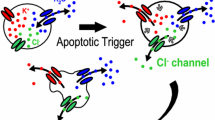
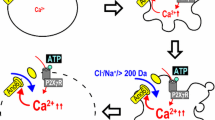
Abstract
Even under anisotonic conditions, most cells can regulate their volume by mechanisms called regulatory volume decrease (RVD) and increase (RVI) after osmotic swelling or shrinkage, respectively. In contrast, the initial processes of necrosis and apoptosis are associated with persistent swelling and shrinkage. Necrotic volume increase (NVI) is initiated by uptake of osmolytes, such as Na+, Cl− and lactate, under conditions of injury, hypoxia, ischaemia, acidosis or lactacidosis. Persistence of NVI is caused by dysfunction of RVD due to impairment of volume-sensitive Cl− channels under conditions of ATP deficiency or lactacidosis. Both lactacidosis-induced RVD dysfunction and necrotic cell death are prevented by pretreatment of cells with the vacuolating cytotoxin-A (VacA) toxin protein purified from Helicobacter pylori, which forms a lactacidosis-resistant anion channel. Apoptotic volume decrease (AVD) is triggered by activation of K+ and Cl− conductances following stimulation with a mitochondrion-mediated or death receptor-mediated apoptosis inducer. Apoptotic cell death can be prevented by blocking the Cl− channels but not the K+-Cl− cotransporters. Thus, the volume regulatory anion channel plays, unless impaired, a cell-rescuing role in the necrotic process by ensuring RVD after swelling induced by necrotic insults, whereas normotonic activation of the anion channel plays a cell-killing role in the apoptotic process by triggering AVD following stimulation with apoptosis inducers.








Similar content being viewed by others
References
Araki T, Hayashi M, Watanabe N, Kanuka H, Yoshino J, Miura M, Saruta T (2002) Down-regulation of Mcl-1 by inhibition of the PI3-K/Akt pathway is required for cell shrinkage-dependent cell death. Biochem Biophys Res Commun 290:1275–1281
Barriere H, Poujeol C, Tauc M, Blasi JM, Counillon L, Poujeol P (2001) CFTR modulates programmed cell death by decreasing intracellular pH in Chinese hamster lung fibroblasts. Am J Physiol 281:C810–C824
Barros LF, Hermosilla T, Castro J (2001) Necrotic volume increase and the early physiology of necrosis. Comp Biochem Physiol [A] Mol Integr Physiol 130:401–409
Benson RSP, Heer S, Dive C, Watson AJM (1996) Characterization of cell volume loss in CEM-C7A cells during dexamethasone-induced apoptosis. Am J Physiol 270:C1190–C1203
Bize I, Dunham PB (1994) Staurosporine, a protein kinase inhibitor, activates K-Cl cotransport in LK sheep erythrocytes. Am J Physiol 266:C759–C770
Bize I, Munoz P, Canessa M, Dunham PB (1998) Stimulation of membrane serine-threonine phosphatase in erythrocytes by hydrogen peroxide and staurosporine. Am J Physiol 274:C440–C446
Bock J, Szabo I, Jekle A, Gulbins E (2002) Actinomycin D-induced apoptosis involves the potassium channel Kv1.3. Biochem Biophys Res Commun 295:526–531
Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA (1995) Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-d-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci USA 92:7162–7166
Bortner CD, Cidlowski JA (1996) Absence of volume regulatory mechanisms contributes to the rapid activation of apoptosis in thymocytes. Am J Physiol 271:C950–C961
Bortner CD, Cidlowski JA (1999) Caspase independent/dependent regulation of K+, cell shrinkage, and mitochondrial membrane potential during lymphocyte apoptosis. J Biol Chem 274:21953–21962
Botchkin LM, Matthews G (1995) Swelling activates chloride current and increases internal calcium in nonpigmented epithelial cells from the rabbit ciliary body. J Cell Physiol 164:286–294
Choi DW (1987) Ionic dependence of glutamate neurotoxicity. J Neurosci 7:369–379
Colom LV, Diaz ME, Beers DR, Neely A, Xie W, Appel SH (1998) Role of potassium channels in amyloid-induced cell death. J Neurochem 70:1925–1934
Cover TL, Blaser MJ (1992) Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem 267:10570–10575
Duvall E, Wyllie AH (1986) Death and the cell. Immunol Today 7:115–119
Ekhterae D, Platoshyn O, Krick S, Yu Y, McDaniel SS, Yuan JX-J (2001) Bcl-2 decreases voltage-gated K+ channel activity and enhances survival in vascular smooth muscle cells. Am J Physiol 281:C157–C165
Fan HT, Morishima S, Kida H, Okada Y (2001) Phloretin differentially inhibits volume-sensitive and cyclic AMP-activated, but not Ca-activated, Cl− channels. Br J Pharmacol 133:1096–1106
Flatman PW, Adragna NC, Lauf PK (1996) Role of protein kinases in regulating sheep erythrocyte K-Cl cotransport. Am J Physiol 271:C255–C263
Fujita H, Yanagisawa A, Ishikawa K (1997) Suppressive effect of a chloride bicarbonate exchanger inhibitor on staurosporine-induced apoptosis of endothelial cells. Heart Vessels Suppl 12:84–88
Garay RP, Nazaret C, Hannaert PA, Cragoe EJ Jr (1988) Demonstration of a [K+,Cl−]-cotransport system in human red cells by its sensitivity to [(dihydroindenyl)oxy]alkanoic acids: regulation of cell swelling and distinction from the bumetanide-sensitive [Na+,K+,Cl−]-cotransport system. Mol Pharmacol 33:696–701
Grammatopoulos T, Morris K, Ferguson P, Weyhenmeyer J (2002) Angiotensin protects cortical neurons from hypoxic-induced apoptosis via the angiotensin type 2 receptor. Mol Brain Res 99:114–124
Gulbins E, Jekle A, Ferlinz K, Grassme H, Lang F (2000) Physiology of apoptosis. Am J Physiol 279:F605–615
Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y (2002) LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 9:163–173
Hortelano S, Zeini M, Castrillo A, Alvarez AM, Bosca L (2002) Induction of apoptosis by nitric oxide in macrophages is independent of apoptotic volume decrease. Cell Death Differ 9:643–650
Houzen H, Kikuchi S, Kanno M, Shinpo K, Tashiro K (1997) Tumor necrosis factor enhancement of transient outward potassium currents in cultured rat cortical neurons. J Neurosci Res 50:990–999
Hughes FM Jr, Cidlowski JA (1998) Glucocorticoid-induced thymocyte apoptosis: protease-dependent activation of cell shrinkage and DNA degradation. J Steroid Biochem Mol Biol 65:207–217
Jackson PS, Strange K (1993) Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. Am J Physiol 265:C1489–C1500
Jackson PS, Strange K (1995) Single-channel properties of a volume-sensitive anion conductance. Current activation occurs by abrupt switching of closed channels to an open state. J Gen Physiol 105:643–660
Jackson PS, Strange K (1995) Characterization of the voltage-dependent properties of a volume-sensitive anion conductance. J Gen Physiol 105:661–676
Jackson PS, Morrison R, Strange K (1994) The volume-sensitive organic osmolyte-anion channel VSOAC is regulated by nonhydrolytic ATP binding. Am J Physiol 267:C1203–C1209
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Kraig RP, Pulsinelli WA, Plum F (1985) Heterogeneous distribution of hydrogen ions and bicarbonate ions during complete brain ischemia. Prog Brain Res 63:155–166
Kraig RP, Petito CK, Plum F, Pulsinelli WA (1987) Hydrogen ions kill brain at concentrations reached in ischemia. J Cereb Blood Flow Metab 7:379–386
Krick S, Platoshyn O, Sweeney M, Kim H, Yuan JX-J (2001) Activation of K+ channels induces apoptosis in vascular smooth muscle cells. Am J Physiol 280:C970–C979
Kroemer G, Dallaporta B, Resche-Rigon M (1998) The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol 60:619–642
Lang F (ed) (1998) Cell Volume Regulation. Karger, Basel, pp264
Lang F, Madlung J, Bock J, Lukewille U, Kaltenbach S, Lang KS, Belka C, Wagner CA, Lang HJ, Gulbins E, Lepple-Wienhues A (2000) Inhibition of Jurkat-T-lymphocyte Na+/H+-exchanger by CD95(Fas/Apo-1)-receptor stimulation. Pflugers Arch 440:902–907
Lauf PK, Bauer J, Adragna NC, Fujise H, Zade-Oppen AM, Ryu KH, Delpire E (1992) Erythrocyte K-Cl cotransport: properties and regulation. Am J Physiol 263:C917–C932
Leaf A (1956) On the mechanism of fluid exchange of tissues in vitro. Biochem J 62:241–248
Li J, Eastman A (1995) Apoptosis in an interleukin-2-dependent cytotoxic T lymphocyte cell line is associated with intracellular acidification. J Biol Chem 270:3203–3211
Liu Y, Oiki S, Tsumura T, Shimizu T, Okada Y (1998) Glibenclamide blocks volume-sensitive Cl− channels by dual mechanisms. Am J Physiol 275:C343–C351
Lomneth R, Medrano S, Gruenstein EI (1990) The role of transmembrane pH gradients in the lactic acid induced swelling of astrocytes. Brain Res 523:69–77
Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y (2000) Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc Natl Acad Sci USA 97:9487–9492
Maeno E, Wakabayashi S, Okada Y (2001) Role of a Na+/H+ exchange in disordered volume regulation associated with apoptosis induced by physical shrinkage (abstract). Jpn J Physiol (Suppl) 51:S113
Marmarou A (1992) Intracellular acidosis in human and experimental brain injury. J Neurotrauma (Suppl) 9:S551–S562
McLaughlin B, Pal S, Tran MP, Parsons AA, Barone FC, Erhardt JA, Aizenman E (2001) p38 activation is required upstream of potassium current enhancement and caspase cleavage in thiol oxidant-induced neuronal apoptosis. J Neurosci 21:3303–3311
Meng X-J, Carruth MW, Weinman SA (1997) Leukotriene D4 activates a chloride conductance in hepatocytes from lipopolysaccharide-treated rats. J Clin Invest 99:2915–2922
Mori S, Morishima S, Takasaki M, Okada Y (2002) Impaired activity of volume-sensitive anion channel during lactacidosis-induced swelling in neuronally differentiated NG108-15 cells. Brain Res 957:1–11
Morishima S, Shimizu T, Kida H, Okada Y (2000) Volume expansion sensitivity of swelling-activated Cl− channel in human epithelial cells. Jpn J Physiol 50:277–280
Nabekura T, Morishima S, Cover TL, Mori S, Kannan H, Komune S, Okada Y (2003) Recovery from lactacidosis-induced glial cell swelling with the aid of exogenous anion channels. Glia 41:247–259
Nedergaard M, Goldman SA (1993) Carrier-mediated transport of lactic acid in cultured neurons and astrocytes. Am J Physiol 265:R282–R289
Nicholson D, Thornberry, NA (1997) Caspases: killer proteases. Trends Biochem Sci 22:299–306
Nietsch HH, Roe MW, Fiekers JF, Moore AL, Lidofsky SD (2000) Activation of potassium and chloride channels by tumor necrosis factor α. Role in liver cell death. J Biol Chem 275:20556–20561
Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G (1997) Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol 68:69–119
Oike M, Droogmans G, Nillius B (1994) The volume-activated chloride current in human endothelial cells depends on intracellular ATP. Pflugers Arch 427:184–186
Oiki S, Kubo M, Okada Y (1995) Mg2+ and ATP-dependence of volume-sensitive Cl− channels in human epithelial cells. Jpn J Physiol 44:S77–S79
Okada Y (1997) Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am J Physiol 273:C755–C789
Okada Y (1998) Cell volume-sensitive chloride channels. In: Lang F (ed) Cell volume regulation. Karger, Basel, pp 21–33
Okada Y (ed) (1998) Cell volume regulation: the molecular mechanism and volume sensing machinery. Elsevier, Amsterdam, p 214
Okada Y, Maeno E (2001) Apoptosis, cell volume regulation and volume-regulatory chloride channels. Comp Biochem Physiol [A] Mol Integr Physiol 130:377–383
Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S (2001) Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J Physiol (Lond) 532:3–16
Olney JW (1986) Inciting excitotoxic cytocide among central neurons. Adv Exp Med Biol 203:631–645
Olney JW, Price MT, Samson L, Labruyere J (1986) The role of specific ions in glutamate neurotoxicity. Neurosci Lett 28:65–71
O’Reilly N, Xia Z, Fiander H, Tauskela J, Small DL (2002) Disparity between ionic mediators of volume regulation and apoptosis in N1E 115 mouse neuroblastoma cells. Brain Res 943:245–256
Paschen W, Djuricic B, Mies G, Schmidt-Kastner R, Linn F (1987) Lactate and pH in the brain: association and dissociation in different pathophysiological states. J Neurochem 48:154–159
Patel AJ, Honoré E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M (1998) A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J 17:4283–4290
Payne JA (1997) Functional characterization of the neuronal-specific K-Cl cotransporter: implications for [K+]o regulation. Am J Physiol 273:C1516–C1525
Platoshyn O, Zhang S, McDaniel SS, Yuan JX-J (2002) Cytochrome c activates K+ channels before inducing apoptosis. Am J Physiol 283:C1298–C1305
Rasola A, Farahi Far D, Hofman P, Rossi B (1999) Lack of internucleosomal DNA fragmentation is related to Cl− efflux impairment in hematopoietic cell apoptosis. FASEB J 13:1711–1723
Rich IN, Worthington-White D, Garden OA, Musk P (2000) Apoptosis of leukemic cells accompanies reduction in intracellular pH after targeted inhibition of the Na+/H+ exchanger. Blood 95:1427–1434
Schrantz N, Blanchard DA, Auffredou M-T, Sharma S, Leca G, Vazquez A (1999) Role of caspases and possible involvement of retinoblastoma protein during TGFβ-mediated apoptosis of human B lymphocytes. Oncogene 18:3511–3519
Schumann MA, Gardner P, Raffin TA (1993) Recombinant human tumor necrosis factor α induces calcium oscillation and calcium-activated chloride current in human neutrophils. The role of calcium/calmodulin-dependent protein kinase. J Biol Chem 268:2134–2140
Shen MR, Chou CY, Ellory JC (2000) Volume-sensitive KCI cotransport associated with human cervical carcinogenesis. Pflugers Arch 440:751–760
Shen M-R, Droogmans G, Eggermont J, Voets T, Ellory JC, Nilius B (2000) Differential expression of volume-regulated anion channels during cell cycle progression of human cervical cancer cells. J Physiol (Lond) 529:385–394
Sheppard DN, Welsh MJ (1992) Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J Gen Physiol 100:573–591
Shimizu T, Numata T, Okada Y (2004) A role of reactive oxygen species in apoptotic activation of volume-sensitive Cl− channel. Proc Natl Acad Sci USA (in press)
Siesjö BK (1985) Acid-base homeostasis in the brain: physiology, chemistry, and neurochemical pathology. Prog Brain Res 63:121–154
Siesjö BK (1988) Acidosis and ischemic brain damage. Neurochem Pathol 9:31–88
Small DL, Tauskela J, Xia Z (2002) Role for chloride but not potassium channels in apoptosis in primary rat cortical cultures. Neurosci Lett 334:95–98
Souktani R, Berdeaux A, Ghaleh B, Giudicelli JF, Guize L, Le Heuzey JY, Henry P (2000) Induction of apoptosis using sphingolipids activates a chloride current in Xenopus laevis oocytes. Am J Physiol 279:C158–C165
Staub F, Mackert B, Kempski O, Peters J, Baethmann A (1993) Swelling and death of neuronal cells by lactic acid. J Neurol Sci 119:79–84
Strange K, Emma F, Jackson PS (1996) Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol 270:C711–C730
Szabo I, Lepple-Wienhues A, Kaba KN, Zoratti M, Gulbins E, Lang F (1998) Tyrosine kinase-dependent activation of a chloride channel in CD95-induced apoptosis in T lymphocytes. Proc Natl Acad Sci USA 95:6169–6174
Tan X, Wang JYJ (1998) The caspase-RB connection in cell death. Trends Cell Biol 8:116–120
Tosteson DC, Hoffman JF (1960) Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J Gen Physiol 44:169–194
Vu CCQ, Bortner CD, Cidlowski JA (2001) Differential involvement of initiator caspases in apoptotic volume decrease and potassium efflux during Fas- and UV-induced cell death. J Biol Chem 276:37602–37611
Wang L, Xu D, Dai W, Lu L (1999) An ultraviolet-activated K+ channel mediates apoptosis of myeloblastic leukemia cells. J Biol Chem 274:3678–3685
Wolf CM, Reynolds JE, Morana SJ, Eastman A (1997) The temporal relationship between protein phosphatase, ICE/CED-3 proteases, intracellular acidification, and DNA fragmentation in apoptosis. Exp Cell Res 230:22–27
Wondergem R, Gong W, Monen SH, Dooley SN, Gonce JL, Conner TD, Houser M, Ecay TW, Ferslew KE (2001) Blocking swelling-activated chloride current inhibits mouse liver cell proliferation. J Physiol (Lond) 532:661–672
Wu KL, Khan S, Lakhe-Reddy S, Wang L, Jarad G, Miller RT, Konieczkowski M, Brown AM, Sedor JR, Schelling JR (2003) Renal tubular epithelial cell apoptosis is associated with caspase cleavage of the NHE1 Na+/H+ exchanger. Am J Physiol 284:F829–F839
Wyllie AH, Kerr JFR, Currie AR (1980) Cell death: the significance of apoptosis. Int Rev Cytol 68:251–306
Yu SP, Yeh C-H, Sensi SL, Gwag BJ, Canzoniero LMT, Farhangrazi ZS, Ying HS, Tian M, Dugan LL, Choi DW (1997) Mediation of neuronal apoptosis by enhancement of outward potassium current. Science 278:114–117
Zhivotovsky B, Gahm A, Ankarcrona M, Nicotera P, Orrenius S (1995) Multiple proteases are involved in thymocyte apoptosis. Exp Cell Res 221:404–412
Acknowledgements
We thank TL Cover for reading the manuscript and providing the VacA protein, RZ Sabirov for reading the manuscript, M Ohara, K Shigemoto and C Kondo for technical assistance, and T Okayasu for secretarial assistance. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by support from the Salt Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okada, Y., Maeno, E., Shimizu, T. et al. Dual roles of plasmalemmal chloride channels in induction of cell death. Pflugers Arch - Eur J Physiol 448, 287–295 (2004). https://doi.org/10.1007/s00424-004-1276-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-004-1276-3