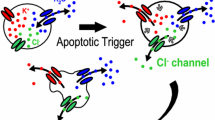
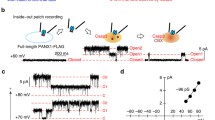
Abstract
Activation of ion channels and pores are essential steps during regulated cell death. Channels and pores participate in execution of apoptosis, necroptosis and other forms of caspase-independent cell death. Within the program of regulated cell death, these channels are strategically located. Ion channels can shrink cells and drive them towards apoptosis, resulting in silent, i.e. immunologically unrecognized cell death. Alternatively, activation of channels can induce cell swelling, disintegration of the cell membrane, and highly immunogenic necrotic cell death. The underlying cell death pathways are not strictly separated as identical stimuli may induce cell shrinkage and apoptosis when applied at low strength, but may also cause cell swelling at pronounced stimulation, resulting in regulated necrosis. Nevertheless, the precise role of ion channels during regulated cell death is far from being understood, as identical channels may support regulated death in some cell types, but may cause cell proliferation, cancer development, and metastasis in others. Along this line, the phospholipid scramblase and Cl−/nonselective channel anoctamin 6 (ANO6) shows interesting features, as it participates in apoptotic cell death during lower levels of activation, thereby inducing cell shrinkage. At strong activation, e.g. by stimulation of purinergic P2Y7 receptors, it participates in pore formation, causes massive membrane blebbing, cell swelling, and membrane disintegration. The LRRC8 proteins deserve much attention as they were found to have a major role in volume regulation, apoptotic cell shrinkage and resistance towards anticancer drugs.



Similar content being viewed by others
References
Zong WX, Thompson CB (2006) Necrotic death as a cell fate. Genes Dev 20:1–15
Galluzzi L, Kepp O, Krautwald S, Kroemer G, Linkermann A (2014) Molecular mechanisms of regulated necrosis. Semin Cell Dev Biol 35:24–32
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Williams GT (1991) Programmed cell death: apoptosis and oncogenesis. Cell 65:1097–1098
Cohen JJ, Duke RC, Fadok VA, Sellins KS (1992) Apoptosis and programmed cell death in immunity. Annu Rev Immunol 10:267–293
Henriquez M, Armisen R, Stutzin A, Quest AF (2008) Cell death by necrosis, a regulated way to go. Curr Mol Med 8:187–206
Gulbins E, Jekle A, Ferlinz K, Grassme H, Lang F (2000) Physiology of apoptosis. Am J Physiol Renal Physiol 279:F605–F615
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G (1999) Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397:441–446
Kondratskyi A, Kondratska K, Skryma R, Prevarskaya N (2014) Ion channels in the regulation of apoptosis. Biochim Biophys Acta 1848:2532–2546
Nagata S (1997) Apoptosis by death factor. Cell 88:355–365
Mehlen P, DE Bredesen (2011) Dependence receptors: from basic research to drug development. Sci Signal 4:mr2
Adams JM (2003) Ways of dying: multiple pathways to apoptosis. Genes Dev 17:2481–2495
Ryter SW, Mizumura K, Choi AM (2014) The impact of autophagy on cell death modalities. Int J Cell Biol. 2014:502676
Frisch SM, Screaton RA (2001) Anoikis mechanisms. Curr Opin Cell Biol 13:555–562
Bertrand K (2011) Survival of exfoliated epithelial cells: a delicate balance between anoikis and apoptosis. J Biomed Biotechnol 2011:534139
Bergsbaken T, Fink SL, Cookson BT (2009) Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7:99–109
Kayagaki N, Warming S, Lamkanfi M, Vande WL, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479:117–121
Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, Monack DM (2012) Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490:288–291
Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, Forsberg LS, Carlson RW, Dixit VM (2013) Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341:1246–1249
Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526:666–671
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526:660–665
Berridge MJ, Bootman MD, Lipp P (1998) Calcium—a life and death signal. Nature 395:645–648
Fang KM, Chang WL, Wang SM, Su MJ, Wu ML (2008) Arachidonic acid induces both Na+ and Ca2+ entry resulting in apoptosis. J Neurochem 104:1177–1189
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312
Lang F, Hoffmann EK (2012) Role of ion transport in control of apoptotic cell death. Compr Physiol 2:2037–2061
Harteneck C, Reiter B (2007) TRP channels activated by extracellular hypo-osmoticity in epithelia. Biochem Soc Trans 35:91–95
Numata T, Shimizu T, Okada Y (2007) TRPM7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am J Physiol Cell Physiol 292:C460–C467
Plant TD (2014) TRPs in mechanosensing and volume regulation. Handb Exp Pharmacol 223:743–766
Desai BN, Krapivinsky G, Navarro B, Krapivinsky L, Carter BC, Febvay S, Delling M, Penumaka A, Ramsey IS, Manasian Y, Clapham DE (2012) Cleavage of TRPM7 releases the kinase domain from the ion channel and regulates its participation in Fas-induced apoptosis. Dev Cell 22:1149–1162
Dubois C, Vanden Abeele F, Prevarskaya N (2013) Targeting apoptosis by the remodelling of calcium-transporting proteins in cancerogenesis. FEBS J 280:5500–5510
Joseph SK, Hajnoczky G (2007) IP3 receptors in cell survival and apoptosis: Ca2+ release and beyond. Apoptosis 12:951–968
Hoffmann EK, Lambert IH, Pedersen SF (2009) Physiology of cell volume regulation in vertebrates. Physiol Rev 89:193–277
Orlov SN, Model MA, Grygorczyk R (2013) CrossTalk opposing view: the triggering and progression of the cell death machinery can occur without cell volume perturbations. J Physiol 591:6123–6125
Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D (1998) Functional significance of cell volume regulatory mechanisms. Physiol Rev 78:247–306
Stutzin A, Hoffmann EK (2006) Swelling-activated ion channels: functional regulation in cell-swelling, proliferation and apoptosis. Acta Physiol (Oxf) 187:27–42
Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S (2001) Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J Physiol 532:3–16
Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y (2000) Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc Natl Acad Sci USA 97:9487–9492
Strange K, Emma F, Jackson PS (1996) Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol 270:C711–C730
Eggermont J, Trouet D, Carton I, Nilius B (2001) Cellular function and control of volume-regulated anion channels. Cell Biochem Biophys 35:263–274
Montague JW, Bortner CD, Hughes FM Jr, Cidlowski JA (1999) A necessary role for reduced intracellular potassium during the DNA degradation phase of apoptosis. Steroids 64:563–569
Bortner CD, Hughes FM Jr, Cidlowski JA (1997) A primary role for K+ and Na+ efflux in the activation of apoptosis. J Biol Chem 272:32436–32442
Poulsen KA, Andersen EC, Hansen CF, Klausen TK, Hougaard C, Lambert IH, Hoffmann EK (2010) Deregulation of apoptotic volume decrease and ionic movements in multidrug-resistant tumor cells: role of chloride channels. Am J Physiol Cell Physiol 298:C14–C25
Ousingsawat J, Wanitchakool P, Kmit A, Romao AM, Jantarajit W, Schreiber S, Kunzelmann K (2015) Anoctamin 6 mediates effects essential for innate immunity downstream of P2X7-receptors in macrophages. Nat Commun 6:6245
Martins JR, Faria D, Kongsuphol P, Reisch B, Schreiber R, Kunzelmann K (2011) Anoctamin 6 is an essential component of the outwardly rectifying chloride channel. Proc Natl Acad Sci USA 108:18168–18172
Hammer C, Wanitchakool P, Sirianant L, Papiol S, Monnheimer M, Faria D, Ousingsawat J, Schramek N, Schmitt C, Margos G, Michel A, Kraiczy P, Pawlita M, Schreiber R, Schulz TF, Fingerle V, Tumani H, Ehrenreich H, Kunzelmann K (2015) A coding variant of ANO10, affecting volume regulation of macrophages, is associated with Borrelia seropositivity. Mol Med 21:26–37
Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, Nagata S (2014) Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 344:1164–1168
Suzuki J, Fujii T, Imao T, Ishihara K, Kuba H, Nagata S (2013) Calcium-dependent phospholipid scramblase activity of TMEM16 family members. J Biol Chem 288:13305–13316
Bortner CD, Cidlowski JA (2004) The role of apoptotic volume decrease and ionic homeostasis in the activation and repression of apoptosis. Pflugers Arch 448:313–318
Hughes FM Jr, Cidlowski JA (1999) Potassium is a critical regulator of apoptotic enzymes in vitro and in vivo. Adv Enzyme Regul 39:157–171
Okada Y (2004) Ion channels and transporters involved in cell volume regulation and sensor mechanisms. Cell Biochem Biophys 41:233–258
Hoffmann EK (2011) Ion channels involved in cell volume regulation: effects on migration, proliferation, and programmed cell death in non adherent EAT cells and adherent ELA cells. Cell Physiol Biochem 28:1061–1078
Beauvais F, Michel L, Dubertret L (1995) Human eosinophils in culture undergo a striking and rapid shrinkage during apoptosis. Role of K+ channels. J Leukoc Biol 57:851–855
Yurinskaya V, Goryachaya T, Guzhova I, Moshkov A, Rozanov Y, Sakuta G, Shirokova A, Shumilina E, Vassilieva I, Lang F, Vereninov A (2005) Potassium and sodium balance in U937 cells during apoptosis with and without cell shrinkage. Cell Physiol Biochem 16:155–162
Szabo I, Lepple-Wienhues A, Kaba KN, Zoratti M, Gulbins E, Lang F (1998) Tyrosine kinase-dependent activation of a chloride channel in CD95-induced apoptosis in T lymphocytes. Proc Natl Acad Sci USA 95:6169–6174
Wang Z (2004) Roles of K+ channels in regulating tumour cell proliferation and apoptosis. Pflugers Arch 448:274–286
Burg ED, Remillard CV, Yuan JX (2006) K+ channels in apoptosis. J Membr Biol 209:3–20
Lang F, Ritter M, Gamper N, Huber SM, Fillon S, Tanneur V, Lepple-Wienhues A, Szabo I, Gulbins E (2000) Cell volume in the regulation of cell proliferation and apoptotic cell death. Cell Physiol Biochem 10:417–428
Borjesson SI, Englund UH, Asif MH, Willander M, Elinder F (2011) Intracellular K+ concentration decrease is not obligatory for apoptosis. J Biol Chem 286:39823–39828
Cahalan MD, Lewis RS (1988) Role of potassium and chloride channels in volume regulation by T lymphocytes. Soc Gen Physiol Ser 43:281–301
Hazama A, Okada Y (1988) Ca2+ sensitivity of volume-regulatory K+ and Cl− channels in cultured human epithelial cells. J Physiol 402:687–702
Nilius B, Oike M, Zahradnik I, Droogmans G (1994) Activation of a Cl− current by hypotonic volume increase in human endothelial cells. J Gen Physiol 103:787–805
Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G (1997) Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol 68:69–119
Jackson PS, Morrison R, Strange K (1994) The volume-sensitive organic osmolyte-anion channel VSOAC is regulated by nonhydrolytic ATP binding. Am J Physiol 267:C1203–C1209
Droogmans G, Prenen J, Eggermont J, Voets T, Nilius B (1998) Voltage-dependent block of endothelial volume-regulated anion channels by calix[4]arenes. Am J Physiol 275:C646–C652
Burow P, Klapperstuck M, Markwardt F (2014) Activation of ATP secretion via volume-regulated anion channels by sphingosine-1-phosphate in RAW macrophages. Pflugers Arch
Jackson PS, Strange K (1993) Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. Am J Physiol 265:C1489–C1500
Pedersen SF, Klausen TK, Nilius B (2015) The identification of VRAC (Volume Regulated Anion Channel): an amazing Odyssey. Acta Physiol (Oxf) 213:868–881
Nilius B, Prenen J, Voets T, Eggermont J, Droogmans G (1998) Activation of volume-regulated chloride currents by reduction of intracellular ionic strength in bovine endothelial cells. J Physiol 506:353–361
Sabirov RZ, Prenen J, Tomita T, Droogmans G, Nilius B (2000) Reduction of ionic strength activates single volume-regulated anion channels (VRAC) in endothelial cells. Pflugers Arch 439:315–320
Voets T, Droogmans G, Raskin G, Eggermont J, Nilius B (1999) Reduced intracellular ionic strength as the initial trigger for activation of endothelial volume-regulated anion channels. Proc Natl Acad Sci USA 96:5298–5303
Mongin AA, Kimelberg HK (2005) ATP regulates anion channel-mediated organic osmolyte release from cultured rat astrocytes via multiple Ca2+-sensitive mechanisms. Am J Physiol Cell Physiol 288:C204–C213
Akita T, Fedorovich SV, Okada Y (2011) Ca2+ nanodomain-mediated component of swelling-induced volume-sensitive outwardly rectifying anion current triggered by autocrine action of ATP in mouse astrocytes. Cell Physiol Biochem 28:1181–1190
Akita T, Okada Y (2011) Regulation of bradykinin-induced activation of volume-sensitive outwardly rectifying anion channels by Ca2+ nanodomains in mouse astrocytes. J Physiol 589:3909–3927
Varela D, Penna A, Simon F, Eguiguren AL, Leiva-Salcedo E, Cerda O, Sala F, Stutzin A (2010) P2X4 activation modulates volume-sensitive outwardly rectifying chloride channels in rat hepatoma cells. J Biol Chem 285:7566–7574
Varela D, Simon F, Riveros A, Jorgensen F, Stutzin A (2004) NAD(P)H oxidase-derived H2O2 signals chloride channel activation in cell volume regulation and cell proliferation. J Biol Chem 279:13301–13304
Voets T, Manolopoulos V, Eggermont J, Ellory C, Droogmans G, Nilius B (1998) Regulation of a swelling-activated chloride current in bovine endothelium by protein tyrosine phosphorylation and G proteins. J Physiol 506:341–352
Burow P, Markwardt F (2014) When S1P meets ATP. Channels (Austin) 8:385–386
Akita T, Okada Y (2014) Characteristics and roles of the volume-sensitive outwardly rectifying (VSOR) anion channel in the central nervous system. Neuroscience 275:211–231
Sirianant L, Ousingsawat J, Wanitchakool P, Schreiber R, Kunzelmann K (2015) Cellular volume regulation by anoctamin 6: Ca2+, phospholipase A2, osmosensing. Pflügers Arch. (in press)
Walters EA, Rome L, Luke RG, Galla JH (1991) Absence of a regulatory role of angiotensin II in acute chloride-depletion alkalosis in rats. Am J Physiol 261:F741–F745
Kunzelmann K, Cabrita I, Wanitchakool P, Ousingsawat J, Sirianant L, Benedetto R, Schreiber R (2016) Ca2+ signaling—a common link to diverse functions of anoctamins. Pflügers Arch. (in press)
Jin X, Shah S, Liu Y, Zhang H, Lees M, Fu Z, Lippiat JD, Beech DJ, Sivaprasadarao A, Baldwin SA, Zhang H, Gamper N (2013) Activation of the Cl− channel ANO1 by Localized calcium signals in nociceptive sensory neurons requires coupling with the IP3 receptor. Sci Signal 6:ra73
Almaca J, Tian Y, AlDehni F, Ousingsawat J, Kongsuphol P, Rock JR, Harfe BD, Schreiber R, Kunzelmann K (2009) TMEM16 proteins produce volume regulated chloride currents that are reduced in mice lacking TMEM16A. J Biol Chem 284:28571–28578
Pedemonte N, Galietta LJ (2014) Structure and function of TMEM16 proteins (anoctamins). Physiol Rev 94:419–459
Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U (2008) TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455:1210–1215
Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ (2014) Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 344:634–638
Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A (2014) SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 157:447–458
Abascal F, Zardoya R (2012) LRRC8 proteins share a common ancestor with pannexins, and may form hexameric channels involved in cell–cell communication. BioEssays 34:551–560
Hyzinski-Garcia MC, Rudkouskaya A, Mongin AA (2014) LRRC8A protein is indispensable for swelling-activated and the ATP-induced release of excitatory amino acids in rat astrocytes. J Physiol 592:4855–4862
Sorensen BH, Thorsteinsdottir UA, Lambert IH (2014) Acquired cisplatin resistance in humane ovarian cancer A2780 cells correlates with shift in Taurine homeostasis and ability to volume regulate. Am J Physiol Cell Physiol 307:C1071–C1080
Planells-Cases R, Lutter D, Guyader C, Gerhards NM, Ullrich F, Elger DA, Kucukosmanoglu A, Xu G, Voss FK, Reincke SM, Stauber T, Blomen VA, Vis DJ, Wessels LF, Brummelkamp TR, Borst P, Rottenberg S, Jentsch TJ (2015) Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt-based anti-cancer drugs. EMBO J e201592409
Sawada A, Takihara Y, Kim JY, Matsuda-Hashii Y, Tokimasa S, Fujisaki H, Kubota K, Endo H, Onodera T, Ohta H, Ozono K, Hara J (2003) A congenital mutation of the novel gene LRRC8 causes agammaglobulinemia in humans. J Clin Invest 112:1707–1713
Kumar L, Chou J, Yee CS, Borzutzky A, Vollmann EH, von Andrian UH, Park SY, Hollander G, Manis JP, Poliani PL, Geha RS (2014) Leucine-rich repeat containing 8A (LRRC8A) is essential for T lymphocyte development and function. J Exp Med 211:929–942
Gottlieb RA, Dosanjh A (1996) Mutant cystic fibrosis transmembrane conductance regulator inhibits acidification and apoptosis in C127 cells: possible relevance to cystic fibrosis. Proc Natl Acad Sci USA 93:3587–3591
Barriere H, Poujeol C, Tauc M, Blasi JM, Counillon L, Poujeol P (2001) CFTR modulates programmed cell death by decreasing intracellular pH in Chinese hamster lung fibroblasts. Am J Physiol 281:C810–C824
Noe J, Petrusca D, Rush N, Deng P, VanDemark M, Berdyshev E, Gu Y, Smith P, Schweitzer K, Pilewsky J, Natarajan V, Xu Z, Obukhov AG, Petrache I (2009) CFTR regulation of intracellular pH and ceramides is required for lung endothelial cell apoptosis. Am J Respir Cell Mol Biol 41:314–323
Grassme H, Jendrossek V, Riehle A, von Kurthy G, Berger J, Schwarz H, Weller M, Kolesnick R, Gulbins E (2003) Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat Med 9:322–330
Yu H, Zeidan YH, Wu BX, Jenkins RW, Flotte TR, Hannun YA, Virella-Lowell I (2009) Defective acid sphingomyelinase pathway with Pseudomonas aeruginosa infection in cystic fibrosis. Am J Respir Cell Mol Biol 41:367–375
Teichgraber V, Ulrich M, Endlich N, Riethmuller J, Wilker B, Oliveira-Munding CC, Van Heeckeren AM, Barr ML, von Kurthy G, Schmid KW, Weller M, Tummler B, Lang F, Grassme H, Doring G, Gulbins E (2008) Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med 14:382–391
Becker KA, Riethmuller J, Luth A, Doring G, Kleuser B, Gulbins E (2010) Acid sphingomyelinase inhibitors normalize pulmonary ceramide and inflammation in cystic fibrosis. Am J Respir Cell Mol Biol 42:716–724
Nahrlich L, Mainz JG, Adams C, Engel C, Herrmann G, Icheva V, Lauer J, Deppisch C, Wirth A, Unger K, Graepler-Mainka U, Hector A, Heyder S, Stern M, Doring G, Gulbins E, Riethmuller J (2013) Therapy of CF-patients with amitriptyline and placebo—a randomised, double-blind, placebo-controlled phase IIb multicenter, cohort-study. Cell Physiol Biochem 31:505–512
Dimagno MJ, Lee SH, Hao Y, Zhou SY, McKenna BJ, Owyang C (2005) A proinflammatory, antiapoptotic phenotype underlies the susceptibility to acute pancreatitis in cystic fibrosis transmembrane regulator (–/–) mice. Gastroenterology 129:665–681
Rottner M, Kunzelmann C, Mergey M, Freyssinet JM, Martinez MC (2007) Exaggerated apoptosis and NF-kappaB activation in pancreatic and tracheal cystic fibrosis cells. FASEB J. 21:2939–2948
Jungas T, Motta I, Duffieux F, Fanen P, Stoven V, Ojcius DM (2002) Glutathione levels and BAX activation during apoptosis due to oxidative stress in cells expressing wild-type and mutant cystic fibrosis transmembrane conductance regulator. J Biol Chem 277:27912–27918
Rottner M, Tual-Chalot S, Mostefai HA, Andriantsitohaina R, Freyssinet JM, Martinez MC (2011) Increased oxidative stress induces apoptosis in human cystic fibrosis cells. PLoS One 6:e24880
Rubera I, Duranton C, Melis N, Cougnon M, Mograbi B, Tauc M (2013) Role of CFTR in oxidative stress and suicidal death of renal cells during cisplatin-induced nephrotoxicity. Cell Death Dis 4:e817
Linsdell P, Hanrahan JW (1998) Glutathione permeability of CFTR. Am J Physiol 275:C323–C326
Hudson VM (2001) Rethinking cystic fibrosis pathology: the critical role of abnormal reduced glutathione (GSH) transport caused by CFTR mutation. Free Radic Biol Med 30:1440–1461
L’hoste S, Chargui A, Belfodil R, Duranton C, Rubera I, Mograbi B, Poujeol C, Tauc M, Poujeol P (2009) CFTR mediates cadmium-induced apoptosis through modulation of ROS level in mouse proximal tubule cells. Free Radic Biol Med 46:1017–1031
L’hoste S, Chargui A, Belfodil R, Corcelle E, Duranton C, Rubera I, Poujeol C, Mograbi B, Tauc M, Poujeol P (2010) CFTR mediates apoptotic volume decrease and cell death by controlling glutathione efflux and ROS production in cultured mice proximal tubules. Am J Physiol Renal Physiol 298:F435–F453
Okiyoneda T, Barriere H, Bagdany M, Rabeh WM, Du K, Hohfeld J, Young JC, Lukacs GL (2010) Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science 329:805–810
Barriere H, Belfodil R, Rubera I, Tauc M, Poujeol C, Bidet M, Poujeol P (2003) CFTR null mutation altered cAMP-sensitive and swelling-activated Cl− currents in primary cultures of mouse nephron. Am J Physiol Renal Physiol 284:F796–F811
Valverde MA, O`Briens JA, Sepulveda FV, Ratcliff RA, Evans MJ, Colledge WH (1995) Impaired cell volume regulation in intestinal crypt epithelia of cystic fibrosis. Proc Natl Acad Sci 92:9038–9041
Gawenis LR, Franklin CL, Simpson JE, Palmer BA, Walker NM, Wiggins TM, Clarke LL (2003) cAMP inhibition of murine intestinal Na/H exchange requires CFTR-mediated cell shrinkage of villus epithelium. Gastroenterology 125:1148–1163
Braunstein GM, Roman RM, Clancy JP, Kudlow BA, Taylor AL, Shylonsky VG, Jovov B, Peter K, Jilling T, Ismailov II, Benos DJ, Schwiebert LM, Fitz JG, Schwiebert EM (2001) Cystic fibrosis transmembrane conductance regulator facilitates ATP release by stimulating a separate ATP release channel for autocrine control of cell volume regulation. J Biol Chem 276:6621–6630
Uramoto H, Okada T, Okada Y (2012) Protective role of cardiac CFTR activation upon early reperfusion against myocardial infarction. Cell Physiol Biochem 30:1023–1038
Suzuki J, Umeda M, Sims PJ, Nagata S (2010) Calcium-dependent phospholipid scrambling by TMEM16F. Nature 468:834–838
Tian Y, Schreiber R, Kunzelmann K (2012) Anoctamins are a family of Ca2+ activated Cl− channels. J Cell Sci 125:4991–4998
Grubb S, Poulsen KA, Juul CA, Kyed T, Klausen TK, Larsen EH, Hoffmann EK (2013) TMEM16F (Anoctamin 6), an anion channel of delayed Ca2+ activation. J Gen Physiol 141:585–600
Shimizu T, Iehara T, Sato K, Fujii T, Sakai H, Okada Y (2013) TMEM16F is a component of a Ca2+-activated Cl− channel but not a volume-sensitive outwardly rectifying Cl− channel. Am J Physiol Cell Physiol 304:C748–C759
Kunzelmann K, Nilius B, Owsianik G, Schreiber R, Ousingsawat J, Sirianant L, Wanitchakool P, Bevers EM, Heemskerk JW (2014) Molecular functions of anoctamin 6 (TMEM16F): a chloride channel, cation channel or phospholipid scramblase? Pflügers Arch 466:407–414
Malvezzi M, Chalat M, Janjusevic R, Picollo A, Terashima H, Menon AK, Accardi A (2013) Ca2+-dependent phospholipid scrambling by a reconstituted TMEM16 ion channel. Nat. Commun. 4:2367
Kmit A, van Kruchten R, Ousingsawat J, Mattheij NJ, Senden-Gijsbers B, Heemskerk JW, Bevers EM, Kunzelmann K (2013) Calcium-activated and apoptotic phospholipid scrambling induced by Ano6 can occur independently of Ano6 ion currents. Cell Death Dis 25(4):e611
Yang H, Kim A, David T, Palmer D, Jin T, Tien J, Huang F, Cheng T, Coughlin SR, Jan YN, Jan LY (2012) TMEM16F forms a Ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation. Cell 151:111–122
Szteyn K, Schmid E, Nurbaeva MK, Yang W, Munzer P, Kunzelmann K, Lang F, Shumilina E (2012) Expression and functional significance of the Ca-activated Cl− channel ANO6 in dendritic cells. Cell Physiol Biochem 30:1319–1332
Juul CA, Grubb S, Poulsen KA, Kyed T, Hashem N, Lambert IH, Larsen EH, Hoffmann EK (2014) Anoctamin 6 differs from VRAC and VSOAC but is involved in apoptosis and supports volume regulation in the presence of Ca. Pflugers Arch 466:1899–1910
Landoure G, Zdebik AA, Martinez TL, Burnett BG, Stanescu HC, Inada H, Shi Y, Taye AA, Kong L, Munns CH, Choo SS, Phelps CB, Paudel R, Houlden H, Ludlow CL, Caterina MJ, Gaudet R, Kleta R, Fischbeck KH, Sumner CJ (2010) Mutations in TRPV4 cause Charcot-Marie-Tooth disease type 2C. Nat Genet 42:170–174
Takayama Y, Shibasaki K, Suzuki Y, Yamanaka A, Tominaga M (2014) Modulation of water efflux through functional interaction between TRPV4 and TMEM16A/anoctamin 1. FASEB J. 28:2238–2248
Yi E, Lee J, Lee CJ (2013) Developmental role of anoctamin-1/TMEM16A in Ca2+-dependent volume change in supporting cells of the mouse cochlea. Exp Neurobiol 22:322–329
Ponce A, Jimenez-Pena L, Tejeda-Guzman C (2012) The role of swelling-activated chloride currents [I(CL, swell)] in the regulatory volume decrease response of freshly dissociated rat articular chondrocytes. Cell Physiol Biochem 30:1254–1270
Brunner JD, Lim NK, Schenck S, Duerst A, Dutzler R (2014) X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature 516:207–212
Elliott JI, Higgins CF (2003) IKCa1 activity is required for cell shrinkage, phosphatidylserine translocation and death in T lymphocyte apoptosis. EMBO Rep 4:189–194
Owsianik G, Prenen J, Hermans C, Eggermont J, Nilius B (2010) Functional characterization of TMEM16 anion channels. FASEB J (Abstract) 608:12
Milenkovic A, Brandl C, Milenkovic VM, Jendrike T, Sirianant L, Wanitchakool P, Zimmermann S, Reif CM, Horling F, Schrewe H, Strünker T, Alvarez L, Schreiber R, Kunzelmann K, Wetzel CH, Weber BHF (2015) Bestrophin1 is the volume-regulated anion channel in mouse sperm and human retinal pigment epithelium. Proc Natl Acad Sci USA 112:E2630–E2639
Clapham DE (1998) The list of potential volume-sensitive chloride currents continues to swell (and shrink). J Gen Physiol 111:623–624
Jentsch TJ, Stein V, Weinreich F, Zdebik AA (2002) Molecular structure and physiological function of chloride channels. Physiol Rev 82:503–568
Nilius B, Droogmans G (2003) Amazing chloride channels: an overview. Acta Physiol Scand 177:119–147
Grunder S, Thiemann A, Pusch M, Jentsch TJ (1992) Regions involved in the opening of CIC-2 chloride channel by voltage and cell volume. Nature 360:759–762
Duan D, Winter C, Cowley S, Hume JR, Horowitz B (1997) Molecular identification of a volume-regulated chloride channel. Nature 390:417–421
Hermoso M, Satterwhite CM, Andrade YN, Hidalgo J, Wilson SM, Horowitz B, Hume JR (2002) ClC-3 is a fundamental molecular component of volume-sensitive outwardly rectifying Cl− channels and volume regulation in HeLa cells and Xenopus laevis oocytes. J Biol Chem 277:40066–40074
Arreola J, Begenisich T, Nehrke K, Nguyen HV, Park K, Richardson L, Yang B, Schutte BC, Lamb FS, Melvin JE (2002) Secretion and cell volume regulation by salivary acinar cells from mice lacking expression of the Clcn3 Cl− channel gene. J Physiol 545:207–216
Jin NG, Kim JK, Yang DK, Cho SJ, Kim JM, Koh EJ, Jung HC, So I, Kim KW (2003) Fundamental role of ClC-3 in volume-sensitive Cl− channel function and cell volume regulation in AGS cells. Am J Physiol Gastrointest Liver Physiol 285:G938–G948
Liu J, Zhang FF, Li L, Yang J, Liu J, Guan YY, Du YH (2013) ClC-3 deficiency prevents apoptosis induced by angiotensin II in endothelial progenitor cells via inhibition of NADPH oxidase. Apoptosis 18:1262–1273
Matsuda JJ, Filali MS, Moreland JG, Miller FJ, Lamb FS (2010) Activation of swelling-activated chloride current by tumor necrosis factor-alpha requires ClC-3-dependent endosomal reactive oxygen production. J Biol Chem 285:22864–22873
Dermietzel R, Hwang TK, Buettner R, Hofer A, Dotzler E, Kremer M, Deutzmann R, Thinnes FP, Fishman GI, Spray DC (1994) Cloning and in situ localization of a brain-derived porin that constitutes a large-conductance anion channel in astrocytic plasma membranes. Proc Natl Acad Sci USA 91:499–503
Okada SF, O’Neal WK, Huang P, Nicholas RA, Ostrowski LE, Craigen WJ, Lazarowski ER, Boucher RC (2004) Voltage-dependent anion channel-1 (VDAC-1) contributes to ATP release and cell volume regulation in murine cells. J Gen Physiol 124:513–526
Oeggerli M, Tian Y, Ruiz C, Wijker B, Sauter G, Obermann E, Guth U, Zlobec I, Sausbier M, Kunzelmann K, Bubendorf L (2012) Role of KCNMA1 in breast cancer. PLoS One 7:e41664
Koehl GE, Spitzner M, Ousingsawat J, Schreiber R, Geissler EK, Kunzelmann K (2010) Rapamycin inhibits oncogenic intestinal ion channels and neoplasia in APCMin/+Mice. Oncogene 29:1553–1560
Ousingsawat J, Spitzner M, Puntheeranurak S, Terracciano L, Tornillo L, Bubendorf L, Kunzelmann K, Schreiber R (2007) Expression of voltage gated potassium channels in human and mouse colonic carcinoma. Clin Cancer Res 13:824–831
Urrego D, Tomczak AP, Zahed F, Stuhmer W, Pardo LA (2014) Potassium channels in cell cycle and cell proliferation. Philos Trans R Soc Lond B Biol Sci 369:20130094
Pardo LA, Stuhmer W (2014) The roles of K+ channels in cancer. Nat Rev Cancer 14:39–48
Kunzelmann K (2005) Ion channels and cancer. J Membr Biol 205:159–173
Turner KL, Sontheimer H (2014) Cl− and K+ channels and their role in primary brain tumour biology. Philos Trans R Soc Lond B Biol Sci 369:20130095
Shukla A, Edwards R, Yang Y, Hahn A, Folkers K, Ding J, Padmakumar VC, Cataisson C, Suh KS, Yuspa SH (2014) CLIC4 regulates TGF-β-dependent myofibroblast differentiation to produce a cancer stroma. Oncogene 33:842–850
Suh KS, Mutoh M, Nagashima K, Fernandez-Salas E, Edwards LE, Hayes DD, Crutchley JM, Marin KG, Dumont RA, Levy JM, Cheng C, Garfield S, Yuspa SH (2004) The organellular chloride channel protein CLIC4/mtCLIC translocates to the nucleus in response to cellular stress and accelerates apoptosis. J Biol Chem 279:4632–4641
Leanza L, Biasutto L, Manago A, Gulbins E, Zoratti M, Szabo I (2013) Intracellular ion channels and cancer. Front Physiol. 4:227
Stavrovskaya AA (2000) Cellular mechanisms of multidrug resistance of tumor cells. Biochemistry (Mosc) 65:95–106
Lee EL, Shimizu T, Ise T, Numata T, Kohno K, Okada Y (2007) Impaired activity of volume-sensitive Cl− channel is involved in cisplatin resistance of cancer cells. J Cell Physiol 211:513–521
Ise T, Shimizu T, Lee EL, Inoue H, Kohno K, Okada Y (2005) Roles of volume-sensitive Cl− channel in cisplatin-induced apoptosis in human epidermoid cancer cells. J Membr Biol 205:139–145
Shimizu T, Lee EL, Ise T, Okada Y (2008) Volume-sensitive Cl− channel as a regulator of acquired cisplatin resistance. Anticancer Res 28:75–83
West RB, Corless CL, Chen X, Rubin BP, Subramanian S, Montgomery K, Zhu S, Ball CA, Nielsen TO, Patel R, Goldblum JR, Brown PO, Heinrich MC, van de RM (2004) The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol 165:107–113
Duvvuri U, Shiwarski DJ, Xiao D, Bertrand C, Huang X, Edinger RS, Rock JR, Harfe BD, Henson BJ, Kunzelmann K, Schreiber R, Seethala RR, Egloff AM, Chen X, Lui VW, Grandis JR, Gollin SM (2012) TMEM16A, induces MAPK and contributes directly to tumorigenesis and cancer progression. Cancer Res 72:3270–3281
Wanitchakool P, Wolf L, Koehl G, Sirianant L, Gaumann A, Schreiber R, Duvvuri U, Kunzelmann K (2014) Role of anoctamins in cancer and apoptosis. Philos Trans R Soc Lond B Biol Sci 369:20130096
Shiwarski DJ, Shao C, Bill A, Kim J, Xiao D, Bertrand C, Seethala RR, Sano D, Myers JN, Ha PK, Grandis JR, Gaither LA, Puthenveedu MA, Duvvuri U (2014) To “Grow” or “Go”: TMEM16A expression as a switch between tumor growth and metastasis in SCCHN. Clin Cancer Res 20:4673–4688
Qu Z, Yao W, Yao R, Liu X, Yu K, Hartzell HC (2014) The Ca-activated Cl channel, ANO1 (TMEM16A), is a double-edged sword in cell proliferation and tumorigenesis. Cancer Med 3:453–461
Britschgi A, Bill A, Brinkhaus H, Rothwell C, Clay I, Duss S, Rebhan M, Raman P, Guy CT, Wetzel K, George E, Popa MO, Lilley S, Choudhury H, Gosling M, Wang L, Fitzgerald S, Borawski J, Baffoe J, Labow M, Gaither LA, Bentires-Alj M (2013) Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc Natl Acad Sci USA 110:E1026–E1034
Matsuba S, Niwa S, Muraki K, Kanatsuka S, Nakazono Y, Hatano N, Fujii M, Zhan P, Suzuki T, Ohya S (2014) Downregulation of Ca2+-activated Cl− channel TMEM16A by the inhibition of histone deacetylase in TMEM16A-expressing cancer cells. J Pharmacol Exp Ther JPET
He Q, Halm ST, Zhang J, Halm DR (2011) Activation of the basolateral membrane Cl conductance essential for electrogenic K secretion suppresses electrogenic Cl secretion. Exp Physiol 96:305–316
Ruiz C, Martins JR, Rudin F, Schneider S, Dietsche T, Fischer CA, Tornillo L, Terracciano LM, Schreiber R, Bubendorf L, Kunzelmann K (2012) Enhanced expression of ANO1 in head and neck squamous cell carcinoma causes cell migration and correlates with poor prognosis. PLoS One 7:e43265
Jacobsen KS, Zeeberg K, Poulsen KA, Hoffmann EK, Schwab A (2013) The role of TMEM16A (ANO1) and TMEM16F (ANO6) in cell migration. Pflugers Arch 465:1753–1762
Berglund E, Akcakaya P, Berglund D, Karlsson F, Vukojevic V, Lee L, Bogdanovic D, Lui WO, Larsson C, Zedenius J, Frobom R, Branstrom R (2014) Functional role of the Ca-activated Cl channel DOG1/TMEM16A in gastrointestinal stromal tumor cells. Exp Cell Res 326:315–325
Sui Y, Sun M, Wu F, Yang L, Di W, Zhang G, Zhong L, Ma Z, Zheng J, Fang X, Ma T (2014) Inhibition of TMEM16A expression suppresses growth and invasion in human colorectal cancer cells. PLoS One 9:e115443
Bill A, Hall ML, Borawski J, Hodgson C, Jenkins J, Piechon P, Popa O, Rothwell C, Tranter P, Tria S, Wagner T, Whitehead L, Gaither LA (2014) Small molecule-facilitated degradation of ANO1 protein: a new targeting approach for anticancer therapeutics. J Biol Chem 289:11029–11041
Cha JY, Wee J, Jung J, Jang Y, Lee B, Hong GS, Chang BC, Choi YL, Shin YK, Min HY, Lee HY, Na TY, Lee MO, Oh U (2015) Anoctamin 1 (TMEM16A) is essential for testosterone-induced prostate hyperplasia. Proc, Natl Acad Sci USA 201423827
Prevarskaya N, Ouadid-Ahidouch H, Skryma R, Shuba Y (2014) Remodelling of Ca2+ transport in cancer: how it contributes to cancer hallmarks? Philos Trans R Soc Lond B Biol Sci 369:20130097
Schreiber R (2005) Ca2+ signaling, intracellular pH and cell volume in cell proliferation. J Membr Biol 205:129–137
Fink SL, Cookson BT (2006) Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol 8:1812–1825
Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A (1999) The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA 96:2396–2401
Fischer H, Clauss W (1990) Regulation of Na+ channels in frog lung epithelium: a target tissue for aldosteron action. Pflügers Arch 416:62–67
Silveira TN, Zamboni DS (2010) Pore formation triggered by Legionella spp. is an Nlrc4 inflammasome-dependent host cell response that precedes pyroptosis. Infect Immun 78:1403–1413
Kahlenberg JM, Dubyak GR (2004) Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol 286:C1100–C1108
Franchi L, Kanneganti TD, Dubyak GR, Nunez G (2007) Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem 282:18810–18818
Eisenhut M, Wallace H (2011) Ion channels in inflammation. Pflugers Arch 461:401–421
Casson CN, Shin S (2013) Inflammasome-mediated cell death in response to bacterial pathogens that access the host cell cytosol: lessons from legionella pneumophila. Front Cell Infect Microbiol 3:111
Chen Q, Jin Y, Zhang K, Li H, Chen W, Meng G, Fang X (2014) Alarmin HNP-1 promotes pyroptosis and IL-1beta release through different roles of NLRP3 inflammasome via P2X7 in LPS-primed macrophages. Innate Immun 20:290–300
Keyel PA, Roth R, Yokoyama WM, Heuser JE, Salter RD (2013) Reduction of streptolysin O (SLO) pore-forming activity enhances inflammasome activation. Toxins (Basel) 5:1105–1118
Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P (2014) Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 15:135–147
Barros LF, Hermosilla T, Castro J (2001) Necrotic volume increase and the early physiology of necrosis. Comp Biochem Physiol A Mol Integr Physiol 130:401–409
Linkermann A, Green DR (2014) Necroptosis. N Engl J Med 370:455–465
Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, Lich JD, Finger J, Kasparcova V, Votta B, Ouellette M, King BW, Wisnoski D, Lakdawala AS, DeMartino MP, Casillas LN, Haile PA, Sehon CA, Marquis RW, Upton J, Daley-Bauer LP, Roback L, Ramia N, Dovey CM, Carette JE, Chan FK, Bertin J, Gough PJ, Mocarski ES, Kaiser WJ (2014) RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell 56:481–495
Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148:213–227
Kaczmarek A, Vandenabeele P, Krysko DV (2013) Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 38:209–223
Linkermann A, Stockwell BR, Krautwald S, Anders HJ (2014) Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat Rev Immunol 14:759–767
Kitur K, Parker D, Nieto P, Ahn DS, Cohen TS, Chung S, Wachtel S, Bueno S, Prince A (2015) Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog 11:e1004820
Kozai D, Ogawa N, Mori Y (2014) Redox regulation of transient receptor potential channels. Antioxid Redox Signal 21:971–986
Bogeski I, Kappl R, Kummerow C, Gulaboski R, Hoth M, Niemeyer BA (2011) Redox regulation of calcium ion channels: chemical and physiological aspects. Cell Calcium 50:407–423
Takahashi N, Kozai D, Kobayashi R, Ebert M, Mori Y (2011) Roles of TRPM2 in oxidative stress. Cell Calcium 50:279–287
Simon F, Varela D, Eguiguren AL, Diaz LF, Sala F, Stutzin A (2004) Hydroxyl radical activation of a Ca2+-sensitive nonselective cation channel involved in epithelial cell necrosis. Am J Physiol Cell Physiol. 287:C963–C970
Barros LF, Stutzin A, Calixto A, Catalan M, Castro J, Hetz C, Hermosilla T (2001) Nonselective cation channels as effectors of free radical-induced rat liver cell necrosis. Hepatology 33:114–122
Miller BA (2006) The role of TRP channels in oxidative stress-induced cell death. J Membr Biol 209:31–41
Patel AJ, Lauritzen I, Lazdunski M, Honore E (1998) Disruption of mitochondrial respiration inhibits volume-regulated anion channels and provokes neuronal cell swelling. J Neurosci 18:3117–3123
Okada Y, Maeno E, Shimizu T, Manabe K, Mori S, Nabekura T (2004) Dual roles of plasmalemmal chloride channels in induction of cell death. Pflugers Arch 448:287–295
McConkey DJ, Orrenius S (1996) The role of calcium in the regulation of apoptosis. J Leukoc Biol 59:775–783
Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG (2014) Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol 16:55–65
Chen X, Li W, Ren J, Huang D, He WT, Song Y, Yang C, Li W, Zheng X, Chen P, Han J (2014) Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res 24:105–121
Diaz RJ, Harvey K, Boloorchi A, Hossain T, Hinek A, Backx PH, Wilson GJ (2014) Enhanced cell volume regulation: a key mechanism in local and remote ischemic preconditioning. Am J Physiol Cell Physiol 306:C1191–C1199
Sciacca MF, Milardi D, Messina GM, Marletta G, Brender JR, Ramamoorthy A, La Rosa C (2013) Cations as switches of amyloid-mediated membrane disruption mechanisms: calcium and IAPP. Biophys J 104:173–184
Su L, Quade B, Wang H, Sun L, Wang X, Rizo J (2014) A plug release mechanism for membrane permeation by MLKL. Structure 22:1489–1500
Karch J, Kanisicak O, Brody MJ, Sargent MA, Michael DM, Molkentin JD (2015) Necroptosis interfaces with MOMP and the MPTP in mediating cell death. PLoS One 10:e0130520
Tait SW, Oberst A, Quarato G, Milasta S, Haller M, Wang R, Karvela M, Ichim G, Yatim N, Albert ML, Kidd G, Wakefield R, Frase S, Krautwald S, Linkermann A, Green DR (2013) Widespread mitochondrial depletion via mitophagy does not compromise necroptosis. Cell Rep 5:878–885
Schulze-Lohoff E, Hugo C, Rost S, Arnold S, Gruber A, Brune B, Sterzel RB (1998) Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am J Physiol 275:F962–F971
Adinolfi E, Pizzirani C, Idzko M, Panther E, Norgauer J, Di Virgilio F, Ferrari D (2005) P2X(7) receptor: death or life? Purinergic Signal 1:219–227
Schreiber R, Faria D, Skryabin BV, Rock JR, Kunzelmann K (2014) Anoctamins support calcium-dependent chloride secretion by facilitating calcium signaling in adult mouse intestine. Pflügers Arch 467:1203–1213
Sirianant L, Ousingsawat J, Tian Y, Schreiber R, Kunzelmann K (2014) TMC8 (EVER2) attenuates intracellular signaling by Zn2+ and Ca2+ and suppresses activation of Cl− currents. Cell Signal 26:2826–2833
Kunzelmann K, Schreiber R (2014) Chloride secretion, anoctamin 1 and Ca2+ signaling. Channels (Austin) 8:387–388
Kunzelmann K, Schreiber R, Kmit A, Jantarajit W, Martins JR, Faria D, Kongsuphol P, Ousingsawat J, Tian Y (2012) Expression and function of epithelial anoctamins. Exp Physiol 97:184–192
Dixon SJ, Stockwell BR (2014) The role of iron and reactive oxygen species in cell death. Nat Chem Biol 10:9–17
Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz PS, Weinlich R, Vanden Berghe T, Vandenabeele P, Pasparakis M, Bleich M, Weinberg JM, Reichel CA, Brasen JH, Kunzendorf U, Anders HJ, Stockwell BR, Green DR, Krautwald S (2014) Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci USA 111:16836–16841
Vorobjeva NV, Pinegin BV (2014) Neutrophil extracellular traps: mechanisms of formation and role in health and disease. Biochemistry (Mosc) 79:1286–1296
Yipp BG, Kubes P (2013) NETosis: how vital is it? Blood 122:2784–2794
Waring P (2005) Redox active calcium ion channels and cell death. Arch Biochem Biophys 434:33–42
Andrabi SA, Dawson TM, Dawson VL (2008) Mitochondrial and nuclear cross talk in cell death: parthanatos. Ann N Y Acad Sci 1147:233–241
Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H, Weinberg JM, Green DR, Kunzendorf U, Krautwald S (2013) Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci USA 110:12024–12029
Laster SM, Wood JG, Gooding LR (1988) Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol 141:2629–2634
Golstein P, Kroemer G (2007) Cell death by necrosis: towards a molecular definition. Trends Biochem Sci 32:37–43
Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G (2007) Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 13:54–61
Hetz CA, Hunn M, Rojas P, Torres V, Leyton L, Quest AF (2002) Caspase-dependent initiation of apoptosis and necrosis by the Fas receptor in lymphoid cells: onset of necrosis is associated with delayed ceramide increase. J Cell Sci 115:4671–4683
Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11:700–714
Acknowledgments
This work was supported by DFG SFB699-A7/A12, DFG KU756/12-1 and Volkswagenstiftung AZ 87 499. We thank Prof. Dr. Stefan Krautwald and PD Dr. Andreas Linkermann (Division of Nephrology and Hypertension, Christian-Albrechts-University Kiel) for providing the NIH-3T3 cell line and critical discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kunzelmann, K. Ion channels in regulated cell death. Cell. Mol. Life Sci. 73, 2387–2403 (2016). https://doi.org/10.1007/s00018-016-2208-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2208-z