Abstract
Thyroid sonography has made substantial progress over the last decades in terms of spatial resolution and additional parameters including vascularity, perfusion, and elasticity of lesions. The improved depictability of thyroid nodules has led to a more detailed sonographic characterization of malignant thyroid nodules considering features like microcalcification, capsular invasion, and reduced elasticity. Thus, ultrasound (US) has become the most important single tool for risk assessment of thyroid nodules. Predominantly cystic and spongiform nodules as well as many nodules with a mixed composition of solid and cystic components can safely be identified as benign on ultrasound and need no further work-up for risk assessment. Predominantly solid nodules, however, require a structured and consistent sonographic assessment including the evaluation of nodule composition, echogenicity, calcifications, shape, margins, and additional sonographic parameters. One should adhere to one of the TIRADS when reporting ultrasound findings on thyroid nodules categorizing each nodule to a certain risk class. However, the risk of malignancy reported in studies for each class may not be readily transferrable to daily routine, since the prevalence of malignant nodules in tertiary care centers is as high as 20% whereas in primary care units the prevalence may be as low as 1 per mille, thus lowering positive predictive values by one to two orders of magnitude. In addition, some suspicious features found in those studies may not be directly transferable to daily practice: microcalcifications are often difficult to discern from benign colloidal spots, even after having run through learning sessions; a taller-than-wide shape of nodules with contact to the dorsal parts of the thyroid gland does not imply malignancy according to a pole concept which has been recently developed.
Thyroid scintigraphy (TS) has long been an indispensable modality for functional characterization of thyroid nodules. It should be applied to rule out malignancy in a substantial proportion of predominantly solid nodules by showing a hyperfunctioning (“hot”) nodule. Such nodules are almost always benign with few exceptions (e.g., “trapping only” nodules). If laboratory findings and scintigraphic appearance of a hot nodule do not match, further diagnostic work-up is warranted including 123I− scintigraphy with late imaging at 24 h and fine-needle aspiration cytology (FNAC). In settings with a rather low prevalence of malignant thyroid nodules such as in primary or secondary care units, a combined use of US and TS may be adopted to rule in suspicious nodules for further work-up by FNAC, including predominantly solid hypofunctioning (“cold”) nodules. MIBI scintigraphy can be used in hypofunctioning nodules with indeterminate cytology and if contraindications preclude patients from FNAC.
TS is a valuable tool to detect functional abnormalities not only in nodules but also in the thyroid gland as a whole by assessing the overall radionuclide uptake. As such, it has long been used to confirm the diagnosis of Graves’ disease and thyroid autonomy. For disseminated autonomy, it is the only tool to definitely verify the diagnosis. In destructive thyroiditis, TS reliably shows a reduced overall radionuclide uptake justifying its application in selected cases with doubtful sonographic or laboratory findings. The user of TS is encouraged to calculate a site-specific normal range for the uptake value normalized to TSH in addition to the raw uptake. Thus, even subtle functional disorders of the thyroid can be detected and graded.
Functional or metabolic imaging is increasingly combined with morphological imaging and is acquired and displayed as volume data rather than planar images including SPECT/CT, PET/CT, and PET/MRI. These combined modalities increase the restricted morphological field of view from ultrasound enabling to reliably image substernal, ectopic, or dystopic localizations of thyroid tissue. Combined modalities also benefit from the increasing spectrum of functional or metabolic tracers including MIBI, iodine isotopes (e.g., 124I-) and newer PET tracers.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Thyroid
- TIRADS
- Ultrasound
- Contrast-Enhanced Ultrasound
- Color Doppler Ultrasound
- Elastography
- Thyroid Scan
- Hybrid Imaging
- Scintigraphy
- SPECT
- SPECT/CT
- PET/CT
4.1 Basics of Thyroid Imaging
Modern integrated imaging of thyroid diseases combines multiparametric thyroid ultrasound as morphological imaging method and molecular imaging with radiopharmaceutical substances to assess the structure and the functional status of the thyroid gland or thyroid lesions. This chapter provides an overview of thyroid ultrasound and molecular imaging for diffuse and nodular thyroid diseases.
4.1.1 Sonography
Since its initial description by Fujimoto et al. in 1967, ultrasound (US) has gained a critical role in the evaluation of diffuse and nodular thyroid diseases. With today’s high-resolution systems, ultrasound examinations are among the most widely used imaging methods worldwide. Ultrasound is cost-effective, free of radiation, can be repeated as often as required, is easy to learn, and allows versatile use. Modern ultrasound devices are equipped with transducers that have a frequency spectrum of typically 2–20 Megahertz (MHz), with the choice of frequency depending on the target structure (location and depth within the body). For thyroid ultrasound high-resolution linear transducers with a frequency of 7–15 MHz have been used. Besides brightness-mode (B-mode, gray-scale ultrasound), multiparametric ultrasound (MPUS) also integrates vascularization (spectral/color/power Doppler ultrasound, SDUS/CDUS/PDUS, superb microvascular imaging, SMI, and contrast-enhanced ultrasound, CEUS) and tissue stiffness (ultrasound elastography) [1,2,3,4].
4.1.1.1 B-Mode Sonography (Gray-Scale Ultrasound)
B-mode sonography is the standard imaging method for the structured examination of thyroid morphology. The thyroid gland as a whole (i.e., position, enlargement, shape, echogenicity, and composition) as well as focal lesions (i.e., size, localization, echogenicity, shape, margins, composition, and calcification) can be described in detail; B-mode sonography is usually displayed in gray-scale mode. B-mode sonography is useful for the assessment of diffuse thyroid disorders (DTD), thyroid nodules, and other thyroid lesions, e.g., inflammatory disorders. The following objectives reflect the indications for thyroid ultrasound set out by the American Thyroid Association (ATA) and American Association of Clinical Endocrinology (AACE):
-
I.
To characterize thyroid nodules to differentiate between benign and malignant lesions (Table 4.1).
-
II.
To evaluate diffuse changes in thyroid parenchyma.
-
III.
To differentiate thyroid lesions from other cervical structures like cervical cysts or a thyroglossal duct.
-
IV.
To detect and to follow up cervical lymph nodes and recurrent disease in patients with thyroid malignancy.
-
V.
To screen high-risk patients with MEN type II, a history of familial thyroid cancer or neck irradiation in childhood.
-
VI.
To guide diagnostic (e.g., fine-needle aspiration cytology, FNAC) and therapeutic (e.g., radiofrequency ablation) procedures [5, 6].
The normal thyroid gland physiologically consists of two lobes and the thyroid isthmus. Occasionally, a residue or rests of the thyroglossal duct (pyramidal lobe) may be seen. The normal thyroid size and volume depend on age, sex, and iodine supply. The volume of the thyroid lobe is approximated by the ellipsoid formula: volume = width (mediolateral) × depth (anteroposterior) × length (craniocaudal) × π/6. In case of enlargement, both, the volume of the thyroid isthmus and the pyramid lobe should be added to the total thyroid volume. The upper limit of the thyroid gland volume in a slightly iodine-deficient area such as Germany is as high as 18 mL and 25 mL in adult women and men, respectively [7]. A lower volume limit is frequently not given. However, some studies have shown that thyroid volumes below 5 mL may not be sufficient for adequate thyroid hormone production. The internal structure of a normal thyroid gland is homogenously bright comparable to the salivary glands and markedly hyperechogenic compared to the adjacent cervical muscles [6, 8].
4.1.1.2 Cine-Mode and Tomographic Ultrasound
Cine-mode ultrasound allows for the continuous acquisition of ultrasound images over time [12]. This provides additional means of documentation and storage of cross-sectional data in multiple planes. With the acquired data sets incorporating the whole thyroid gland, structured assessment can be carried out in retrospective and longitudinal comparison of corresponding image planes becomes more accurate. Continuous image acquisition combined with probe tracking technology provides the basis for tomographic ultrasound imaging [13]. Data acquired in an axial plane can thus be reconstructed in multiple planes and fused with functional imaging data as needed. Integrated SPECT/Ultrasound and PET/Ultrasound fusion imaging have been shown to be feasible and may gain wider use once available on a larger scale [14, 15]. Volume data derived from standardized cine-mode acquisitions are the basis for automatic segmentation algorithms and—in the future—artificial intelligence applications.
4.1.1.3 Color Doppler Ultrasound (CDUS) and Contrast-Enhanced Ultrasound (CEUS)
CDUS gives information about the vascularization of the thyroidal tissue by visualizing intraparenchymal as well as the perithyroidal vessels. The underlying technique is based on the change in frequency of a sound wave once reflected by the motion of red blood cells in vessels. Using color coding (red: toward the transducer, blue: away from the transducer), the direction of blood flow is shown in an overlay of Doppler and gray-scale images (Fig. 4.1a). In power Doppler mode only one color (e.g., orange) is used to code both flow directions.
The vascularity patterns of thyroid nodules can be categorized into four types:
-
I.
Type 1: No vascularity (absence of intra-/perinodular vascularity).
-
II.
Type 2: Perinodular vascularity (circumferential vascularity of the nodule).
-
III.
Type 3: Mild intranodular vascularity (with or without perinodular vascularity).
-
IV.
Type 4: Marked intranodular vascularity (with or without perinodular vascularity) [3, 9].
In CEUS, an intravenous microbubble contrast agent is used, which enhances the backscattering of ultrasound waves resulting in an amplification of flow signals. This means, it allows visualization of blood flow in thyroid vessels and within the microvasculature. The enhancement pattern reflects the different vascular phases (i.e., arterial, portal-venous, and venous) comparable to contrast-enhanced computed tomography. CEUS is a safe procedure, however, rarely allergic events are possible [3, 16]. Image evaluation for thyroid nodule assessment includes qualitative (quality of enhancement, washout behavior, and comparison to the paranodular tissue) and quantitative (time-to-peak, wash-in slope, peak intensity, mean transit time, and area under time-intensity curves) parameters [17]. Normal thyroid tissue shows only small areas with flow termed as “normal background flow” as shown in Fig. 4.1b [18].
4.1.1.4 Ultrasound Elastography
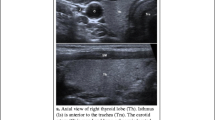
With Ultrasound Elastography (UE) it is possible to measure the elasticity of tissues. The concept of UE was first introduced by Orphir et al. in 1991. The principle is based on an external compression of the tissue, which leads to a measurable deformation. This deformation is more pronounced in soft tissues than in hard structures. Since a change in the elasticity of tissue typically correlates with pathologic features, UE can aid in differential diagnosis [19]. Three methods are distinguished in UE on the basis of the external stimulus: strain-based UE (USE, mechanically induced force, measuring tissue strain), acoustic radiation force impulse (ARFI, ultrasound induced, measuring tissue displacement), and shear wave-based UE (SWE, ultrasound induced, measuring quantitative shear wave propagation) [20, 21]. UE reflects the histologic composition of a tissue (i.e., cells, membranes, and ultrastructures). Normal thyroid parenchyma presents homogenously soft on UE (in case of USE mostly green, sometimes mixed green/yellow/red, Fig. 4.2). For thyroid nodule elasticity evaluation two qualitative scores are commonly used. The Asteria score ranges from elasticity score 1 representing soft (i.e., benign) tissue to elasticity 4 representing hard (i.e., suspicious) tissue. The three-tier Rago Score ranges from an elasticity score 1 representing elasticity in whole or large part of the nodule (i.e., benign) to score 3 without elasticity in the nodule and in the posterior shadowing (i.e., suspicious) [22, 23]. For quantitative SWE it is known, that the stiffer (i.e., suspicious) the tissue is, the higher the propagation of shear waves. Data for normal thyroid tissue are sparse. The World Federation for Ultrasound in Medicine and Biology reported a range for normal thyroid tissue 1.60 ± 0.18 m/s for point-SWE and 2.6 ± 1.8 m/s for 2-D SWE; a single cutoff for differentiation of thyroid nodules are so far not established [24,25,26].
(a, b) Normal thyroid gland. (a) Strain elastography (left: transverse orientation in B-mode sonography; right: superimposed strain elastography) showing a homogenously green/red colored (soft) thyroid lobe and blue colored (hard) trachea. (b) Shear wave elastography: transverse image of the left thyroid lobe (left: B-mode sonography and calculated elasticity modulus (kPa) and velocity of the propagation of the shear wave (m/s); right: box with three regions of interest to calculated velocity in the normal thyroid tissue
4.1.2 Radionuclide Imaging
4.1.2.1 99mTc-Pertechnetate/123Iodine
Thyroid scintigraphy (TS) allows for global and regional imaging of the sodium iodide transporter (NIS) activity within the thyroid gland in vivo. TS has long been performed with iodine isotopes (131I−, 123I−). For standard TS, nowadays, 99mTc-pertechnetate is preferred over iodine isotopes due to its advantageous physical properties (short physical half-life [6 h], pure gamma emission [140 keV], radiation exposure as low as approx. 1 mSv for a standard application of 70 MBq, i.e., 0.013 mSv/MBq, its easy availability and lower costs. As a mimicker of ionic iodine, 99mTc-pertechnetate enters the thyroid cell through the basolateral cell via the NIS. In contrast to iodine, 99mTc-pertechnetate is not further organified and leaves the thyroid cell with a rather short effective half-life (hours) compared to about half a day in 123I− or a couple of days in131I− [27, 28]. Since the import and the organification of iodine are key for thyroid hormone synthesis, the NIS activity and thus the uptake of 99mTc-pertechnetate is deemed representative of overall hormone production. TS thus provides overall and regional functional information about the thyroid gland. Under physiological conditions, the activity of the NIS is positively regulated by TSH. Only a small proportion of NIS activity appears to be independent of TSH regulation as indicated by a low (<0.5%, see below), but detectable scintigraphic uptake of 99mTc-pertechnetate even in subjects with full TSH suppression (physiological background autonomy) [29, 30]. The 99mTc-pertechnetate uptake can be quantified and is often given as a percentage of injected radioactivity. Sometimes a “normal” range is also given for the “raw” 99mTc-pertechnetate uptake, e.g., 0.5%–4% [31]. The raw uptake can only be interpreted with knowledge of the current TSH value. To avoid bias by fluctuations of TSH, TSH assessment should be closely connected with scintigraphy—with both exams ideally performed at the same time and at the same institution(s).
The authors suggest calculating 99mTc-pertechnetate uptake normalized to TSH (i.e., % per mU/L) in addition to raw uptake. The according normal range should be obtained from a site-specific cohort of about 20–30 subjects with normal thyroid function and may range from 0.3–3.0% per mU/L [32]. In addition to TSH regulation, NIS activity is also under inverse regulation by the intrathyroidal iodine concentration (“autoregulation”) which in turn is representative of the iodine supply. High iodine concentrations may deteriorate image quality given the inverse effect on NIS activity. The influence of iodine may reach a point, where a scintigraphic image becomes “blocked” (uptake 0%), e.g., after administration of contrast agents containing iodine. This may also occur after the use of NIS blocking agents such as perchlorate. As a conclusion, any “excess” iodine intake should be either avoided prior to scintigraphic imaging or scintigraphy should be postponed for at least 1–3 months in cases of severe iodine contamination to yield high-quality images and usable uptake values.
Thyroid hormones exert their influence on thyroid uptake mainly via TSH regulation. As TSH influence can be accounted for by the use of TSH-normalized uptake values, a properly substituted patient is not necessarily required to withdraw thyroid hormones before TS. However, one should bear in mind that roughly two-thirds of thyroid hormone mass is made up of iodine. This may result in a somewhat hypernormal iodine status reducing image quality and (normalized) uptake values via “autoregulation” (see above). For this reason, a mild reduction of thyroid hormone substitution may be appropriate before TS. In contrast to 99mTc-pertechnetate scintigraphy, TS with radioiodine isotopes not only reflects the import of iodine but also organification allowing for late imaging at and beyond 24 h. Owing to organification, thyroid uptake may increase about ten-fold on scintigraphies with radioiodine compared to 99mTc-pertechnetate resulting in much better thyroid-to-background contrast. Radioiodine scintigraphy is usually performed with 123I− (pure gamma emitter) for its lower radiation burden and better imaging quality compared to 131I− (90% beta- and 10% gamma emitter). 131I− is reserved for thyroid imaging in a therapeutic setting, e.g., testing before and during radioiodine therapy. 123I− TS should be used for evaluating mediastinal or ectopic/dystopic thyroid tissue allowing for better delineation against bloodpool activity (mediastinum) and non-thyroidal uptake (e.g., salivary glands) compared with 99mTc-pertechnetate (see below). There may be a role of 123I− scintigraphy for delineating small autonomously functioning thyroid nodules (AFTNs) below 1 cm in diameter. As shown below, AFTNs can mimic thyroid cancer on ultrasound imaging. Small AFTNs cannot be reliably diagnosed by 99mTc-pertechnetate scintigraphy owing to limited contrast resolution and TSH may not be indicative of autonomous function due to limited autonomous volume [33, 34]. Due to the higher contrast on late images, 123I− scintigraphies could delineate such small AFTNs and prevent them from further work-up since AFTNs are presumed to be benign. Another role of 123I− scintigraphy may be ruling out of “trapping only” nodules (see below). Trapping only nodules may mimic true AFTNs by showing a high initial uptake on TS but rapid washout over time as seen on late imaging (e.g., 24 h). Since trapping only nodules harbor a significant risk of malignancy ranging between 5 and 10% it is advised to use 123I− TS if the autonomous nature of a nodule is doubtable [28, 35]. On scintigraphy, the intensity of radionuclide uptake within nodules is visually rated relative to the surrounding thyroid tissue and described as “hyperfunctioning” (“hot”), “hypofunctioning” (“cold”), or isofunctioning (“indifferent”). Note that “hyperfunctioning” indicates a relatively increased thyroid hormone synthesis but not automatically an absolutely increased one. In fact, when thyroid parenchyma is damaged, e.g., by Hashimoto’s thyroiditis, a “hot” nodule may be the only site with normal and preserved hormone synthesis. Vice versa, a cold nodule mostly, but not always indicates a decreased hormone synthesis. In summary, indications for thyroid scintigraphy are shown in Table 4.2.
4.1.2.2 99mTc-Methoxy-Isobutyl-Isonitrile (MIBI) Imaging
99mTc-methoxy-isobutyl-isonitrile (MIBI) is a cationic lipophile complex agent that accumulates in mitochondria by passive diffusion. It has a high first-pass extraction and the MIBI uptake in viable cells depends on the blood flow. Besides its use as a marker for myocardial perfusion and hyperactive parathyroidal tissue, numerous studies have described MIBI uptake in various tumors such as lungs, brain, breast, bone, and thyroid. It is suggested that in addition to the number of mitochondria, their negative membrane potential is also responsible for the binding of MIBI [37,38,39]. The usefulness of MIBI imaging for risk stratification of hypofunctioning thyroid nodules has been investigated for more than 30 years. For qualitative image evaluation, the MIBI uptake in the thyroid nodule is compared to the MIBI uptake in the paranodular thyroid tissue and classified as hyper-, iso-, and hypointense with the latter ruling out malignancy with high probability. A semiquantitative method has been published by Campenni et al. taking the MIBI kinetics into account also (see below) [40,41,42,43]. A further thyroid-specific application for MIBI imaging is to differentiate amiodarone-induced hyperthyroidism (AIT) type 1 and type 2 [44]
4.1.3 Hybrid Imaging
4.1.3.1 Hybrid Imaging with 99mTc-Pertechnetate and 123I-NaI
Single photon emission computed tomography (SPECT) with integrated computed tomography (SPECT/CT) enables co-registration of anatomic and functional data and provides accurate attenuation correction of the detected tracer distribution (Table 4.3). It has substantially improved patient care in the management of thyroid cancer. A lot of reported advantages over conventional planar imaging after 131I− post-thyroidectomy radioactive iodine ablation have led to the use of SPECT/CT for precise localization and characterization of radioactive iodine foci and therefore influence staging, risk stratification, and clinical management [45]. SPECT/CT systems use the same gamma camera component generally used for planar and tomographic imaging of single photon emitting radiotracers, without additional radiation exposure for SPECT. In Germany, a volume CT dose index (CTDIvol) of 3 mGy has been defined for auxiliary CT imaging for attenuation correction and anatomic co-registration [46]. For benign thyroid conditions and pretherapeutic thyroid imaging SPECT/CT is not routinely used but SPECT/CT can be helpful in a number of indications, especially for substernal or ectopic thyroid tissue [28]. And it can be useful in detecting the dominant nodule in a goiter with multiple nodules and in distinguishing ectopic thyroid tissue from adjacent structures that may physiologically show an elevated iodine concentration, such as salivary glands, salivary collections in the mouth, and swallowed saliva in the esophagus. CT can also provide additional information, e.g., on tracheal compression and shift.
4.1.3.2 Hybrid Imaging with 18F-FDG-PET
Hybrid imaging with positron-emission tomography/computed tomography (PET/CT) and PET/magnetic resonance imaging (PET/MRI) combines imaging of function, e.g., metabolic information, and anatomic structure to provide accurate localization, characterization, and diagnosis in a “one-stop” imaging approach. Under normal conditions, thyroid tissue shows low or absent uptake of FDG. Due to the increase in 18F-fluorodeoxyglucose (18F-FDG)-PET examinations in patients with malignant and non-malignant diseases, incidental FDG uptake in the thyroid gland is seen more frequently and reported in up to 3% of the examinations. FDG uptake patterns in thyroid are generally classified as focal or diffuse. Currently, 18F-FDG-PET/CT is not recommended for initial or preoperative risk assessment of newly detected thyroid nodules or diffuse thyroid diseases. For some thyroid indications, 18F-FDG-PET/CT shows promising results, especially for cytologically indeterminate nodules (see below).
Currently, the radiolabelled glucose analog 18F-FDG-PET/CT is established in clinical practice and widely used for initial staging, restaging, recurrence detection, and assessment of treatment outcomes in a variety of malignant diseases. Combining metabolic and anatomical information, 18F-FDG-PET/CT detects malignant tumor lesions by identifying regions with increased glycolytic metabolism and expression of membrane glucose transporter (GLUT) proteins [47]. However, increased FDG uptake is not only found in malignant diseases but also in infectious or inflammatory conditions. Combined PET/MRI provides complementary data acquired at the same time and offers advantages over PET/CT with regard to increased anatomical details and radiation dose reduction by omitting radiation exposure through the CT component. The radiation exposure related to a PET/CT scan is dependent on the administered activity of FDG and on the intended use of the CT scan. Exposure can be chosen very low for attenuation correction only or mere anatomical correlation, but is higher for a diagnostic CT scan including intravenous contrast agent administration. The effective dose for the CT component thus ranges from 1 to 20 mSv [48]. Scan range can reach from a limited area confined to the tumor, to torso imaging up to whole-body imaging. For Germany, new diagnostic reference levels (DRL) for typical nuclear medicine examinations have been published in 2021. DRL for PET tracers are expressed as administered activity per body weight and for scintigraphies or SPECT as upper threshold levels for activities [46]. The effective dose from FDG application in adults is 0.019 mSv/MBq according to ICRP publication 106, i.e., about 4.3 mSv for an administered activity of 225 MBq [48] (Table 4.3).
4.2 Integrated Imaging of Thyroid Disorders
4.2.1 Diffuse Thyroid Diseases
The most frequent diffuse disorder of the thyroid gland is an enlargement of the glandular volume (goiter). This disorder can easily be assessed by ultrasound using the standard formula for volume calculation (thyroid volume = transverse diameter × anteroposterior diameter × longitudinal diameter × /π/6) [3]. In theory, growth of the thyroid gland is diffuse in first instance but becomes more and more nodular over time. However, nodules mostly occur already in non-enlarged thyroid glands. Up to two-thirds of a population may have thyroid nodules above 5 mm in diameter whereas thyroid gland enlargement affects only 17% in a mildly iodine-deficient population [49]. Therefore, in clinical routine, diffuse growth and development of nodules have a parallel time course rather than a consecutive one. For the sake of clarity, diffuse and nodular growth are treated separately in the following. Goitrous growth is seen in about one-tenth of the world population and it is driven by two main causes: genetic factors and iodine supply [50]. Other factors such as smoking and childbearing have also been shown to play a role [51]. Since iodine deficiency in areas endemic to goiter often dates back over centenaries or even thousands of years [52], it can be hypothesized that longstanding iodine deficiency is also a driving force behind genetic factors promoting goitrous growth. Goiters attributable to iodine deficiency can be assumed to be normofunctioning in the presence of normal or rather high TSH values. TS can demonstrate iodine avidity of the thyroid gland by a rather high uptake (e.g., uptake % twice or more per TSH unit) representing high NIS activity. However, TS is rarely considered necessary for such a diagnosis. Nevertheless, in doubt of the reason for goitrous growth, TS readily distinguishes between iodine deficiency and other reasons such as inflammatory causes (e.g., autoimmune disease, see below) by a high normalized uptake versus a low uptake. This may have implications for the treatment of the goiter–e.g., iodine vs. levothyroxine. In more advanced or longstanding endemic goiters autonomously functioning thyroid tissue above a certain baseline level of autonomy (see above) occasionally develops. By definition, autonomously functioning thyroid tissue produces thyroid hormone without being controlled by TSH regulation. The detection of autonomy is the core indication for TS—mostly performed with 99mTc-pertechnetate as the preferred radionuclide. In many cases, autonomy can be assigned to a single thyroid nodule or multiple thyroid nodules (i.e., unifocal autonomy and multifocal autonomy, see below) on the basis of an elevated circumscribed uptake on the scintigram. However, the thyroid gland as a whole can also be affected by autonomy, then termed “diffuse” or “disseminated” autonomy. While this disorder is frequently encountered in endemic areas, it is rather uncommon in non-endemic areas. In non-endemic areas, this presentation is often summarized under abortive/antibody-negative forms of Graves’ disease (GD) or multinodular autonomy [53, 54]. However, diffuse autonomy exhibits unique features not shared by GD such as a normal echogenicity on thyroid ultrasound, insidious onset, a rather stable clinical course over time and no curative restitution after the use of thyreostatics. Compared to multinodular autonomy there is no focal “hot” lesion on scintigraphy although the scintigraphic appearance may be somewhat patchy. However, diffuse autonomy may occur in combination with AFTN [55] then being named focally- or multifocally-disseminated autonomy. Diffuse thyroid autonomy is a mere scintigraphic diagnosis (see Fig. 4.3). In the absence of serological/biochemical or sonographic hints for GD, diffuse autonomy can be assumed when a goiter shows a moderately increased global uptake (raw uptake typically between 1 and 3%) not attributable to thyroid nodules—in combination with a low or subnormal TSH value. Thyroid antibodies are typically negative. However, in patients with positive TPO antibodies, some overlap with abortive forms of GD exist as well as with iodine avidity in endemic goiter. In goiters without nodules, TS may be considered when TSH is low, e.g., below 1 mU/L, in order to rule out diffuse thyroid autonomy—e.g., in elderly patients before anti-goitrous therapy with iodine is initiated or in other instances such as impending iodine contamination from contrast agents or disinfecting agents.
(a, b) Seventy-year-old man with borderline low TSH over several years. At presentation TSH is 0.23 mU/L. Thyroid antibodies are negative and the patient has no appreciable symptoms. (a) Ultrasound examination reveals grade 2–3 goiter (60 mL) with beginning nodular transformation mainly in the caudal portions of the gland. The thyroid parenchyma is otherwise unremarkable on thyroid ultrasound. (b) On scintigraphy [the hook marks the jugular groove], the uptake is somewhat inhomogeneous with more pronounced accumulation in the cranial and mid portions, i.e., the portions with the least nodular transformation. The raw uptake (1.5%) appears moderate but normalization to TSH reveals a clearly elevated normalized uptake of 6.5% per mU/L. On the basis of the scintigraphy, the diagnosis of disseminated autonomy is made
From studies in nodular goiter it is known that thyroid autonomy may occur in the presence of TSH values up to approx. 2 mU/L. This TSH value may also be adopted as a threshold below which diffuse thyroid autonomy may be present in goiters [56, 57]. However, in goiters with TSH in the mid-range TS cannot reliably differentiate between hidden diffuse autonomy and iodine avidity since both exhibit a rather high normalized uptake (see above). A suppression study may be added for a definitive diagnosis (see below). Ruling out disseminated autonomy in large goiters with TSH in the mid-range may have implications for goiter therapy and on how to deal with iodine contamination and its potential to enhance thyroid function. TS is not only able to detect autonomy but also to grade autonomous functions. The overall excess of hormone production originating from AFTNs and/or diffuse autonomy can be estimated by calculating the radionuclide uptake. As a threshold, a raw uptake between or above 1–2% is considered a relevant autonomy when TSH is suppressed to or below 0.1 mU/L [56]. In this regard, it plays no role whether TSH is suppressed endogenously or exogenously by administering thyroid hormone. The actual uptake threshold may vary between different regions depending on the prevailing iodine supply. Threshold values are lower in regions with sufficient iodine supply (e.g., 1%) as compared to regions with insufficient supply (e.g., 2%) [58, 59]. In subjects with TSH being naturally suppressed by autonomy, raw uptake will almost certainly be above 1%. If not, recent iodine contamination has to be taken into account—possibly lowering or even blocking the uptake [60]. The most common reason for thyrotoxicosis in non-endemic areas is GD [61]. GD is a hormone-productive autoimmune thyroid disease relying on the formation of TSH receptor-stimulating antibodies (TRAbs). Typically, it can readily be diagnosed by a high uptake on TS (generally ≫ 2%) in combination with a marked thyrotoxicosis, diffuse goiter with lowered echogenicity (Fig. 4.4a) with or without micronodulation, and positive Trabs. If CDUS of the thyroid gland shows a diffuse increase in vascularization (“thyroid inferno,” Fig. 4.4b), in such settings TS can be omitted—in particular in juveniles—since the diagnosis is sufficiently certain [3, 8]. The thyroid gland stiffness is significantly higher in GD than in healthy controls [62], but this could also be shown for Hashimoto’s thyroiditis (HT) and subacute thyroiditis (SAT) [63,64,65]. There are mild forms of GD showing only minor ultrasound signs, no elevated vascularization, borderline TRAbs, and high antibodies against thyroid peroxidase (TPO) and/or human Thyroglobulin (hTg). In such cases, differentiation from destructive autoimmune thyroid disease—in particular HT, which can also present with a hypervascularization, and hormone release as the underlying cause for thyrotoxicosis is difficult without TS [3]. Scintigraphy reliably shows an elevated uptake even in mild forms of GD (Fig. 4.4c) vs. a low uptake in destructive thyroid disease [66]. However, the uptake must be related to the TSH value (“normalized uptake”) in order to become meaningfully interpreted. The only pitfall in this regard may be a recent (<3 months) iodine contamination resulting in a temporarily blocked scintigraphy in GD. CDUS is helpful for the evaluation of disease remission, recurrence, and response to treatment in patients with GD. However, in the early stage of disease, HT shows a diffuse increase in vascularization that decreases in the course of disease due to extensive fibrosis [3].
(a–c) A 30-year-old male presenting with weight loss (20 kg within a few weeks), nervousness, and finger trembling. Pulse rate at 120 bpm. On ultrasound (a) the thyroid gland is enlarged with reduced echogenicity—comparable to the overlying strap muscle. Vascularity of the thyroid gland is markedly increased (b). Severe hyperthyroidism (TSH suppressed, fT3 31 pg/mL) with high TRAb levels (25 IU/L). On scintigraphic imaging (c) there is a markedly increased uptake (11.5%) yielding a normalized uptake (% per mU/L TSH) approaching “infinity”
Autoimmune (destructive) thyroid disease as well as postpartal thyroiditis and silent thyroiditis are the most common causes for acquired hypothyroidism—besides thyroid surgery [67]. The ultrasound patterns of autoimmune thyroiditis are:
-
(markedly) hypoechoic and more or less heterogeneous parenchyma
-
(“Swiss cheese” or “honeycombing,” 1–6 mm) changes
-
Pseudomacronodular changes
-
Fibrotic (pseudolobulated) changes
-
Speckled appearance (infrequently)
-
Increased stiffness of the thyroid
Thyroid volume may be normal, increased (HT), or decreased (Ord’s disease). Ultrasound may detect perithyroidal satellite lymph nodes in patients with HT (e.g., “Delphian lymph node” above the cranial isthmus) [8, 65, 68].
The onset of autoimmune thyroiditis may be insidious or prompt. The latter often entails an initial interval (some weeks) of thyrotoxicosis as a result of hormone liberation from damaged thyroid follicles. Commonly, the insidious and the prompt form end up in hypothyroidism. TS mostly is not needed for the diagnosis of autoimmune thyroiditis since patients usually present with typical ultrasound and laboratory findings (TPO-antibodies, Tg-antibodies, hypothyroidism). A possible initial interval of thyrotoxicosis frequently goes unnoticed but can otherwise prompt confusion with GD. In this situation, TS is valuable to differentiate GD from early autoimmune thyroiditis by a high vs. a low uptake. As in GD, there are mild (e.g., silent thyroiditis) or even abortive forms of autoimmune thyroiditis (e.g., postpartum thyroiditis) with no recognizable ultrasound signs or without marked elevation of TPO-antibodies (and /or Tg-antibodies). If TSH elevation is borderline there may be doubt whether hypothyroidism is present. Any diagnosis—in favor or against a mild form of thyroiditis—is arbitrary in this situation and may rely on clinical symptoms and signs alone. Although not part of clinical routine work-up, TS may be helpful in selected cases by showing a lowered normalized uptake which reflects parenchymal damage versus a normal uptake in unaffected glands. However, when using TS in this case, high standardization of uptake calculation, site-specific normalized uptake ranges, and exclusion of iodine contamination are mandatory. Subacute thyroiditis (SAT, de Quervain’s thyroiditis, Fig. 4.5a–d) is of unclear origin, mostly occurring a few weeks after a viral infection—including COVID-19—and usually exhibiting a painful clinical course over weeks [69]. At the inflammatory stage, even ultrasound examination may be painful for the patient almost always showing rather large, irregularly confined hypoechoic lesions in an enlarged thyroid gland (Fig. 4.5a) and higher stiffness in UE. CDUS shows decreased flow in the hypoechoic areas (Fig. 4.5b). The disease often starts unilaterally but tends to involve both sides over time or to shift to the contralateral side. Within a few months, the ultrasound pattern and enlargement of the thyroid resolve with some strands of fibrosis reflecting the residual thyroid damage. Functionally, SAT mostly has a marked initial interval of thyrotoxicosis [8, 63, 70, 71]. In COVID-19-induced cases, an abortive even painless form of SAT may be seen [72]. If ultrasound features and clinical symptoms are equivocal, TS may be necessary to differentiate SAT from GD. Following the initial phase, a post-inflammatory decline in hormone production often gives rise to increased TSH secretion above normal but usually returns to normal levels within weeks. There is debate over how often a (slight) parenchymal damage after SAT may persist [70]. In individual patients, TS may help to detect thyroid damage on the basis of a decreased normalized uptake (Fig. 4.5c).
(a–d) Seventy-three-year-old male patient presenting with neck pain. TSH 0.01 mU/L, free T3 and free T4 normal, thyroid antibodies not elevated. (a) B-mode ultrasound (right thyroid lobe, transverse image) showing thyroid gland enlargement (volume right lobe: 17 mL, left lobe: 12 mL) and confluent hypoechoic areas in both lobes. (b) CDUS without increased vascularization. (c) Absent tracer uptake at thyroid scintigraphy. (d) Three months follow-up shows a hypoechoic thyroid shrunken to normal volume (right lobe: 5 mL, left lobe: 5 mL) and TSH 6.15 mU/L and low-free T3 2.35 pg/mL)
4.2.1.1 Diffuse Incidental Thyroid 18F-FDG-Uptake
Diffuse thyroid uptake (Fig. 4.6) is most often associated with benign disease corresponding to inflammatory uptake, e.g., in autoimmune thyroid disorders like HT or hyper-/hypothyroidism [73, 74]. For further clarification, a sonographic examination should be carried out. Sonographically, these patients often show characteristic diffuse heterogeneity; FNAC or intervention is generally not required. In addition, a correlation between thyroid function and the presence of antibodies against thyroid antigens is helpful.
(a–c) Diffusely increased 18F-FDG uptake in thyroid of 53-year-old woman referred for evaluation of melanoma. Patient is taking Levothyroxine 50 mcg/d. TSH 2.03 mU/L, TPO- and Tg-antibodies were elevated at 649 U/mL (reference range, <60 U/mL) and 66 U/mL (reference range, <29 U/mL) because of known Hashimoto’s thyroiditis. (a) Transaxial CT image, (b) transaxial fused PET/CT image, (c) maximum intensity projection image presented diffuse homogeneous tracer uptake, with SUVmax 6.7 in the thyroid gland
4.2.1.2 Nodular Thyroid Diseases
4.2.1.2.1 Autonomously Functioning Thyroid Nodules
As shown by a recent multicenter study, depending on the size, Autonomously Functioning Thyroid Nodules (AFTNs) may constitute up to 27% of all thyroid nodules in Germany [75]. The development of AFTNs is based on somatic mutations. In contrast to many other organs, the thyroid gland harbors a high spontaneous mutation rate which even increases in an environment of iodine deficiency or other goitrogens. As the mutation load increases, clones with positively activating mutations may outgrowth the surrounding thyroid tissue and give rise to AFTNs with elevated hormone production [76]. Since activated thyroid hormone production leads to an increased import of iodine by the NIS, AFTNs are readily detectable on TS as a “hot spot” (hyperfunctioning). If significant, the increased hormone synthesis in AFTNs leads to TSH downregulation which in turn reduces hormone synthesis in the non-autonomous thyroid tissue. However, TSH secretion by the pituitary gland is mostly not suppressed but normal or low normal [34, 77, 78]. Only, about one-half of AFTNs lead to TSH suppression which often is referred to as a “decompensated” AFTN [75, 78]. On B-mode ultrasound and CDUS, AFTNs may mimic suspicious nodules [33], including cases with a rather high vascularization centrally [79]. In other cases, AFTNs are unremarkable with a small rim-like perfusion [80]. AFTNs cannot be safely identified by thyroid elastography as shown in several studies. Ruhlmann et al. found that up to 70% of the examined AFTNs were rated as hard (i.e., suspicious) according to Rago and Asteria score. A more recent study by Trimboli et al. described that 45% of the AFTNs were hard on UE and 30% showed an intermediate classification [77, 81]. Another study by Ruhlmann and colleagues investigated whether UE can be used to predict the treatment response of AFTNs to radioiodine therapy. They found no significant difference between the groups of responders and non-responders for visual Rago classification [82]. CEUS is not routinely used for the diagnosis of autonomous thyroid nodules. AFTNs have a negligibly low malignancy risk—usually below 1% [28, 83]. Overall, AFTNs can be regarded as safely benign and further diagnostic work-up to exclude malignancy is not necessary. As mentioned above, AFTNs develop through mutations. However, in rare instances, a further—inactivating—mutation may occur which disrupts the production line for hormone synthesis. This small subgroup of AFTNs is referred to as “trapping only” nodules and constitutes about 5% of all AFTNs in the presence of a non-suppressed TSH. Trapping only nodules exhibit both an increased iodine import and a decreased organification/hormone synthesis. As a consequence, on 99mTc-pertechnetate scintigraphy and early phase (up to 4 h after injection) radioiodine scintigraphy trapping only nodules appear hyperfunctioning and indistinguishable from true AFTNs. On delayed radioiodine scintigraphies (e.g., 24 h), however, trapping only nodules become “cold” representing the decreased organification of iodine. Trapping only nodules is functionally not relevant and thus no candidate for radioiodine therapy. The small number of hyperfunctioning thyroid carcinomas is probably attributable to trapping only nodules, since such nodules harbor a significant risk of malignancy of about 30% [35]. The diagnosis of an AFTN should be made with care for its importance with regard to a safe exclusion of malignancy. For this reason, hyperfunctioning nodules with a disproportion between size and intensity on the one hand and TSH level on the other hand should be regarded as potential trapping only nodules. Scintigraphy with 123I− including late-phase scans 24 h after injection can be performed to demonstrate or rule out trapping only nodules. If no late-phase radioiodine scan is available, in a potential trapping only nodule, FNAC should be considered if suspicious sonographic features are present [See Fig. 4.7].
(a, b) (a) Female patient with a hypoechoic and solid thyroid nodule in the left lower lobe (10 × 10 × 14 mm, TSH 0.8 mU/L, left image: transverse orientation, right image: longitudinal orientation). (b) 123I− scintigraphy 60 min (left image) and 24 h (right image) after application of 12 MBq 123I− shows a focal tracer uptake in the left lower lobe representing a true AFTN, thus ruling out a trapping only nodule
Hyperfunctioning focal lesions in destructive thyroiditis mostly are not AFTNs but rather pseudonodules (“white knight”) [84, 85]. Often, they are the only place with preserved hormone synthesis rendering them hyperfunctioning on TS. As with trapping only nodules, further work-up should be considered in such nodules if suspicious sonographic features are present.
4.2.2 Imaging in the Risk Assessment of Thyroid Nodules
4.2.2.1 Ultrasound Risk Stratification Systems (B-Mode Ultrasound, UE, CEUS)
Many lesions within the thyroid gland can be diagnosed as almost safely benign on ultrasound. Cystic lesions often can be excluded from further work-up for risk assessment since thyroid carcinomas are predominantly solid in nature. This applies not only to pure cysts but also to cysts with a concentric solid part at the periphery or with some polygonal solid parts (ATA 2016) (Figs. 4.8 and 4.9). However, with an increasing solid component, evaluation of the solid parts according to Table 4.3 is necessary in order to further assess the risk of malignancy. A spongiform thyroid nodule is almost always benign. It is characterized by close proximity of numerous tiny cystic spaces with membranes in between and only sparse solid parts, giving it the ultrasound appearance of a sponge (Fig. 4.10). Spongiform nodules should also be excluded from further work-up for risk assessment.
In case of significant (e.g., >50%) solid parts within a thyroid lesion, a structured and consistent sonographic risk assessment is recommended according to Table 4.3. As a rule, the risk of malignancy for a thyroid nodule increases as the number of suspicious features increases. However, it is difficult to define the exact number or constellation of sonographic risk factors in order to sharply differentiate benign from malignant nodules since suspicious features are also shared by many benign nodules. According to a low prevalence of malignant thyroid nodes (0.1–1%) in primary care institutions, a higher number of risk factors or the addition of further modalities may be adequate as compared to third-level care centers with an a priori malignancy risk as high 20%.
Ultrasound risk stratification systems (US-RSSs) such as the Thyroid Imaging Reporting and Data System (TIRADS) are increasingly used in clinical routine for the risk assessment of thyroid nodules in order to select candidates for further diagnostic work-up and reduce unnecessary FNACs. With all US-RSSs the combination and/or the number of certain ultrasound criteria are documented (i.e., composition, echogenicity, margin, spots/calcification, and shape) because no single criterion alone is sufficiently predictive for malignancy [86] (Table 4.4).
The correct application and interpretation of these five ultrasound criteria for the assessment of malignancy risk in thyroid nodules is crucial for TIRADS categorization but is more sophisticated than it may look at first glance. The categorization of a nodule as solid is rather straightforward as it relies on the subjective estimation of the cystic proportion of a nodule. For labelling as “solid” a nodule should exhibit less than 10% cystic portions (ATA 2016: <5%). “Solidity” has a sensitivity for the detection of malignancy around 90% but a very low specificity of around 20% [87]. For this reason, solidity cannot serve as a sole criterion for indicating FNAC. Classifying a nodule as hypoechoic heavily relies on subjective assessment since many nodules are heterogenous in pattern, exhibit cystic parts which may lower overall echogenicity or show artifacts from e.g. calcifications. In addition, the assessment is complicated in thyroiditis such that the surrounding thyroid tissue is no longer representative of normal echogenicity. It is not surprising that the assessment of echogenicity has a high interobserver variability (kappa around 0.6) but can be somewhat improved by consensus meetings [88]. Irregular margins including microlobulation and external thyroid extension as a hint for malignancy is a rather inconstant feature of thyroid carcinomas (sensitivity around 50%) but is among the most specific criteria. However, it is difficult to discriminate irregular margins from more ill-defined margins in inflammatory lesions (e.g., SAT) or from macrolobulated margins in composite nodules—again resulting in a high interobserver variability. This variability can substantially be improved by consensus meetings. Microcalcifications are characteristic of papillary thyroid carcinoma and histologically most likely represent psammoma bodies. The assessment of microcalcifications is the most cumbersome among the ultrasound criteria for malignancy. Thus, it has the highest interobserver variability with a kappa of around 0.4 which virtually cannot be improved by consensus meetings [88]. In many nodules, even for specialists, it is difficult to discern microcalcifications from frequent similar findings in benign nodules such as colloidal spots, small macrocalcifications, scars, and ultrasound artifacts resulting from tiny membranes/crystals, etc. (Fig. 4.11).
(a, b) This nodule in the left thyroid lobe (a) shows signs of benignity such as a cystic portion >10% and a predominantly isoechogenic appearance of the solid component. However, it shows echogenic foci which may not readily allow discrimination from microcalcification. Whereas most of the foci exhibit no dorsal ultrasound phenomena one such focus does show (a clear dorsal reverberation artifact (“comet tail”) classifying it as a benign colloidal spot. The localization within a small cystic collection underlines the colloidal nature. With this spot in mind, the other echogenic spots in this nodule and in further nodules in the same lobe (b) should also be classified as most probably colloidal/benign. Otherwise, classification of such tiny hyperechoic foci may be difficult
A taller-than-wide shape of a nodule is defined by a nodule growth more into the depth than into the width on axial imaging. In some settings, this configuration is referred to as “non parallel orientation” in relation to the shape of the thyroid lobe. This antiparallel orientation has been associated with malignant growth [9]. Recently, however, it has been shown that up to 17% of benign nodules also show a taller-than-wide shape. It could be demonstrated that such benign nodules while growing often follow a dorsal protrusion of the thyroid lobe (e.g., Zuckerkandl’s tubercle). In this regard, they are still parallel with respect to the anatomically given preconditions (Fig. 4.12). A pole concept of nodule growth (Fig. 4.13) was introduced allowing to exclude the vast majority of taller-than-wide nodules from FNAC [89].
(a, b) This large nodule in the left lobe is solid, echonormal, without calcifications, and well circumscribed. Its lower part grows toward a posteroinferior direction making it taller than wide (1.6 cm × 2.5 cm, red arrows). Formally, the nodule shows two signs of malignancy (solidity and taller-than-wide shape) and should undergo FNAC. However, the posteroinferior direction of growth is typical for nodules at the posterior surface of the thyroid rendering this feature non-suspicious in this case. At a primary care center, one single sign of malignancy generally does not justify FNAC. Nevertheless, this hypofunctioning (b, 99mTc-pertechnetate scintigraphy) nodule underwent FNAC. FNAC was unremarkable showing regressive changes of otherwise normal follicular epithelium (Bethesda II)
Pole concept of goiter growth. Nodule growth follows the shape of the respective pole. A taller-than-wide configuration of nodules at a posterior or posterior/inferior horn should not be regarded as a suspicious feature without further hints of malignancy. Schematic depiction of nodule growth along the pole model
The different US-RSSs are divided into score-based (i.e., ACR TI-RADS and Kwak TIRADS), pattern-based (Horvath TIRADS, ATA Guideline), and mixed systems (EU-TIRADS, K-TIRADS). In combination with the size of the thyroid nodule, a recommendation for the next diagnostic steps is provided (i.e., FNAC or follow-up by ultrasound) [10, 11, 83, 84, 90, 91]. Many studies have investigated the diagnostic performance of the systems and all have been shown to be useful for risk stratification [92,93,94,95]. Migda et al. investigated the diagnostic performance of Kwak TIRADS in their meta-analysis including four retrospective and two prospective studies. They found sensitivities, specificities, and diagnostic odds ratios (DOR) of 97–99% (pooled sensitivity 98%), 16–91% (pooled specificity 55%), and 12–372 (pooled DOR 51) [96]. There are significant differences regarding the recommendation for FNAC. In this point, ACR TI-RADS seems to be superior compared to other systems [95, 97]. However, there are limitations that must be considered when using TIRADS. These include the missing integration of TS for functional assessment of the nodules (see below), the varying performance in different histologic subtypes of thyroid cancer (e.g., poor performance for follicular thyroid cancer), and interobserver variability (see above) [33, 88, 98].
As a complementary method, UE could help with risk stratification of thyroid nodules (Fig. 4.14). In their meta-analysis that included 14 prospective and one retrospective studies, Lin et al. reported sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for quantitative elastography (SWE and ARFI) of 84%, 88%, 28–45%, and 98–99% [99]. For the group of thyroid nodules with indeterminate results on FNAC, a meta-analysis on a total of 486 thyroid nodules from eight studies showed pooled sensitivity of 69%, specificity of 75%, PPV of 63%, and NPV of 82%, respectively. Thus, the accuracy of elastography appears to be lower for this subset of nodules, but the number of cases was very small [100]. The crucial parameter for the use of UE is the high NPV for a soft nodule, both for qualitative and quantitative elastography. Proper performance of the respective method for UE and knowledge of the underlying technical principles and limitations is essential. Typical limitations for UE are:
-
Presence of calcifications
-
Cystic changes (fluid is not compressible)
-
Unfavorable location of the nodule (i.e., dorsal position of the thyroid nodule)
(a–d) Twenty-seven-year-old female patient, TSH 1.18 mU/L, Calcitonin <1.0 pg/mL; (a) B-mode sonography (left: transverse orientation; right: longitudinal orientation) shows a solitary thyroid nodule in the lower left lobe (7 × 8 × 12 mm). EU-TIRADS 5 (hypoechoic and solid composition, taller-than-wide configuration). (b) Color Doppler ultrasound of the thyroid nodule shows no hypervascularization; (c) Strain elastography shows homogeneous green (soft) normal thyroid tissue and blue (hard) thyroid nodule. (d) 99mTc-pertechnetate scintigraphy shows a reduced tracer uptake in the lower left lobe (i.e., hypofunctioning thyroid nodule). Fine-needle aspiration cytology was non-diagnostic (Bethesda I). Histology revealed papillary thyroid cancer (pT1b pN0)
Another limitation is—comparable to TIRADS—that follicular thyroid cancer may be soft on UE and therefore lead to false negative results [21]. When comparing different elastographic methods, SWE proves to be less operator-dependent than SE in most studies [4, 20]. With CEUS, the enhancement of ultrasound contrast agent in the thyroid nodule compared to the paranodular tissue can be described. The qualitative parameters of enhancement include intensity, homogeneity, as well as retention of the contrast agent. Hypoenhancement (sensitivity 82%, specificity 85%, accuracy 84%) and a heterogeneous pattern (sensitivity 88%, specificity 93%, accuracy 90%) as well as early arterial central washout are suspicious for malignancy. The hypoenhancement of malignant nodules goes back to a lack of blood supply because of necrosis and embolus formation within the tumor. A ring enhancement pattern (sensitivity 83%, specificity 94%, accuracy 89%) is suggestive of a benign thyroid nodule. Additionally, quantitative parameters are evaluated including time-intensity curves using a region of interest in the thyroid nodule and comparison to the adjacent thyroid tissues [3, 101, 102]. Trimboli et al. included 1515 thyroid nodules with preoperative CEUS in their systematic review and meta-analysis. For the quantitative methods, the pooled sensitivity and specificity were found to be 85% and 82%, respectively, the pooled PPV was 83% and the NPV 85% [103]. Although performed as a single-center study, the results of a study by Xu et al. combining TIRADS and CEUS are promising in highly preselected cohorts (sensitivity 91%, specificity 90%, PPV 93%, and NPV 87%) [104]. However, to date no single feature has been found to be sufficiently sensitive or specific and CEUS is still an active field of research [101].
4.2.2.2 Value of 99mTc-Pertechnetate/123I−Scintigraphy
TS, in many guidelines, is no longer a mandatory part of assessing the malignancy risk of a thyroid nodule. Notably, ATA guidelines from 2015 mention TS as an auxiliary but not mandatory tool only in nodules accompanied by a low or lowered TSH [83]. The purpose of TS in such a setting is to virtually rule out malignancy in cases where a nodule is found to be hyperfunctioning on scintigraphy. However, in other countries (e.g., Germany), TS is recommended for all thyroid nodules at ≥1 cm in diameter [36]. How are these differences explicable regarding the purpose of TS? The answer may be found in epidemiologic statistics.
A merely ultrasound-based preselection of thyroid nodules for FNAC is derived from studies with a carcinoma-to-nodule ratio as high as 1:6 [84, 90]. Assessment by FNAC is recommended once a certain malignancy risk can be expected (i.e., PPV), e.g., 2–10% [ultrasound risk category 4a according to Kwak] or 7% [ultrasound risk category 4a according to Horvath]. At institutions with an a priori risk as high as above (1:6), e.g., at tertiary care units, such an ultrasound routine for FNAC preselection results in a reasonably low absolute number of false positive FNAC results (specificity 60–70%) and thus unnecessary surgeries. This is attributable to the rather high predictive values for malignancy of the ultrasound threshold used for indications for FNAC in such settings. In addition, the high sensitivity of FNAC (>90%) would prevent from overlooking a relevant number of thyroid carcinomas. In a non-preselected population, however, carcinoma-to-nodule ratios are as low as 1:1000 [49]. Recalculating the PPV of ultrasound risk category 4a according to Kwak, yields a value as low as roughly 1–2 per thousand for such low risk-settings, i.e., by a factor 20 or more lower as compared to the values from the original study. In such low-risk settings, adopting ultrasound thresholds for FNAC from the above studies with high risk would result in an overwhelming majority of the selected nodules being benign. According to the rather low specificity of FNAC, there would be a great number of unnecessary surgeries, e.g., about 200 per carcinoma vs. 3 per carcinoma in the above study settings. As a consequence, one would be urged to adjust ultrasound criteria toward a more restrictive application in order to reduce unnecessary surgeries but this, in turn, would increase the number of thyroid carcinomas being overlooked. For this reason, in primary care units, the preselection of thyroid nodules by ultrasound alone appears to be suboptimal. TS is necessary to rule out a significant percentage of thyroid nodules (about 25% in Germany) from further diagnostic work-up by the finding of a hyperfunctioning nodule. Such AFTNs harbor a negligible malignancy risk and generally need no cytological or histological examination [28]. In particular, as described previously, AFTNs can present with intermediate or highly suspicious ultrasound features. In the prospective study by Schenke et al., 58% of the AFTNs in the subgroup of euthyroid patients were classified as intermediate (4B) and high risk (4C, 5) according to Kwak TIRADS [33]. Although the rate of AFTNs in the cohort of 1029 euthyroid thyroid nodules retrospectively examined by Noto et al. was low (5% of all nodules), the rate of recommended FNAC for AFTNs for ATA guidelines, EU-TIRADS, and ACR TI-RADS was remarkable (64.7%, 43.1%, and 25.5% respectively). Also, none of the ultrasound features studied was associated with hyperfunctionality, emphasizing the importance of TS as a complementary diagnostic procedure in the diagnostic algorithm [105] (Fig. 4.15).
(a–c) This 60-year-old man has a solitary hypoechoic and solid nodule (10 × 11 × 12 mm) in the dorsal right thyroid lobe (a). The configuration of the nodule is slightly taller than wide which may be triggered by the gross protrusion of the thyroid lobe toward the back (b, EU-TIRADS 5). Formally, this nodule exhibits three of five ultrasound risk factors making FNAC necessary. However, on TS, the nodule is hyperfunctioning compared to the surrounding tissue (c) indicating an AFTN and obviating the need for FNAC. TSH was at 0.8 mU/L× meaning no functional relevance of the autonomy. The non-suppressed TSH as well as a normal normalized uptake (1.6% per mU/L TSH) are plausible owing to the small volume of the nodule and the only slightly elevated uptake therein
Even the exclusion of 25% of thyroid nodules from FNAC by identifying AFTNs on TS cannot profoundly reduce the number of unnecessary surgeries when adopting ultrasound criteria from a setting with high risk to a setting with low risk of malignancy. For this reason, TS has long been used in low-risk settings not only to rule out hyperfunctioning nodules but also to rule in hypofunctioning thyroid nodules for further work-up by FNAC. In nodules ≥1 cm in diameter, the sensitivity of a hypofunctioning nodule for malignancy is more than 80% [106]. The addition of a hypofunctioning nodule as a reason for FNAC thus compensates for the loss of sensitivity by a more restrictive use of ultrasound criteria. The finding of a hypofunctioning nodule, however, in conjunction with substantial cystic proportion should not per se lead to FNAC, since such a finding is readily explicable by the missing uptake in the fluid and thus has no predictive value.
4.2.2.3 Value of MIBI Imaging
Many studies have shown that an absent/low MIBI uptake in hypofunctioning thyroid nodules as well as a high washout of MIBI in semiquantitative analyses are predictive of a benign finding (high NPV) [40, 107]. On the other hand, if a higher uptake of MIBI in a nodule is detected, surgery is recommended to exclude malignancy; but, overall specificity is low, especially for unselected thyroid nodules [42]. To increase specificity it is recommended to select thyroid nodules for MIBI imaging by sonographic appearance (i.e., TIRADS intermediate or high-risk nodules), functional status (exclusion of AFTNs), and FNAC (i.e., indeterminate results) [41, 43, 108]. In a very recent study, the diagnostic performance of MIBI imaging was analyzed at 12 European study sites. The authors included 365 hypofunctioning thyroid nodules, that were classified as intermediate-risk (38%) or high-risk (62%) according to EU-TIRADS and had indeterminate results on FNAC. MIBI imaging evaluation was performed visually and semiquantitatively. As a main result of the study, the authors stated that a negative result on visual image evaluation can rule out malignancy with a very high probability (NPV 96% on planar and SPECT imaging). Additionally, the semiquantitative approach can improve the overall diagnostic performance by providing a more accurate differentiation between malignant and benign thyroid nodules (cutoff -19%: sensitivity 100%, specificity 89%, PPV 82%, NPV 100%, ACC 93%) [109] (Fig. 4.16).
(a–c) A 59-year-old female patient with multinodular goiter, TSH 0.50 mU/l, Calcitonin <1.0 pg/ml. (a) B-mode sonography showed two thyroid nodules (upper images: right central lobe, 14 x 10 x 15 mm, EU-TIRADS 3; lower images: left lower pole, 12x12x14 mm, EU-TIRADS 4). (c) 99mTc-pertechnetate scintigraphy with 64 MBq, both thyroid nodules were identified as hypofunctioning (black circles). (d) MIBI imaging: planar early image (left, 10 minutes p.i.) and late image (right, 60 minutes p.i.) show MIBI uptake in the thyroid nodule in the left lobe that visually decreases with time. Semiquantitative analysis reveals a washout index of −28% (MIBI negative). Note: The thyroid nodule in the right lobe is visually MIBI negative in the early and late images. Surgery revealed benign multinodular goiter
4.2.2.4 Incidental Focal Uptake on 18F-FDG Imaging
Focal thyroid incidental FDG uptake (FTI) is detected in 1–2% of patients and defined as an incidentaloma which is in contrast to diffuse uptake associated with a high risk of malignancy ranging 20–40%. A meta-analysis with 34 studies and 215,057 included patients showed a pooled risk of malignancy of 36.2% and a prevalence of FTI of 1.9%. No significant differences among iodine-deficient or iodine-sufficient countries and various geographic regions were found [110]. A systematic review of 125,754 patients without known thyroid disease had an unexpected focal hypermetabolic activity in 2.1%. Approximately one in three (~35%) revealed thyroid malignancy with a higher standardized uptake value (SUV) in malignancies compared to benign nodules (6.9 ± 4.7 vs. 4.8 ± 3.1, p < 0.001) [111]. SUV may be helpful in predicting malignant versus benign histology but in clinical settings a reliable discrimination is not always possible. Therefore, an ultrasound exam is recommended for patients with new focal thyroid lesions on 18F-FDG-PET to further assess thyroid tissue, depending on clinical and prognostic contexts. In this case, nodules ≥1 cm with focal 18F-FDG uptake require further work-up and nodules <1 cm should be monitored like thyroid nodules with a high-risk pattern in sonography [83, 112]. We suggest performing TS with 99mTc-pertechnetate or 123I− to detect AFTNs, rule out malignancy with a NPV of 96-99% [28], and preselect for FNAC. Additionally, in hypofunctioning nodules molecular imaging with 99mTc-MIBI may be useful in discriminating malignant from benign nodules.
4.2.2.5 Thyroid Nodules with Indeterminate Cytology
The management of cytologically indeterminate nodules represents a dilemma for thyreologists. The term indeterminate nodule summarizes the results of the FNAC for Bethesda categories III and IV [113]. On the one hand, a 15–40% risk of nodule malignancy leads to overtreatment in most patients when hemithyroidectomy is performed including the risk of operative complications. On the other hand, a conservative management is associated with a residual risk for malignancy. In recent years the utility of 18F-FDG-PET was examined in predicting the correct diagnosis of nodules with indeterminate cytology. The rationale for using the glucose analog FDG is that most malignant tumors characteristically have increased glucose utilization. This is in part related to the overexpression of GLUT glucose transporters and increased hexokinase activity.
In one recent systematic review and meta-analysis which included 225 patients a pooled sensitivity, specificity, PPV, NPV, and accuracy of 95%, 48%, 39%, 96%, and 60% were shown, respectively. Specifically, in patients who had nodules >15 mm sensitivity increased to 100%. A negative 18F-FDG-PET/CT examination can improve diagnostic accuracy in these patients and can reliably exclude T2 thyroid malignancies [114]. Vice versa, an 18F-FDG-PET-positivity did not identify malignant nodules with sufficient certainty, as approximately 50% of these patients had benign lesions. A prospective study in 87 patients compared the preoperative accuracy of 18F-FDG-PET/CT, ultrasound, and 99mTc-MIBI scan for thyroid nodules with indeterminate cytology. The authors showed that overall sensitivity (94%) and NPV (98%) were significantly higher than with ultrasound and 99mTc-MIBI imaging. Therefore, a negative 18F-FDG-PET/CT correctly predicts benign nodules and could prevent unnecessary (hemi)thyroidectomies. As in previous studies, 18F-FDG-PET/CT showed a low PPV (37%). Consequently, 18F-FDG-positive nodules should undergo (hemi)thyreoidectomy with histopathological confirmation [115]. Currently, 18F-FDG-PET/CT for preoperative risk assessment shows promising results but needs further evaluation and is not routinely recommended for cytologically indeterminate nodules.
In another preoperative comparison study in hypofunctioning thyroid nodules, in a limited number of patients (n = 23) with larger thyroid nodule size (median 32 mm diameter), a similar NPV and the same sensitivity in detecting malignant nodules was shown for 18F-FDG-PET/CT and MIBI scans. The authors suggest that 18F-FDG-PET/CT imaging is not superior to 99mTc-MIBI scintigraphy in differentiating thyroid nodules. For the reasons of costs and availability MIBI should thus be the first choice in preoperative evaluation of hypofunctioning thyroid nodules complementary to FNAC [116].
4.2.3 Medication-Induced Thyroid Dysfunction
The antiarrhythmic drug amiodarone is iodine-rich (a single 200 mg tablet contains 75 mg bound iodine and 7 mg free iodine) and lipophilic, thus accumulating in the thyroid gland. It causes thyroid dysfunction in 15–20% of treated patients: amiodarone-induced thyrotoxicosis (AIT), type 1 is an iodine-induced thyrotoxicosis in patients with promoting underlying thyroid abnormalities. Type 2 is a destructive thyroiditis with prolonged hormone liberation and mixed type. Type 1 is typically found in iodine-deficient areas whereas amiodarone-induced hypothyroidism, AIH, is more frequent in iodine-rich areas (Table 4.5).
Differentiation of the type of AIT is important because of the different treatments (Antithyroid drugs vs. oral glucocorticoids) [117, 119, 120]. Besides the laboratory findings, both, ultrasonography and nuclear medicine imaging have been used to confirm the diagnosis of amiodarone-associated thyroid dysfunctions. AIH often occurs in patients with preexisting chronic autoimmune thyroiditis according to typical structural changes on ultrasound. However, a normal appearance on ultrasound may be present. In AIT type 1 underlying (latent) GD or nodular goiter can be detected in thyroid ultrasound whereas AIT type 2 often presents with normal thyroid tissue [117]. Additional CDUS can be helpful by specifying different patterns of blood flow. Pattern 0 (absent vascularity) is associated with AIT type 2, and pattern 1 (uneven patchy parenchymal flow), 2 (diffuse, homogeneous distribution or increased flow, similar to GD), or 3 (marked increased signal and diffuse homogeneous distribution) are typically associated with AIT type 1 [121]. TS with 99mTc-pertechnetate mostly shows low or absent uptake owing to the high intrathyroidal content of iodine (“autoregulation”). In type 1 AIT, occasionally, there may be appreciable or normal uptake reflecting the underlying disease [117]. In case of unclear findings, molecular imaging with 99mTc-labelled MIBI can be used. This lipophilic cation complex accumulates in the mitochondria of viable cells and thus in hyperfunctioning thyroid tissue, i.e., toxic nodular goiter and GD, whereas MIBI uptake is reduced in type 2 destructive thyroiditis (Piga et al. 2008, Pattison et al. 2014). In their study of 20 patients with AIT, Piga et al. identified the visual MIBI uptake as the only method to differentiate AIT type 1 from AIT type 2 [44]. A more operator-independent method was published in 2018 by Censi and colleagues. They calculated a target-to-background ratio with a cut-off of 0.482 to differentiate AIT type 1 (> 0.482) from AIT type 2 with a specificity of 100% and a sensitivity of 91.7% [122].
Targeted cancer therapies with immune-checkpoint inhibitors (ICIs) and tyrosine kinase inhibitors (TKIs) play an important role in the therapy of various malignancies. However, during therapy, immune-related adverse effects (irAEs) are common including endocrine disorders (10%). Thyroid dysfunction is the most common endocrine irAE. The overall incidence of primary hypothyroidism and hyperthyroidism is reported as 8% and 3%, respectively. Typically, inflammatory destructive thyroiditis is the most common manifestation with an initial thyrotoxicosis followed by a hypothyroid phase. In rare cases, ICIs and TKI can cause autoimmune-mediated GD, also presenting with thyrotoxicosis in the initial course of the disease. Besides the typical course of thyroid dysfunction, thyroid ultrasound, and TS can help in the differential diagnosis, especially in cases with negative Trab (PD-1 inhibitors). Comparable to other causes of thyroiditis, the CDUS and 99mTc-pertechnetate uptake is decreased in the destructive form, whereas it is increased in autoimmune-mediated GD. CTLA-4 inhibitors are known to cause an inflammation of the pituitary gland (hypophysitis) that can lead to secondary hypothyroidism with the typical laboratory constellation of a low/normal TSH and low free T4. Also, hypophysitis and thyroiditis can be detected by 18F-FDG-PET/CT showing diffusely increased uptake that could serve as a surrogate prognostic marker during ICI and TKI treatments [123,124,125,126,127].
4.2.4 Aberrant Localization of Thyroid Tissue and Congenital Hypothyroidism
During embryonic development, the thyroid gland arises from endodermal tissue at the pharyngeal floor [128]. It subsequently descends through the foramen cecum at the tongue base, following the anterior trachea before ultimately separating into two lobes. Anywhere along this passage—the so-termed thyroglossal duct—ectopic thyroid tissue can persist. This may present as minimal cellular remnants detectable by scintigraphic imaging only or a macroscopic pyramidal lobe on structural imaging. More rarely cervical cysts remain patent along the thyroglossal duct baring the possibility of benign or malignant thyroid tissue growth over time. Embryonic remnants of thyroid cells at the tongue base can give rise to lingual struma clinically presenting with globus sensation and swallowing complaints in affected patients.
Occasionally, thyroid tissue may be found outside of its ontogenetic path. To make a distinction, such thyroid tissue is referred to as dystopic throughout this chapter as compared to ectopic tissue within the ontogenetic path and to orthotopic location of the thyroid gland next to the second to fifth tracheal ring. Dystopic thyroid tissue often occurs just below the lower poles of any of both thyroid lobes and may easily be overlooked on ultrasound. It is often nodular in nature and may be connected to the lower pole by a fibrous band. Ontogenetically, such dystopic tissue can be considered as having descended too far. It is frequently encountered in the upper anterior but also in the middle mediastinum and may give rise to intrathoracic goiter [129]. Dystopic intrathoracic goiter must be distinguished from extensive downward growth of an a priori orthotopic multinodular goiter. Both, bulky retrosternal goiter (arising from orthotopic goiter) as well as dystopic intrathoracic goiter may impair tracheal, esophageal, or vascular passage. However, as their blood supply differs, retrosternal goiter most often can be removed by a cervical approach, whereas intrathoracic goiter often requires sternotomy.
Rarely, dystopic thyroid tissue may be found within the trachea, within the heart, below the diaphragm or at other sites [130, 131]. Particular ontogenetic incidents may be causative, e.g., dislocation of thyroid cells during separation of the thyroid gland (see above) into the trachea or carriage of thyroid cells within the cardiac precursor tissue.
123I− is the radionuclide of choice when ectopic or dystopic thyroid tissue is suspected. Due to superior properties such as higher gamma energy (159 keV) compared to 99mTechnetium (140 keV), 123I− allows for better penetration of osseous structures and detection of retrosternal, intrathoracic and abdominal thyrogenous masses (Fig. 4.17). Sensitivity and specificity of scintigraphy increase if SPECT/CT hybrid imaging is available for anatomic correlation and attenuation correction [132]. Once sufficient iodine uptake to benign lesions is detected, radioiodine therapy is a therapeutic option. Successful treatment of both retrosternal and lingual strumae has been carried out with radioiodine therapy [133, 134]
123I− imaging; (a) planar image from anterior, (b) SPECT+MRI fusion image showing intense iodine uptake to a benign lingual struma in a 49-year-old patient presenting with swallowing complaints (after having undergone total thyroidectomy for multinodular goiter). The patient underwent partial tongue ground resection and radioiodine therapy
Further rare localization of thyroid tissue may be present in ovarian struma. First described by Boettlin et al. in the late nineteenth century, struma ovarii is classified as a rare form of mature teratoma with a predominant fraction of thyroid tissue on histologic assessment. Struma ovarii is mostly detected as an incidental finding after resection of a clinically manifest ovarian mass, in some cases incidental uptake to the ovary is seen in patients undergoing radioiodine therapy for differentiated thyroid cancer. Since up to 5% of all ovarian strumae exhibit features of malignancy histological work-up is warranted [135].
Congenital hypothyroidism is a heterogeneous disorder that can present with either eutopic, dystopic, or dysplastic thyroid tissues. Early diagnosis and treatment are essential to maintain regular neurological and cognitive development in affected infants [136]. Neonatal 123I− scintigraphy can be used to localize functional thyroid tissue in clinically suspected cases. Absent uptake is seen in thyroid aplasia, necessitating lifelong hormone substitution therapy. If 123I− uptake indicates the presence of thyroid tissue, congenital hypothyroidism can remain in a transient state [137].
4.2.5 Tracers Beyond 18F-FDG—Present and Future Directions
In some challenging cases, traditional radiotracers may fail in detecting the presence and extent of disease. Beyond 18F-FDG there are several other PET tracers available, which have been studied and described (Table 4.6). For their use in thyroid nodule risk assessment, there are almost only case reports or studies with a small number of patients.
However, the vast expanse of tumor microenvironment provides even more opportunities to exploit several other pathways. The relevant radiopharmaceuticals are listed in Table 4.6. Most PET radiopharmaceuticals are used for the detection of residual disease in malignancy and not for primary risk stratification.
124I− emits positrons and allows PET/CT or PET/MRI imaging in high resolution and for quantification. It is used as a diagnostic tool to localize disease and also for dosimetry which can be carried out for each individual tumor or metastatic focus without stunning effects [138].
The superiority of low-activity 124I-PET/low-dose CT in functional–morphological assessment of thyroid nodules has been recently showed. A multiobserver study to investigate 124I-PET/Ultrasound fusion imaging demonstrated more hyper- and hypofunctional nodules and less indifferent or not ratable nodules with additional use of 124I-PET/Ultrasound together with conventional imaging vs. using conventional imaging only (ultrasound, TS, and laboratory parameters). The authors showed a significantly lower suggestion of follow-up and more proposals of invasive treatments, e.g., FNAC and surgery [139]. 124I-PET/Ultrasound fusion improves the accuracy of the functional assessment of thyroid nodules even in unfavorable localizations and thus influences the suggested treatment for patients with ambiguous findings in conventional diagnostics.
18F-tetrafluoroborate (18F-TFB) is a promising iodide analog for PET imaging and has been recently proposed as a novel PET tracer for human NIS imaging [140]. 18F-TFB is retained but shows no thyroid organification. Therefore, 18F-TFB may show benefit in so-called “Thyreoglobulin Elevated and Negative Iodine Scintigraphy” (TENIS) while not requiring thyroid-stimulating hormone (TSH) stimulation to detect metastatic lesions after therapy. But clinical evaluation of 18F-TFB is needed in larger patient cohorts [141].
Since the 1990s somatostatin receptors (SSTRs) are known to play a role in regulation and proliferation of normal thyroid cells and tumor tissue [142]. In neuroendocrine tumors (NETs), SSTRs are often overexpressed on the surface of tumor cells. This has led to the development of different radiolabelled somatostatin analogs for diagnostics and/or therapy. Medullary thyroid carcinoma (MTC) is a neuroendocrine tumor arising from parafollicular C-cells of the thyroid glands. The experience in diagnostic performance with 68Ga-labelled DOTA peptides in preoperative MTC staging is very limited. In the situation of disease recurrence, 68Ga-DOTATATE-PET/CT showed promising results, especially in higher levels of tumor marker serum calcitonin [143]. Globally the diagnostic performance is inferior compared with other NETs and 68Ga DOTATATE-PET/CT performs inferior to 18F-DOPA-PET in recurrent MTC lesions [144].
18F-DOPA, which explores amino acid uptake, decarboxylation, and storage, when applied in MTC prior to surgery demonstrated a high sensitivity of 88% in detection of primary tumor sites and sensitivity in detection of central and lateral lymph node metastasis of 53% and 73%, compared to 20% and 39%, respectively, for ultrasonography [145]. At present, neither 18F-FDG-PET/CT nor 18F-DOPA-PET/CT is recommended for primary staging in MTC [146, 147].
PSMA-PET/CT imaging in prostate cancer has become widely established in diagnostics, especially in recurrence situations, due to the precise imaging of the tumor spread. PSMA is a type II transmembrane glycoprotein and is overexpressed in prostate cancer. PSMA could be expressed by vascular endothelium in other solid tumors such as thyroid carcinoma [148].
As 18F-FDG-PET, 68Ga-PSMA- or 18F-PSMA-PET harbor the capability of detecting thyroid lesions (PSMA thyroid incidentalomas - PTI). A systematic review demonstrated among 23 detected thyroid incidentalomas 5 proven primary thyroid carcinomas (4 papillary thyroid carcinomas, 1 follicular thyroid carcinoma) [149].
18F-choline PET/CT is mostly used for evaluation of prostate cancer. But an increased cell membrane choline metabolism can also be seen in other conditions like in patients with primary hyperparathyroidism and thyroid incidentalomas.
In a retrospective study, the authors identified 9 incidentalomas, 8 of them underwent further investigation and two cases were revealed to be malignant [150].
The percentage of carcinomas identified with choline PET (25%) is similar to FDG-PET (~35%).
However, it must be mentioned that PSMA and choline radiotracers are mainly used in men with prostate cancer and therefore women have so far not been included in these analyses.
Fibroblast activation protein (FAP) is expressed in the stroma of many malignancies and histopathological studies have shown FAP-positive cancer-associated fibroblasts in over 90% of epithelial tumors. FAP inhibitors (FAPIs) specifically bind to the enzymatic domain of FAP with a rapid and almost complete internalization. FAPI-based PET imaging has yielded high uptake and image contrast in several cancers. FAPI as a novel tracer may be helpful in applications from noninvasive tumor characterization up to radioligand therapy. In differentiated thyroid cancers only low-to-moderate FAPI uptake was observed [151]. Currently, major issues are the complementary use of 18F-FDG-PET/CT in localizing disease recurrence and detecting metastatic lesions in patients with “Thyreoglobulin Elevated and Negative Iodine Scintigraphy” (TENIS) [152].
Change history
21 September 2023
A correction has been published.
References
Fujimoto Y, Oka A, Omoto R, Hirose M. Ultrasound scanning of the thyroid gland as a new diagnostic approach. Ultrasonics. 1967;5:177–80.
Chan V, Perlas A. Basics of ultrasound imaging. In: Atlas of ultrasound-guided procedures in interventional pain management. New York: Springer Science + Business Media; 2011. p. 13–9.
Chung J, Lee YJ, Choi YJ, Ha EJ, Suh CH, Choi M, et al. Clinical applications of Doppler ultrasonography for thyroid disease: consensus statement by the Korean Society of Thyroid Radiology. Ultrasonography. 2020;39(4):315–30.
Zhao CK, Xu HX. Ultrasound elastography of the thyroid: principles and current status. Ultrasonography. 2019;38(2):106–24.
Yuen HY, Wong KT, Ahuja AT. Sonography of diffuse thyroid disease. Australas J Ultrasound Med. 2016;19(1):13–29.
Dighe M, Barr R, Bojunga J, Cantisani V, Chammas MC, Cosgrove D, et al. Thyroid ultrasound: state of the art part 1 - thyroid ultrasound reporting and diffuse thyroid diseases. Med Ultrason. 2017;19(1):79–93.
Dietlein M, Dressler J, Grünwald F, Joseph K, Leisner B, Moser E, et al. Leitlinie zur Schilddrüsendiagnostik - Deutsche Gesellschaft für Nuklearmedizin e.V. [Internet] Deutsche Gesellschaft für Nuklearmedizin eV 2022 [zitiert 18. August 2022]. Verfügbar unter: http://nuklearmedizin.de/leistungen/leitlinien/html/schild_diagn.php?navId=53.
Chaudhary V, Bano S. Thyroid ultrasound. Indian J Endocrinol Metab. 2013;17(2):219–27.
Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean society of thyroid radiology consensus statement and recommendations. Korean J Radiol. 2016;17(3):370–95.
Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J. 2017;6(5):225–37.
Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS Committee. J Am Coll Radiol. 2017;14(5):587–95.
Seifert P, Maikowski I, Winkens T, Kühnel C, Gühne F, Drescher R, et al. Ultrasound cine loop standard operating procedure for benign thyroid diseases—evaluation of non-physician application. Diagnostics. 2021;11(1):67.
Köhler M, Gomes Ataide EJ, Ziegle J, Boese A, Friebe M. Novel assistive device for tomographic ultrasound neck imaging vs. freehand. Curr Dir Biomed Eng. 2020;6(3):20203008.
Freesmeyer M, Winkens T, Kühnel C, Opfermann T, Seifert P. Technetium-99m SPECT/US hybrid imaging compared with conventional diagnostic thyroid imaging with scintigraphy and ultrasound. Ultrasound Med Biol. 2019;45(5):1243–52.
Seifert P, Winkens T, Kühnel C, Gühne F, Freesmeyer M. I-124-PET/US fusion imaging in comparison to conventional diagnostics and Tc-99m Pertechnetate SPECT/US fusion imaging for the function assessment of thyroid nodules. Ultrasound Med Biol. 2019;45(9):2298–308.
Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29(01):28–44.
Radzina M, Ratniece M, Putrins DS, Saule L, Cantisani V. Performance of contrast-enhanced ultrasound in thyroid nodules: review of current state and future perspectives. Cancers. 2021;13(21):5469.
Clark KJ, Cronan JJ, Scola FH. Color Doppler sonography: anatomic and physiologic assessment of the thyroid. J Clin Ultrasound. 1995;23(4):215–23.
Ophir J, Céspedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: A quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13(2):111–34.
Bamber J, Cosgrove D, Dietrich C, Fromageau J, Bojunga J, Calliada F, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound Elastography. Part 1: basic principles and technology. Ultraschall Med. 2013;34(02):169–84.
Cantisani V, Grazhdani H, Drakonaki E, D’Andrea V, Di Segni M, Kaleshi E, et al. Strain US Elastography for the characterization of thyroid nodules: advantages and limitation. Int J Endocrinol. 2015;2015:908575.
Asteria C, Giovanardi A, Pizzocaro A, Cozzaglio L, Morabito A, Somalvico F, et al. US-elastography in the differential diagnosis of benign and malignant thyroid nodules. Thyroid. 2008;18(5):523–31.
Rago T, Scutari M, Santini F, Loiacono V, Piaggi P, Di Coscio G, et al. Real-time elastosonography: useful tool for refining the presurgical diagnosis in thyroid nodules with indeterminate or nondiagnostic cytology. J Clin Endocrinol Metab. 2010;95(12):5274–80.
Cosgrove D, Barr R, Bojunga J, Cantisani V, Chammas MC, Dighe M, et al. WFUMB guidelines and recommendations on the clinical use of ultrasound Elastography: part 4. Thyroid. Ultrasound Med Biol. 2017;43(1):4–26.
Cantisani V, Lodise P, Grazhdani H, Mancuso E, Maggini E, Di Rocco G, et al. Ultrasound elastography in the evaluation of thyroid pathology. Current status. Eur J Radiol. 2014;83(3):420–8.
Giusti M, Orlandi D, Melle G, Massa B, Silvestri E, Minuto F, et al. Is there a real diagnostic impact of elastosonography and contrast-enhanced ultrasonography in the management of thyroid nodules? J Zhejiang Univ Sci B. 2013;14(3):195–206.
Mahlstedt J, Schmidt H, Joseph K. Untersuchungen zur Verläßlichkeit des 99m Tc-Speichertests als Schätzer der thyreoidalen Stimulation. RöFo - Fortschritte Auf Dem Geb Röntgenstrahlen Bildgeb Verfahr. 1979;131(11):536–44.
Giovanella L, Avram AM, Iakovou I, Kwak J, Lawson SA, Lulaj E, et al. EANM practice guideline/SNMMI procedure standard for RAIU and thyroid scintigraphy. Eur J Nucl Med Mol Imaging. 2019;46(12):2514–25.
Meng W. 333460392X - Schilddrüsenerkrankungen. Pathophysiologie, Diagnostik, Therapie - Meng, Wieland [Internet]. 3. Aufl. Jena: Gustav Fischer Verlag GmbH; 1992 [zitiert 21. März 2022]. Verfügbar unter: https://www.eurobuch.com/buch/isbn/333460392X.html.
Vassart G, Pardo L, Costagliola S. A molecular dissection of the glycoprotein hormone receptors. Trends Biochem Sci. 2004;29(3):119–26.
Atkins HL. Technetium-99m pertechnetate uptake and scanning in the evaluation of thyroid function. Semin Nucl Med. 1971;1(3):345–55.
Stahl A, Nagel H, Negele T. Schilddrüse-Atlas.info [Internet]. 2.Auflage. München: Nuk-Verlag; 2021 [zitiert 10. Juli 2022]. Verfügbar unter: https://nuk-verlag.de.
Schenke S, Seifert P, Zimny M, Winkens T, Binse I, Goerges R. Risk stratification of thyroid nodules using thyroid imaging reporting and data system (TIRADS): the omission of thyroid scintigraphy increases the rate of falsely suspected lesions. J Nucl Med. 2019;60:342.
Görges R, Kandror T, Kuschnerus S, Zimny M, Pink R, Palmedo H, et al [Scintigraphically „hot “thyroid nodules mainly go hand in hand with a normal TSH]. Nuklearmedizin. 2011;50(5):179–88.
Reschini E, Ferrari C, Castellani M, Matheoud R, Paracchi A, Marotta G, et al. The trapping-only nodules of the thyroid gland: prevalence study. Thyroid. 2006;16(8):757–62.
Dietlein M, Eschner W, Lassmann M, Verburg FA, Luster M. Procedure guideline for thyroid scintigraphy (version 4) 2014. 2014. Verfügbar unter: www.nuklearmedizin.de/leistungen/leitlinien/docs/031-011I_S1_Schiilddrüsenszintigraphie_2014-10.pdf.
Rager O, Radojewski P, Dumont RA, Treglia G, Giovanella L, Walter MA. Radioisotope imaging for discriminating benign from malignant cytologically indeterminate thyroid nodules. Gland Surg. 2019;8(Suppl 2):S118–25.
Moretti JL, Hauet N, Caglar M, Rebillard O, Burak Z. To use MIBI or not to use MIBI? That is the question when assessing tumour cells. Eur J Nucl Med Mol Imaging. 2005;32(7):836–42.
Sarikaya A, Huseyinova G, Irfanoğlu ME, Erkmen N, Cermik TF, Berkarda S. The relationship between 99Tc(m)-sestamibi uptake and ultrastructural cell types of thyroid tumours. Nucl Med Commun. 2001;22(1):39–44.
Schmidt M, Schenke S. Update 2019 zur MIBI-Szintigrafie bei hypofunktionellen Schilddrüsenknoten. Nuklearmedizin. 2019;42:174–82.
Treglia G, Caldarella C, Saggiorato E, Ceriani L, Orlandi F, Salvatori M, et al. Diagnostic performance of (99m)Tc-MIBI scan in predicting the malignancy of thyroid nodules: a meta-analysis. Endocrine. 2013;44(1):70–8.
Kim SJ, Lee SW, Jeong SY, Pak K, Kim K. Diagnostic performance of technetium-99m methoxy-isobutyl-Isonitrile for differentiation of malignant thyroid nodules: a systematic review and meta-analysis. Thyroid. 2018;28(10):1339–48.
Campennì A, Siracusa M, Ruggeri RM, Laudicella R, Pignata SA, Baldari S, et al. Differentiatinjg malignant from benign thyroid nodules with indeterminate cytology by 99mTc-MIBI scan: a new quantitative method for improving diagnostic accuracy. Sci Rep. 2017;7(1):6147.
Piga M, Cocco MC, Serra A, Boi F, Loy M, Mariotti S. The usefulness of 99mTc-sestaMIBI thyroid scan in the differential diagnosis and management of amiodarone-induced thyrotoxicosis. Eur J Endocrinol. 2008;159(4):423–9.
Choudhury PS, Gupta M. Differentiated thyroid cancer theranostics: radioiodine and beyond. Br J Radiol. 2018;91(1091):20180136.
Bekanntmachung der aktualisierten diagnostischen Referenzwerte für nuklearmedizinische Untersuchungen vom 15. 2021, p. 8.
Wahl RL. Targeting glucose transporters for tumor imaging: “sweet” idea, “sour” result. J Nucl Med. 1996;37(6):1038–41.
Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–54.
Guth S, Theune U, Aberle J, Galach A, Bamberger CM. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Investig. 2009;39(8):699–706.
Carlé A, Krejbjerg A, Laurberg P. Epidemiology of nodular goitre. Influence of iodine intake. Best Pract Res Clin Endocrinol Metab. 2014;28(4):465–79.
Knudsen N, Brix TH. Genetic and non-iodine-related factors in the aetiology of nodular goitre. Best Pract Res Clin Endocrinol Metab. 2014;28(4):495–506.
Spelsberg F, Negele T, Ritter MM. Die Schilddrüse in Klinik und Praxis. Heidelberg: Karl F. Haug Fachbuchverlag; 2000.
Kraiem Z, Glaser B, Yigla M, Pauker J, Sadeh O, Sheinfeld M. Toxic multinodular Goiter: A variant of autoimmune hyperthyroidism*. J Clin Endocrinol Metab. 1987;65(4):659–64.
Meller J, Jauho A, Hüfner M, Gratz S, Becker W. Disseminated thyroid autonomy or graves’ disease: reevaluation by a second generation TSH receptor antibody assay. Thyroid. 2000;10(12):1073–9.
Reinhardt MJ, Joe A, von Mallek D, Zimmerlin M, Manka-Waluch A, Palmedo H, et al. Dose selection for radioiodine therapy of borderline hyperthyroid patients with multifocal and disseminated autonomy on the basis of 99mTc-pertechnetate thyroid uptake. Eur J Nucl Med Mol Imaging. 2002;29(4):480–5.
Meller J, Becker W. The continuing importance of thyroid scintigraphy in the era of high-resolution ultrasound. Eur J Nucl Med Mol Imaging. 2002;29(S2):S425–38.
Giovanella L, D’Aurizio F, Campenni’ A, Ruggeri RM, Baldari S, Verburg FA, et al. Searching for the most effective thyrotropin (TSH) threshold to rule-out autonomously functioning thyroid nodules in iodine deficient regions. Endocrine. 2016;54(3):757–61.
Happel C, Kranert WT, Bockisch B, Korkusuz H, Grünwald F. 131I- und 99mTc-Uptake in fokalen Schilddrüsenautonomien: Entwicklung in Deutschland seit den 1980er Jahren. Nuklearmedizin. 2016;55(06):236–41.
Gotthardt M, Stübinger M, Pansegrau J, Buchwald B, Goecke J, Pfestroff A, et al. Decrease of (99m)Tc-uptake in autonomous thyroid tissue in Germany since the 1970s. Clinical implications for radioiodine therapy. Nukl Nucl. 2006;45(3):122–5.
Vassaux G, Zwarthoed C, Signetti L, Guglielmi J, Compin C, Guigonis JM, et al. Iodinated contrast agents perturb iodide uptake by the thyroid independently of free iodide. J Nucl Med. 2018;59(1):121–6.
Antonelli A, Ferrari SM, Ragusa F, Elia G, Paparo SR, Ruffilli I, et al. Graves’ disease: epidemiology, genetic and environmental risk factors and viruses. Best Pract Res Clin Endocrinol Metab. 2020;34(1):101387.
Kılınçer A, Durmaz MS, Baldane S, Kıraç CO, Cebeci H, Koplay M. Evaluation of the stiffness of thyroid parenchyma with shear wave Elastography using a free-region of interest technique in graves disease. J Ultrasound Med. 2020;40:471.
Xie P, Xiao Y, Liu F. Real-time ultrasound elastography in the diagnosis and differential diagnosis of subacute thyroiditis. J Clin Ultrasound. 2011;39(8):435–40.
Ruchala M, Szczepanek-Parulska E, Zybek A, Moczko J, Czarnywojtek A, Kaminski G, et al. The role of sonoelastography in acute, subacute and chronic thyroiditis: a novel application of the method. Eur J Endocrinol. 2012;166(3):425–32.
Kara T, Ateş F, Durmaz MS, Akyürek N, Durmaz FG, Özbakır B, et al. Assessment of thyroid gland elasticity with shear-wave elastography in Hashimoto’s thyroiditis patients. J Ultrasound. 2020;23(4):543–51.
Ramtoola S, Maisey MN, Clarke SEM, Fogelman I. The thyroid scan in Hashimotoʼs thyroiditis: the great mimic. Nucl Med Commun. 1988;9(9):639–46.
Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550–62.
Lupo MA, Levine RA. Ultrasound of diffuse thyroid enlargement: thyroiditis. In: Jack Baskin Sr H, Duick DS, Levine RA, Thyroid ultrasound and ultrasound-guided FNA. New York, NY: Springer; 2013. [zitiert 22. März 2022]. S. 99–125. Verfügbar unter: https://doi.org/10.1007/978-1-4614-4785-6_6.
Stasiak M, Lewiński A. New aspects in the pathogenesis and management of subacute thyroiditis. Rev Endocr Metab Disord. 2021;22(4):1027–39.
Schenke S, Klett R, Braun S, Zimny M. Are there predictive factors for long-term hormone-replacement? Nuklearmedizin. 2013;52(4):137–40.
Giovanella L, Ruggeri RM, Ovčariček PP, Campenni A, Treglia G, Deandreis D. Prevalence of thyroid dysfunction in patients with COVID-19: a systematic review. Clin Transl Imaging. 2021;9(3):233–40.
Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after Sars-COV-2 infection. J Clin Endocrinol Metab. 2020;105(7):2367–70.
Yasuda S, Shohtsu A, Ide M, Takagi S, Takahashi W, Suzuki Y, et al. Chronic thyroiditis: diffuse uptake of FDG at PET. Radiology. 1998;207(3):775–8.
Kurata S, Ishibashi M, Hiromatsu Y, Kaida H, Miyake I, Uchida M, et al. Diffuse and diffuse-plus-focal uptake in the thyroid gland identified by using FDG-PET: prevalence of thyroid cancer and Hashimoto’s thyroiditis. Ann Nucl Med. 2007;21(6):325–30.
Schenke SA, Kreissl MC, Grunert M, Hach A, Haghghi S, Kandror T, et al. Distribution of functional status of thyroid nodules and malignancy rates of hyperfunctioning and hypofunctioning thyroid nodules in Germany. Nuklearmedizin. 2022;61:376.
Krohn K, Wohlgemuth S, Gerber H, Paschke R. Hot microscopic areas of iodine-deficient euthyroid goitres contain constitutively activating TSH receptor mutations. J Pathol. 2000;192(1):37–42.
Trimboli P, Paone G, Zatelli MC, Ceriani L, Giovanella L. Real-time elastography in autonomously functioning thyroid nodules: relationship with TSH levels, scintigraphy, and ultrasound patterns. Endocrine. 2017;58(3):488–94.
Treglia G, Trimboli P, Verburg FA, Luster M, Giovanella L. Prevalence of normal TSH value among patients with autonomously functioning thyroid nodule. Eur J Clin Investig. 2015;45(7):739–44.
Reschke K, Klose S, Kopf D, Lehnert H. Role of ultrasound in the diagnosis of thyroid autonomy. Exp Clin Endocrinol Diabetes. 1998;106(Suppl 04):S42–4.
Ianni F, Perotti G, Prete A, Paragliola RM, Ricciato MP, Carrozza C, et al. Thyroid scintigraphy: an old tool is still the gold standard for an effective diagnosis of autonomously functioning thyroid nodules. J Endocrinol Invest. 2013;36:233. https://doi.org/10.3275/8471.
Ruhlmann M, Stebner V, Görges R, Farahati J, Simon D, Bockisch A, et al. Diagnosis of hyperfunctional thyroid nodules: impact of US-elastography. Nuklearmedizin. 2014;53(5):173–7.
Ruhlmann M, Stebner V, Görges R, Bockisch A, Rosenbaum-Krumme SJ, Nagarajah J. Ultrasound-elastography for predicting response of hyperfunctional thyroid nodules to radioiodine therapy: initial results. Austin J Nucl Med Radiother. 2016;3(2):01–4.
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
Horvath E, Majlis S, Rossi R, Franco C, Niedmann JP, Castro A, et al. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab. 2009;94(5):1748–51.
Durfee SM, Benson CB, Arthaud DM, Alexander EK, Frates MC. Sonographic appearance of thyroid cancer in patients with Hashimoto thyroiditis. J Ultrasound Med. 2015;34(4):697–704.
Remonti LR, Kramer CK, Leitão CB, Pinto LCF, Gross JL. Thyroid ultrasound features and risk of carcinoma: a systematic review and meta-analysis of observational studies. Thyroid. 2015;25(5):538–50.
Seifert P, Schenke S, Zimny M, Stahl A, Grunert M, Klemenz B, Diagnostic Performance of Kwak, EU, ACR, and Korean TIRADS, et al. As well as ATA guidelines for the ultrasound risk stratification of non-autonomously functioning thyroid nodules in a region with long history of iodine deficiency: a German multicenter trial. Cancers. 2021;13(17):4467.
Seifert P, Görges R, Zimny M, Kreissl MC, Schenke S. Interobserver agreement and efficacy of consensus reading in Kwak-, EU-, and ACR-thyroid imaging recording and data systems and ATA guidelines for the ultrasound risk stratification of thyroid nodules. Endocrine. 2020;67(1):143–54.
Petersen M, Schenke SA, Zimny M, Görges R, Grunert M, Groener D, et al. Introducing a pole concept for nodule growth in the thyroid gland: taller-than-wide shape, frequency, location and risk of malignancy of thyroid nodules in an area with iodine deficiency. J Clin Med. 2022;11(9):2549.
Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. 2011;260(3):892–9.
Ha EJ, Chung SR, Na DG, Ahn HS, Chung J, Lee JY, et al. 2021 Korean thyroid imaging reporting and data system and imaging-based management of thyroid nodules: Korean Society of Thyroid radiology consensus statement and recommendations. Korean J Radiol. 2021;22(12):2094.
Castellana M, Castellana C, Treglia G, Giorgino F, Giovanella L, Russ G, et al. Performance of five ultrasound risk stratification systems in selecting thyroid nodules for FNA. A meta-analysis. J Clin Endocrinol Metab. 2020;105:dgz170.
Chung SR, Ahn HS, Choi YJ, Lee JY, Yoo RE, Lee YJ, et al. Diagnostic performance of the modified Korean thyroid imaging reporting and data system for thyroid malignancy: a multicenter validation study. Korean J Radiol. 2021;22(9):1579.
Kim PH, Suh CH, Baek JH, Chung SR, Choi YJ, Lee JH. Unnecessary thyroid nodule biopsy rates under four ultrasound risk stratification systems: a systematic review and meta-analysis. Eur Radiol. 2021;31(5):2877–85.
Middleton WD, Teefey SA, Reading CC, Langer JE, Beland MD, Szabunio MM, et al. Comparison of performance characteristics of American College of Radiology TI-RADS, Korean Society of Thyroid Radiology TIRADS, and American Thyroid Association guidelines. AJR Am J Roentgenol. 2018;210(5):1148–54.
Migda B, Migda M, Migda MS, Slapa RZ. Use of the Kwak thyroid image reporting and data system (K-TIRADS) in differential diagnosis of thyroid nodules: systematic review and meta-analysis. Eur Radiol. 2018;28(6):2380–8.
Xu T, Wu Y, Wu RX, Zhang YZ, Gu JY, Ye XH, et al. Validation and comparison of three newly-released thyroid imaging reporting and data systems for cancer risk determination. Endocrine. 2019;64(2):299–307.
Schenke SA, Klett R, Wagner PR, Mott S, Zimny M, Feek U, et al. Characteristics of different histological subtypes of thyroid nodules classified with 99mTc-methoxy-isobutyl-isonitrile imaging and thyroid imaging reporting and data system. Nucl Med Commun. 2021;42(1):73–80.
Lin P, Chen M, Liu B, Wang S, Li X. Diagnostic performance of shear wave elastography in the identification of malignant thyroid nodules: a meta-analysis. Eur Radiol. 2014;24(11):2729–38.
Trimboli P, Treglia G, Sadeghi R, Romanelli F, Giovanella L. Reliability of real-time elastography to diagnose thyroid nodules previously read at FNAC as indeterminate: a meta-analysis. Endocrine. 2015;50(2):335–43.
Sidhu P, Cantisani V, Dietrich C, Gilja O, Saftoiu A, Bartels E, et al. The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (long version). Ultraschall Med. 2018;39(02):e2–44.
Schleder S, Janke M, Agha A, Schacherer D, Hornung M, Schlitt HJ, et al. Preoperative differentiation of thyroid adenomas and thyroid carcinomas using high resolution contrast-enhanced ultrasound (CEUS). Clin Hemorheol Microcirc. 2015;61(1):13–22.
Trimboli P, Castellana M, Virili C, Havre RF, Bini F, Marinozzi F, et al. Performance of contrast-enhanced ultrasound (CEUS) in assessing thyroid nodules: a systematic review and meta-analysis using histological standard of reference. Radiol Med. 2020;125(4):406–15.
Xu Y, Qi X, Zhao X, Ren W, Ding W. Clinical diagnostic value of contrast-enhanced ultrasound and TI-RADS classification for benign and malignant thyroid tumors: one comparative cohort study. Medicine (Baltimore). 2019;98(4):e14051.
Noto B, Eveslage M, Pixberg M, Gonzalez Carvalho JM, Schäfers M, Riemann B, et al. Prevalence of hyperfunctioning thyroid nodules among those in need of fine needle aspiration cytology according to ATA 2015, EU-TIRADS, and ACR-TIRADS. Eur J Nucl Med Mol Imaging. 2020;47(6):1518–26.
Seifert P, Freesmeyer M. Preoperative diagnostics in differentiated thyroid carcinoma. Nuklearmedizin. 2017;56(6):201–10.
Wale A, Miles KA, Young B, Zammit C, Williams A, Quin J, et al. Combined (99m)Tc-methoxyisobutylisonitrile scintigraphy and fine-needle aspiration cytology offers an accurate and potentially cost-effective investigative strategy for the assessment of solitary or dominant thyroid nodules. Eur J Nucl Med Mol Imaging. 2014;41(1):105–15.
Giovanella L, Campenni A, Treglia G, Verburg FA, Trimboli P, Ceriani L, et al. Molecular imaging with (99m)Tc-MIBI and molecular testing for mutations in differentiating benign from malignant follicular neoplasm: a prospective comparison. Eur J Nucl Med Mol Imaging. 2016;43(6):1018–26.
Schenke SA, Campennì A, Tuncel M, Bottoni G, Sager S, Bogovic Crncic T, et al. Diagnostic performance of 99mTc-Methoxy-Isobuty-Isonitrile (MIBI) for risk stratification of hypofunctioning thyroid nodules: a European Multicenter Study. Diagnostics. 2022;12(6):1358.
Treglia G, Bertagna F, Sadeghi R, Verburg FA, Ceriani L, Giovanella L. Focal thyroid incidental uptake detected by 18F-fluorodeoxyglucose positron emission tomography: meta-analysis on prevalence and malignancy risk. Nuklearmedizin. 2013;52(04):130–6.
Soelberg KK, Bonnema SJ, Brix TH, Hegedüs L. Risk of malignancy in thyroid Incidentalomas detected by 18F-fluorodeoxyglucose positron emission tomography: a systematic review. Thyroid. 2012;22(9):918–25.
Hoang JK, Langer JE, Middleton WD, Wu CC, Hammers LW, Cronan JJ, et al. Managing incidental thyroid nodules detected on imaging: white paper of the ACR Incidental Thyroid Findings Committee. J Am Coll Radiol. 2015;12(2):143–50.
Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27(11):1341–6.
Vriens D, de Wilt JHW, van der Wilt GJ, Netea-Maier RT, Oyen WJG, de Geus-Oei LF. The role of [18F]-2-fluoro-2-deoxy-d-glucose-positron emission tomography in thyroid nodules with indeterminate fine-needle aspiration biopsy: systematic review and meta-analysis of the literature. Cancer. 2011;117(20):4582–94.
Piccardo A, Puntoni M, Treglia G, Foppiani L, Bertagna F, Paparo F, et al. Thyroid nodules with indeterminate cytology: prospective comparison between 18F-FDG-PET/CT, multiparametric neck ultrasonography, 99mTc-MIBI scintigraphy and histology. Eur J Endocrinol. 2016;174(5):693–703.
Sager S, Vatankulu B, Erdogan E, Mut S, Teksoz S, Ozturk T, et al. Comparison of F-18 FDG-PET/CT and Tc-99m MIBI in the preoperative evaluation of cold thyroid nodules in the same patient group. Endocrine. 2015;50(1):138–45.
Bartalena L, Bogazzi F, Chiovato L, Hubalewska-Dydejczyk A, Links TP, Vanderpump M. 2018 European thyroid association (ETA) guidelines for the management of amiodarone-associated thyroid dysfunction. Eur Thyroid J. 2018;7(2):55–66.
Colunga Biancatelli RM, Congedo V, Calvosa L, Ciacciarelli M, Polidoro A, Iuliano L. Adverse reactions of amiodarone. J Geriatr Cardiol. 2019;16(7):552–66.
Tanda ML, Piantanida E, Lai A, Liparulo L, Sassi L, Bogazzi F, et al. Diagnosis and management of amiodarone-induced thyrotoxicosis: similarities and differences between North American and European thyroidologists. Clin Endocrinol. 2008;69(5):812–8.
Hoermann R. Amiodaron und Schilddrüsenfunktion. Nuklearmedizin. 2004;27(2):78–85.
Tsang W, Houlden RL. Amiodarone-induced thyrotoxicosis: a review. Can J Cardiol. 2009;25(7):421–4.
Censi S, Bodanza V, Manso J, Gusella S, Watutantrige-Fernando S, Cavedon E, et al. Amiodarone-induced thyrotoxicosis: differential diagnosis using 99mTc-SestaMIBI and target-to-background ratio (TBR). Clin Nucl Med. 2018;43(9):655–62.
Stelmachowska-Banaś M, Czajka-Oraniec I. Management of endocrine immune-related adverse events of immune checkpoint inhibitors: an updated review. Endocr Connect. 2020;9(10):R207–28.
Kurimoto C, Inaba H, Ariyasu H, Iwakura H, Ueda Y, Uraki S, et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci. 2020;111(5):1468–77.
Hattersley R, Nana M, Lansdown AJ. Endocrine complications of immunotherapies: a review. Clin Med. 2021;21(2):e212–22.
Brancatella A, Viola N, Brogioni S, Montanelli L, Sardella C, Vitti P, et al. Graves’ disease induced by immune checkpoint inhibitors: a case report and review of the literature. Eur Thyroid J. 2019;8(4):192–5.
Petranović Ovčariček P, Deandreis D, Giovanella L. Thyroid dysfunctions induced by molecular cancer therapies: a synopsis for nuclear medicine thyroidologists. Eur J Nucl Med Mol Imaging. 2021;48(11):3355–60.
Nilsson M, Fagman H. Development of the thyroid gland. Development. 2017;144(12):2123–40.
Zamora E, Ghandili S, Zamora MA, Chun KJ. Incidental primary intrathoracic goiter: dual-isotope scintigraphy and early-MIBI SPECT/CT. World J Nucl Med. 2022;21(2):148–51.
Richmond I, Whittaker JS, Deiraniya AK, Hassan R. Intracardiac ectopic thyroid: a case report and review of published cases. Thorax. 1990;45(4):293–4.
Theurer S, Siebolts U, Lorenz K, Dralle H, Schmid KW. Ektopes Gewebe der Schilddrüse und der Nebenschilddrüsen. Pathologe. 2018;39(5):379–89.
Harisankar CNB, Preethi GR, George M. Hybrid SPECT/CT evaluation of dual ectopia of thyroid in the absence of orthotopic thyroid gland. Clin Nucl Med. 2012;37(6):602–3.
Bonnema SJ, Knudsen DU, Bertelsen H, Mortensen J, Andersen PB, Bastholt L, et al. Does radioiodine therapy have an equal effect on substernal and cervical goiter volumes? Evaluation by magnetic resonance imaging. Thyroid. 2002;12(4):313–7.
Gandhi A, Wong KK, Gross MD, Avram AM. Lingual thyroid ectopia: diagnostic SPECT/CT imaging and radioactive iodine treatment. Thyroid. 2016;26(4):573–9.
Roth LM, Talerman A. The enigma of struma ovarii. Pathology. 2007;39(1):139–46.
van Trotsenburg P, Stoupa A, Léger J, Rohrer T, Peters C, Fugazzola L, et al. congenital hypothyroidism: a 2020–2021 consensus guidelines update—an ENDO-European reference network initiative endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid. 2021;31(3):387–419.
Schoen EJ, Clapp W, To TT, Fireman BH. The key role of newborn thyroid scintigraphy with isotopic iodide (123I) in defining and managing congenital hypothyroidism. Pediatrics. 2004;114(6):e683–8.
Binse I, Poeppel TD, Ruhlmann M, Gomez B, Umutlu L, Bockisch A, et al. Imaging with 124I in differentiated thyroid carcinoma: is PET/MRI superior to PET/CT? Eur J Nucl Med Mol Imaging. 2016;43(6):1011–7.
Winkens T, Seifert P, Hollenbach C, Kühnel C, Gühne F, Freesmeyer M. The FUSION iENA study: comparison of I-124-PET/US fusion imaging with conventional diagnostics for the functional assessment of thyroid nodules by multiple observers. Nuklearmedizin. 2019;58(6):434–42.
Jiang H, Schmit NR, Koenen AR, Bansal A, Pandey MK, Glynn RB, et al. Safety, pharmacokinetics, metabolism and radiation dosimetry of 18F-tetrafluoroborate (18F-TFB) in healthy human subjects. EJNMMI Res. 2017;7(1):90.
Dittmann M, Gonzalez Carvalho JM, Rahbar K, Schäfers M, Claesener M, Riemann B, et al. Incremental diagnostic value of [18F]tetrafluoroborate PET-CT compared to [131I]iodine scintigraphy in recurrent differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2020;47(11):2639–46.
Salavati A, Puranik A, Kulkarni HR, Budiawan H, Baum RP. Peptide receptor radionuclide therapy (PRRT) of medullary and nonmedullary thyroid cancer using radiolabeled somatostatin analogues. Semin Nucl Med. 2016;46(3):215–24.
Tran K, Khan S, Taghizadehasl M, Palazzo F, Frilling A, Todd JF, et al. Gallium-68 Dotatate PET/CT is superior to other imaging modalities in the detection of medullary carcinoma of the thyroid in the presence of high serum calcitonin. Hell J Nucl Med. 2015;18(1):19–24.
Treglia G, Castaldi P, Villani MF, Perotti G, de Waure C, Filice A, et al. Comparison of 18F-DOPA, 18F-FDG and 68Ga-somatostatin analogue PET/CT in patients with recurrent medullary thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2012;39(4):569–80.
Rasul S, Hartenbach S, Rebhan K, Göllner A, Karanikas G, Mayerhoefer M, et al. [18F]DOPA PET/ceCT in diagnosis and staging of primary medullary thyroid carcinoma prior to surgery. Eur J Nucl Med Mol Imaging. 2018;45(12):2159–69.
Wells SA, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma: the American Thyroid Association guidelines task force on medullary thyroid carcinoma. Thyroid. 2015;25(6):567–610.
Giovanella L, Treglia G, Iakovou I, Mihailovic J, Verburg FA, Luster M. EANM practice guideline for PET/CT imaging in medullary thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2020;47(1):61–77.
Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics. 2018;38(1):200–17.
Bertagna F, Albano D, Giovanella L, Bonacina M, Durmo R, Giubbini R, et al. 68Ga-PSMA PET thyroid incidentalomas. Hormones. 2019;18(2):145–9.
Albano D, Durmo R, Bertagna F, Giubbini R. 18F-choline PET/CT incidental thyroid uptake in patients studied for prostate cancer. Endocrine. 2019;63(3):531–6.
Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60(6):801–5.
Li M, Younis MH, Zhang Y, Cai W, Lan X. Clinical summary of fibroblast activation protein inhibitor-based radiopharmaceuticals: cancer and beyond. Eur J Nucl Med Mol Imaging. 2022;49(8):2844–68.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Schenke, S.A., Groener, D., Grunert, M., Stahl, A.R. (2023). Integrated Thyroid Imaging: Ultrasound and Scintigraphy. In: Giovanella, L. (eds) Integrated Diagnostics and Theranostics of Thyroid Diseases. Springer, Cham. https://doi.org/10.1007/978-3-031-35213-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-031-35213-3_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-35212-6
Online ISBN: 978-3-031-35213-3
eBook Packages: MedicineMedicine (R0)