. doi:10.1002/art.23797.
Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204(12):2803–12. doi:10.1084/jem.20071397.
Paulissen SM, van Hamburg JP, Dankers W, Lubberts E. The role and modulation of CCR6+ Th17 cell populations in rheumatoid arthritis. Cytokine. 2015;74(1):43–53. doi:10.1016/j.cyto.2015.02.002.
Melis L, Vandooren B, Kruithof E, Jacques P, De Vos M, Mielants H, et al. Systemic levels of IL-23 are strongly associated with disease activity in rheumatoid arthritis but not spondyloarthritis. Ann Rheum Dis. 2010;69(3):618–23. doi:10.1136/ard.2009.107649.
Jandus C, Bioley G, Rivals JP, Dudler J, Speiser D, Romero P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58(8):2307–17. doi:10.1002/art.23655.
• Abji F, Pollock RA, Liang K, Chandran V, Gladman DD. C-X-C motif chemokine 10 is a possible biomarker for the development of psoriatic arthritis among patients with psoriasis. Arthritis Rheumatol. 2016. doi:10.1002/art.39800. This recent study documents the association of CXCL10 with conversion diagnosis of PsA that might indicate a key role for CXCL10 in PsA.
Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23(5):471–8. doi:10.1097/BOR.0b013e328349c2b1.
Ritter SY, Subbaiah R, Bebek G, Crish J, Scanzello CR, Krastins B, et al. Proteomic analysis of synovial fluid from the osteoarthritic knee: comparison with transcriptome analyses of joint tissues. Arthritis Rheum. 2013;65(4):981–92. doi:10.1002/art.37823.
Seitz M, Loetscher P, Dewald B, Towbin H, Ceska M, Baggiolini M. Production of interleukin-1 receptor antagonist, inflammatory chemotactic proteins, and prostaglandin E by rheumatoid and osteoarthritic synoviocytes—regulation by IFN-gamma and IL-4. J Immunol. 1994;152(4):2060–5.
Bay-Jensen AC, Reker D, Kjelgaard-Petersen CF, Mobasheri A, Karsdal MA, Ladel C, et al. Osteoarthritis year in review 2015: soluble biomarkers and the BIPED criteria. Osteoarthritis Cartilage. 2016;24(1):9–20. doi:10.1016/j.joca.2015.10.014.
Erdem H, Pay S, Serdar M, Simsek I, Dinc A, Musabak U, et al. Different ELR (+) angiogenic CXC chemokine profiles in synovial fluid of patients with Behcet’s disease, familial Mediterranean fever, rheumatoid arthritis, and osteoarthritis. Rheumatol Int. 2005;26(2):162–7. doi:10.1007/s00296-004-0524-3.
Daghestani HN, Kraus VB. Inflammatory biomarkers in osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1890–6. doi:10.1016/j.joca.2015.02.009.
Koch AE, Kunkel SL, Shah MR, Fu R, Mazarakis DD, Haines GK, et al. Macrophage inflammatory protein-1 beta: a C-C chemokine in osteoarthritis. Clin Immunol Immunopathol. 1995;77(3):307–14.
Hampel U, Sesselmann S, Iserovich P, Sel S, Paulsen F, Sack R. Chemokine and cytokine levels in osteoarthritis and rheumatoid arthritis synovial fluid. J Immunol Methods. 2013;396(1–2):134–9. doi:10.1016/j.jim.2013.08.007.
Benigni G, Dimitrova P, Antonangeli F, Sanseviero E, Milanova V, Blom A, et al. CXCR3/CXCL10 axis regulates neutrophil-natural killer cell crosstalk determining the severity of experimental osteoarthritis. J. Immunol. 2017. doi:10.4049/jimmunol.1601359.
Maeda A, Bandow K, Kusuyama J, Kakimoto K, Ohnishi T, Miyawaki S, et al. Induction of CXCL2 and CCL2 by pressure force requires IL-1beta-MyD88 axis in osteoblasts. Bone. 2015;74:76–82. doi:10.1016/j.bone.2015.01.007.
Lisignoli G, Toneguzzi S, Grassi F, Piacentini A, Tschon M, Cristino S, et al. Different chemokines are expressed in human arthritic bone biopsies: IFN-gamma and IL-6 differently modulate IL-8, MCP-1 and rantes production by arthritic osteoblasts. Cytokine. 2002;20(5):231–8.
Huh YH, Lee G, Lee KB, Koh JT, Chun JS, Ryu JH. HIF-2alpha-induced chemokines stimulate motility of fibroblast-like synoviocytes and chondrocytes into the cartilage-pannus interface in experimental rheumatoid arthritis mouse models. Arthritis Res Ther. 2015;17:302. doi:10.1186/s13075-015-0816-x.
Xu YK, Ke Y, Wang B, Lin JH. The role of MCP-1-CCR2 ligand-receptor axis in chondrocyte degradation and disease progress in knee osteoarthritis. Biol Res. 2015;48:64. doi:10.1186/s40659-015-0057-0.
Miller RE, Tran PB, Das R, Ghoreishi-Haack N, Ren D, Miller RJ, et al. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc Natl Acad Sci USA. 2012;109(50):20602–7. doi:10.1073/pnas.1209294110.
Sandell LJ, Xing X, Franz C, Davies S, Chang LW, Patra D. Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1beta. Osteoarthritis Cartilage. 2008;16(12):1560–71. doi:10.1016/j.joca.2008.04.027.
Shen PC, Wu CL, Jou IM, Lee CH, Juan HY, Lee PJ, et al. T helper cells promote disease progression of osteoarthritis by inducing macrophage inflammatory protein-1 gamma. Osteoarthritis Cartilage. 2011;19(6):728–36. doi:10.1016/j.joca.2011.02.014.
Hsu YH, Hsieh MS, Liang YC, Li CY, Sheu MT, Chou DT, et al. Production of the chemokine eotaxin-1 in osteoarthritis and its role in cartilage degradation. J Cell Biochem. 2004;93(5):929–39. doi:10.1002/jcb.20239.
Chao PZ, Hsieh MS, Cheng CW, Lin YF, Chen CH. Regulation of MMP-3 expression and secretion by the chemokine eotaxin-1 in human chondrocytes. J Biomed Sci. 2011;18:86. doi:10.1186/1423-0127-18-86.
Mazzetti I, Magagnoli G, Paoletti S, Uguccioni M, Olivotto E, Vitellozzi R, et al. A role for chemokines in the induction of chondrocyte phenotype modulation. Arthritis Rheum. 2004;50(1):112–22. doi:10.1002/art.11474.
Lisignoli G, Piacentini A, Cristino S, Grassi F, Cavallo C, Cattini L, et al. CCL20 chemokine induces both osteoblast proliferation and osteoclast differentiation: Increased levels of CCL20 are expressed in subchondral bone tissue of rheumatoid arthritis patients. J Cell Physiol. 2007;210(3):798–806. doi:10.1002/jcp.20905.
Merz D, Liu R, Johnson K, Terkeltaub R. IL-8/CXCL8 and growth-related oncogene alpha/CXCL1 induce chondrocyte hypertrophic differentiation. J Immunol. 2003;171(8):4406–15.
• Wigerblad G, Bas DB, Fernades-Cerqueira C, Krishnamurthy A, Nandakumar KS, Rogoz K, et al. Autoantibodies to citrullinated proteins induce joint pain independent of inflammation via a chemokine-dependent mechanism. Ann Rheum Dis. 2015. doi:10.1136/annrheumdis-2015-208094. This paper adds a new level of complexity in the role of ACPA present in RA, demonstrating that ACPA can activate osteoclasts to produce mediators of pain and identifying them as members of the chemokine family.
Recklies AD, Golds EE. Induction of synthesis and release of interleukin-8 from human articular chondrocytes and cartilage explants. Arthritis Rheum. 1992;35(12):1510–9.
Hanaoka R, Kasama T, Muramatsu M, Yajima N, Shiozawa F, Miwa Y, et al. A novel mechanism for the regulation of IFN-gamma inducible protein-10 expression in rheumatoid arthritis. Arthritis Res Ther. 2003;5(2):R74–81.
Kwak HB, Ha H, Kim HN, Lee JH, Kim HS, Lee S, et al. Reciprocal cross-talk between RANKL and interferon-gamma-inducible protein 10 is responsible for bone-erosive experimental arthritis. Arthritis Rheum. 2008;58(5):1332–42. doi:10.1002/art.23372.
Pablos JL, Santiago B, Galindo M, Torres C, Brehmer MT, Blanco FJ, et al. Synoviocyte-derived CXCL12 is displayed on endothelium and induces angiogenesis in rheumatoid arthritis. J Immunol. 2003;170(4):2147–52.
Kim HR, Kim KW, Kim BM, Jung HG, Cho ML, Lee SH. Reciprocal activation of CD4+ T cells and synovial fibroblasts by stromal cell-derived factor 1 promotes RANKL expression and osteoclastogenesis in rheumatoid arthritis. Arthritis Rheumatol. 2014;66(3):538–48. doi:10.1002/art.38286.
Kanbe K, Takagishi K, Chen Q. Stimulation of matrix metalloprotease 3 release from human chondrocytes by the interaction of stromal cell-derived factor 1 and CXC chemokine receptor 4. Arthritis Rheum. 2002;46(1):130–7.
Sawai H, Park YW, He X, Goronzy JJ, Weyand CM. Fractalkine mediates T cell-dependent proliferation of synovial fibroblasts in rheumatoid arthritis. Arthritis Rheum. 2007;56(10):3215–25. doi:10.1002/art.22919.
Pingiotti E, Cipriani P, Marrelli A, Liakouli V, Fratini S, Penco M, et al. Surface expression of fractalkine receptor (CX3CR1) on CD4+/CD28 T cells in RA patients and correlation with atherosclerotic damage. Ann N Y Acad Sci. 2007;1107:32–41. doi:10.1196/annals.1381.004.
Castor CW, Smith EM, Hossler PA, Bignall MC, Aaron BP. Connective tissue activation. XXXV. Detection of connective tissue activating peptide-III isoforms in synovium from osteoarthritis and rheumatoid arthritis patients: patterns of interaction with other synovial cytokines in cell culture. Arthritis Rheum. 1992;35(7):783–93.
Iwamoto T, Okamoto H, Kobayashi S, Ikari K, Toyama Y, Tomatsu T, et al. A role of monocyte chemoattractant protein-4 (MCP-4)/CCL13 from chondrocytes in rheumatoid arthritis. FEBS J. 2007;274(18):4904–12. doi:10.1111/j.1742-4658.2007.06013.x.
Volin MV, Huynh N, Klosowska K, Chong KK, Woods JM. Fractalkine is a novel chemoattractant for rheumatoid arthritis fibroblast-like synoviocyte signaling through MAP kinases and Akt. Arthritis Rheum. 2007;56(8):2512–22. doi:10.1002/art.22806.
Li CH, Xu LL, Zhao JX, Sun L, Yao ZQ, Deng XL, et al. CXCL16 upregulates RANKL expression in rheumatoid arthritis synovial fibroblasts through the JAK2/STAT3 and p38/MAPK signaling pathway. Inflamm Res. 2015. doi:10.1007/s00011-015-0905-y.
Elshabrawy HA, Chen Z, Volin MV, Ravella S, Virupannavar S, Shahrara S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis. 2015;18(4):433–48. doi:10.1007/s10456-015-9477-2.
Borzi RM, Mazzetti I, Cattini L, Uguccioni M, Baggiolini M, Facchini A. Human chondrocytes express functional chemokine receptors and release matrix-degrading enzymes in response to C-X-C and C-C chemokines. Arthritis Rheum. 2000;43(8):1734–41. doi:10.1002/1529-0131(200008)43:8<1734::AID-ANR9>3.0.CO;2-B.
Takebe K, Rai MF, Schmidt EJ, Sandell LJ. The chemokine receptor CCR5 plays a role in post-traumatic cartilage loss in mice, but does not affect synovium and bone. Osteoarthritis Cartilage. 2015;23(3):454–61. doi:10.1016/j.joca.2014.12.002.
Thomas NP, Li P, Fleming BC, Chen Q, Wei X, Xiao-Hua P, et al. Attenuation of cartilage pathogenesis in post-traumatic osteoarthritis (PTOA) in mice by blocking the stromal derived factor 1 receptor (CXCR4) with the specific inhibitor, AMD3100. J Orthop Res. 2015;33(7):1071–8. doi:10.1002/jor.22862.
Wei L, Sun X, Kanbe K, Wang Z, Sun C, Terek R, et al. Chondrocyte death induced by pathological concentration of chemokine stromal cell-derived factor-1. J Rheumatol. 2006;33(9):1818–26.
Chang X, Shen J, Yang H, Xu Y, Gao W, Wang J, et al. Upregulated expression of CCR3 in osteoarthritis and CCR3 mediated activation of fibroblast-like synoviocytes. Cytokine. 2016;77:211–9. doi:10.1016/j.cyto.2015.09.012.
Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood. 2000;96(8):2673–81.
• Sherwood J, Bertrand J, Nalesso G, Poulet B, Pitsillides A, Brandolini L, et al. A homeostatic function of CXCR2 signalling in articular cartilage. Ann Rheum Dis. 2015;74(12):2207–15. This paper represents a change in the current vision of the role of chemokines on cartilage stability. In particular, a CXCR2 ligand is presented as a key factor for the maintenance of cartilage homeostasis rather than being a catabolic factor.
Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states—maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25(2):141–54. doi:10.1016/j.berh.2011.02.005.
Atzeni F, Masala IF, Salaffi F, Di Franco M, Casale R, Sarzi-Puttini P. Pain in systemic inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. 2015;29(1):42–52. doi:10.1016/j.berh.2015.04.016.
Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–84. doi:10.1016/j.cell.2009.09.028.
Biber K, Boddeke E. Neuronal CC chemokines: the distinct roles of CCL21 and CCL2 in neuropathic pain. Front Cell Neurosci. 2014;8:210. doi:10.3389/fncel.2014.00210.
Luo X, Tai WL, Sun L, Pan Z, Xia Z, Chung SK et al. Crosstalk between astrocytic CXCL12 and microglial CXCR4 contributes to the development of neuropathic pain. Mol Pain. 2016;12. doi:10.1177/1744806916636385.
Watkins TA, Barres BA. Nerve regeneration: regrowth stumped by shared receptor. Curr Biol. 2002;12(19):R654–6.
Atzeni F, Cazzola M, Benucci M, Di Franco M, Salaffi F, Sarzi-Puttini P. Chronic widespread pain in the spectrum of rheumatological diseases. Best Pract Res Clin Rheumatol. 2011;25(2):165–71. doi:10.1016/j.berh.2010.01.011.
Kiguchi N, Maeda T, Kobayashi Y, Fukazawa Y, Kishioka S. Macrophage inflammatory protein-1alpha mediates the development of neuropathic pain following peripheral nerve injury through interleukin-1beta up-regulation. Pain. 2010;149(2):305–15. doi:10.1016/j.pain.2010.02.025.
Staniland AA, Clark AK, Wodarski R, Sasso O, Maione F, D’Acquisto F, et al. Reduced inflammatory and neuropathic pain and decreased spinal microglial response in fractalkine receptor (CX3CR1) knockout mice. J Neurochem. 2010;114(4):1143–57. doi:10.1111/j.1471-4159.2010.06837.x.
Clark AK, Staniland AA, Malcangio M. Fractalkine/CX3CR1 signalling in chronic pain and inflammation. Curr Pharm Biotechnol. 2011;12(10):1707–14.
Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2001;21(14):5027–35.
Zhang N, Inan S, Cowan A, Sun R, Wang JM, Rogers TJ, et al. A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc Natl Acad Sci USA. 2005;102(12):4536–41. doi:10.1073/pnas.0406030102.
•• Melik Parsadaniantz S, Rivat C, Rostene W, Reaux-Le Goazigo A. Opioid and chemokine receptor crosstalk: a promising target for pain therapy? Nat Rev Neurosci. 2015;16(2):69–78. doi:10.1038/nrn3858. A very interesting and well-written review of the nociceptive pathways activated by chemokines linked to opioid tolerance.
• Miotla Zarebska J, Chanalaris A, Driscoll C, Burleigh A, Miller RE, Malfait AM, et al. CCL2 and CCR2 regulate pain-related behaviour and early gene expression in post-traumatic murine osteoarthritis but contribute little to chondropathy. Osteoarthritis Cartilage. 2016. doi:10.1016/j.joca.2016.10.008. This is an interesting paper that supports the current concept that different members of the chemokine family contribute differently to the pathogenesis of OA, as highlighted by the role of CCL2 in pain-related behavior but not in cartilage damage.
Li L, Jiang BE. Serum and synovial fluid chemokine ligand 2/monocyte chemoattractant protein 1 concentrations correlates with symptomatic severity in patients with knee osteoarthritis. Ann Clin Biochem. 2015;52(Pt 2):276–82. doi:10.1177/0004563214545117.
•• Szekanecz Z, Koch AE. Successes and failures of chemokine-pathway targeting in rheumatoid arthritis. Nat Rev Rheumatol. 2016;12(1):5–13. A well-written and complete review on the role of chemokines in RA and of the therapeutic approaches to target these molecules.
Vergunst CE, Gerlag DM, Lopatinskaya L, Klareskog L, Smith MD, van den Bosch F, et al. Modulation of CCR2 in rheumatoid arthritis: a double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum. 2008;58(7):1931–9. doi:10.1002/art.23591.
Gerlag DM, Hollis S, Layton M, Vencovsky J, Szekanecz Z, Braddock M, et al. Preclinical and clinical investigation of a CCR5 antagonist, AZD5672, in patients with rheumatoid arthritis receiving methotrexate. Arthritis Rheum. 2010;62(11):3154–60. doi:10.1002/art.27652.
Fleishaker DL, Garcia Meijide JA, Petrov A, Kohen MD, Wang X, Menon S, et al. Maraviroc, a chemokine receptor-5 antagonist, fails to demonstrate efficacy in the treatment of patients with rheumatoid arthritis in a randomized, double-blind placebo-controlled trial. Arthritis Res Ther. 2012;14(1):R11. doi:10.1186/ar3685.
Xue CB, Wang A, Han Q, Zhang Y, Cao G, Feng H, et al. Discovery of INCB8761/PF-4136309, a potent, selective, and orally bioavailable CCR2 antagonist. ACS Med Chem Lett. 2011;2(12):913–8. doi:10.1021/ml200199c.
Santella 3rd JB, Gardner DS, Duncia JV, Wu H, Dhar M, Cavallaro C, et al. Discovery of the CCR1 antagonist, BMS-817399, for the treatment of rheumatoid arthritis. J Med Chem. 2014;57(18):7550–64. doi:10.1021/jm5003167.
Tak PP, Balanescu A, Tseluyko V, Bojin S, Drescher E, Dairaghi D, et al. Chemokine receptor CCR1 antagonist CCX354-C treatment for rheumatoid arthritis: CARAT-2, a randomised, placebo controlled clinical trial. Ann Rheum Dis. 2013;72(3):337–44. doi:10.1136/annrheumdis-2011-201605.
Yellin M, Paliienko I, Balanescu A, Ter-Vartanian S, Tseluyko V, Xu LA, et al. A phase II, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of MDX-1100, a fully human anti-CXCL10 monoclonal antibody, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64(6):1730–9. doi:10.1002/art.34330.
Nanki T, Imai T, Kawai S. Fractalkine/CX3CL1 in rheumatoid arthritis. Mod Rheumatol. 2016:1–6. doi:10.1080/14397595.2016.1213481.
Dairaghi DJ, Zhang P, Wang Y, Seitz LC, Johnson DA, Miao S, et al. Pharmacokinetic and pharmacodynamic evaluation of the novel CCR1 antagonist CCX354 in healthy human subjects: implications for selection of clinical dose. Clin Pharmacol Ther. 2011;89(5):726–34. doi:10.1038/clpt.2011.33.
Jiao Z, Wang W, Jia R, Li J, You H, Chen L, et al. Accumulation of FoxP3-expressing CD4+CD25+ T cells with distinct chemokine receptors in synovial fluid of patients with active rheumatoid arthritis. Scand J Rheumatol. 2007;36(6):428–33. doi:10.1080/03009740701482800.
Bernardini G, Antonangeli F, Bonanni V, Santoni A. Dysregulation of chemokine/chemokine receptor axes and NK cell tissue localization during diseases. Front Immunol. 2016;7:402. doi:10.3389/fimmu.2016.00402.
Acknowledgements
The authors are supported by Inter-Pasteurien Concerted Actions Grant A05_11, France, and by grants from the Ministero dell’Istruzione, dell’Università e della Ricerca–Fondo per gli Investimenti della Ricerca di Base (Futuro in Ricerca and Grant MIUR-L.297 FAR), and from the University of Rome “La Sapienza”.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Giovanni Bernardini, Giorgia Benigni, Rossana Scrivo, Guido Valesini, and Angela Santoni declare that there is no conflict of interest.
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki Declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
This article is part of the Topical Collection on Rheumatoid Arthritis
Rights and permissions
About this article
Cite this article
Bernardini, G., Benigni, G., Scrivo, R. et al. The Multifunctional Role of the Chemokine System in Arthritogenic Processes. Curr Rheumatol Rep 19, 11 (2017). https://doi.org/10.1007/s11926-017-0635-y
Published:
DOI: https://doi.org/10.1007/s11926-017-0635-y