Abstract
Introduction
Efficient therapy of recurrent differentiated thyroid cancer (DTC) is dependent on precise molecular imaging techniques targeting the human sodium iodide symporter (hNIS), which is a marker both of thyroid and DTC cells. Various iodine isotopes have been utilized for detecting DTC; however, these come with unfavorable radiation exposure and image quality ([131I]iodine) or limited availability ([124I]iodine). In contrast, [18F]tetrafluoroborate (TFB) is a novel radiolabeled PET substrate of hNIS, results in PET images with high-quality and low radiation doses, and should therefore be suited for imaging of DTC. The aim of the present study was to compare the diagnostic performance of [18F]TFB-PET to the clinical reference standard [131I]iodine scintigraphy in patients with recurrent DTC.
Methods
Twenty-five patients with recurrent DTC were included in this retrospective analysis. All patients underwent [18F]TFB-PET combined with either CT or MRI due to newly discovered elevated TG levels, antiTG levels, sonographically suspicious cervical lymph nodes, or combinations of these findings. Correlative [131I]iodine whole-body scintigraphy (dxWBS) including SPECT-CT was present for all patients; correlative [18F]FDG-PET-CT was present for 21 patients. Histological verification of [18F]TFB positive findings was available in 4 patients.
Results
[18F]TFB-PET detected local recurrence or metastases of DTC in significantly more patients than conventional [131I]iodine dxWBS and SPECT-CT (13/25 = 52% vs. 3/25 = 12%, p = 0.002). The diagnosis of 6 patients with cervical lymph node metastases that showed mildly increased FDG metabolism but negative [131I]iodine scintigraphy was changed: [18F]TFB-PET revealed hNIS expression in the metastases, which were therefore reclassified as only partly de-differentiated (histological confirmation present in two patients). Highest sensitivity for detecting recurrent DTC had the combination of [18F]TFB-PET-CT/MRI with [18F]FDG-PET-CT (64%).
Conclusion
In the present cohort, [18F]TFB-PET shows higher sensitivity and accuracy than [131I]iodine WBS and SPECT-CT in detecting recurrent DTC. The combination of [18F]TFB-PET with [18F]FDG-PET-CT seems a reasonable strategy to characterize DTC tumor manifestations with respect to their differentiation and thereby also individually plan and monitor treatment. Future prospective studies evaluating the potential of [18F]TFB-PET in recurrent DTC are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The diagnostic workup of patients with recurrent differentiated thyroid cancer (DTC) requires molecular imaging strategies targeting the human sodium iodide symporter (hNIS), which is a key molecular marker of thyroid and DTC cells [1]. The radiopharmaceuticals [124I]iodide, [123I]iodide, and [131I]iodide are substrates of hNIS and thus are predominately used for molecular imaging of recurrent DTC [2]. However, positron emitting [124I]iodine is of subordinate diagnostic significance, as it is only available at few sites and delivers image acquisitions with a low signal to noise ratio [2]. Therefore, diagnostic [131I]iodine or [123I]iodine whole-body scintigraphy (dxWBS) and single photon emission tomography (SPECT-CT) are the current clinical reference standards for the diagnostic workup of DTC patients. Yet both iodine dxWBS and SPECT-CT achieve only unsatisfactory image quality, especially when compared to positron emission tomography (PET) imaging approaches [3,4,5,6]. Moreover, [131I]iodine causes significant radiation exposure, and the administration might require an in-patient stay due to radiation protection legislation [7].
The detection of local recurrence or metastases of DTC by iodine imaging is of great relevance for the planning of localized therapies, like surgery or radiation therapy [2]. Moreover, negative iodine imaging despite elevated tumor marker levels is a prerequisite for diagnosing the “Thyroglobulin Elevated and Negative Iodine Scintigraphy” (TENIS) syndrome, which requires fundamentally different diagnostic and therapy concepts [8,9,10]. Therefore, a failure in detecting hNIS-expressing local recurrences or metastases of DTCs might result both in a missed opportunity for localized therapy and an erroneously assumed TENIS syndrome.
Recently, [18F]fluoride labeled tetrafluoroborate (TFB) has been employed in the primary staging of patients with DTC [11]. [18F]TFB is likewise a substrate of hNIS and therefore an analogue to [124I]iodide or [131I]iodide [12]. Yet in contrast to iodine nuclides, [18F]TFB can easily be synthesized by cyclotrons equipped with PET radiochemistry and offers a favorable half-life, dosage and biodistribution, as well as superior PET image quality [13,14,15]. However, it remains unclear, if [18F]TFB-PET-CT is superior to conventional [131I]iodine dxWBS/SPECT-CT in post-radioiodine ablation (RAI) patients suffering from recurrent DTC.
Therefore, the aim of the present study was to evaluate the potential of [18F]TFB-PET in patients with newly discovered recurrent DTC. To this end, the clinical reference standard [131I]iodine dxWBS/SPECT-CT was compared on an individual basis to [18F]TFB-PET.
Methods
Patients
A consecutive number of 25 [18F]TFB-PET acquisitions combined with either CT or MRI of patients suffering from DTC (9 males and 16 females, median age 57) were included in this retrospective study (inclusion period: July 2018 to June 2019). Patients were referred to [18F]TFB-PET due to newly discovered increasing levels of thyroglobulin (TG), autoantibodies against thyroglobulin (antiTG), suspicious cervical lymph nodes, or combinations of the latter. Decision for [18F]TFB-PET examination was done on a case-by-case basis due to aforementioned clinical indication. Detailed patient characteristics are given by Table 1. The median time from thyroidectomy to [18F]TFB-PET was 5.1 [6.3] years. The median cumulated therapeutic dosage of [131I]iodine that was employed in the cohort until [18F]TFB-PET was 10 [10.4] GBq. All patients were treated with Levothyroxine to suppress thyroid-stimulating hormone (TSH) levels [2].
[18F]TFB preparation
[18F]TFB was prepared according to a literature procedure described previously by isotopic exchange of BF4− with [18F]fluoride in hot hydrochloric acid and purified using an alumina column [16]. The product activity ranged between 6 and 8 GBq/batch. Radiochemical purities were determined by TLC and HPLC. The HPLC system was composed of a S1122 pump (Sykam, Fürstenfeldbruck, Germany); a S-2500 UV detector (KNAUER, Berlin, Germany); a GabiStar γ-detector (Elysia-Raytest GmbH, Straubenhardt, Germany); an Exsil ODS column, 5 μm, 250 × 4.0 mm (SGE Europe Ltd., Bucks, UK) with a mobile phase of 1-mM tetrabutylammonium hydroxide and 1.3 mM potassium hydrogen phthalate in water (pH 7.5) flowing at 1.0 ml/min, detection at λ = 254 nm; and the GINA Star software (Elysia-Raytest GmbH, Straubenhardt, Germany) ([18F]fluoride, tR = 4.7 min; [18F]TFB, tR = 11.2 min). Thin-layer chromatography (TLC) was performed on alumina TLC strips (Polygram Alox N/UV254, 40 × 80 mm, Machery-Nagel, Düren, Germany) with methanol as mobile phase. Strips were scanned using a radio TLC scanner (Elysia-Raytest GmbH, Straubenhardt, Germany). Rf([18F]fluoride) = 0.0; Rf([18F]TFB) = 0.8.
[18F]TFB PET and [131I]iodine imaging
Thyrotropin alfa was administered 48 and 24 h before the administration of [18F]TFB and [131I]iodine. A median activity of 317.0 [63.0] MBq [18F]TFB was administered intravenously. The PET acquisition was initiated 40 min after tracer injection (Biograph mCT flow or Biograph mMR, Siemens Healthineers, Erlangen, Germany; acquisition speed: 2 min/bed position or 1.1 mm/s). Low-dose CT or MRI was acquired for attenuation correction of PET data. A median activity of 315.0 [516.8] MBq [131I]iodine was administered immediately after completion of the PET acquisition (a month delayed in one patient; only patient #12 received a therapeutic dosage of 3 GBq [131I]iodine). WBS and SPECT-CT acquisitions were done in accordance with guidelines (Discovery NM/CT 670 Pro, GE Healthcare, Chalfont St Giles, GB or Symbia T2, Siemens Healthineers, Erlangen, Germany) [17].
Biochemical analysis
Blood levels of TSH, free triiodothyronine (fT3), free thyroxine (fT4), TG, and antiTG were measured on the day of the first thyrotropin alfa administration (TG/TSH, Elecsys assays and cobas e 801, Roche Diagnostics, Rotkreuz, CH; TG, assay and KRYPTOR hTg sensitive, BRAHMS GmbH/Thermo Fisher Scientific, Hennigsdorf, Germany). Stimulated TG levels were measured before [131I]iodine WBS acquisition.
Statistical analysis
SPSS statistics 24 (IBM, NY, USA) was used for descriptive metrics and statistical testing. The McNemar and Wilcoxon method were used to test for statistically significant differences with the “exact” option of SPSS. H0 was rejected if p < 0.05. Values are presented as median with standard deviation (SD) in squared brackets.
Results
A total number of 25 patients underwent [18F]TFB-PET, combined with either CT (n = 24) or MRI (n = 1; due to logistic reasons). In the following, PET-CT is used as phrase to refer to both PET-CT and PET-MRI acquisitions. Detailed patient characteristics are given by Tables 1 and 2. Patients were referred to imaging due to elevated TG levels (n = 19), elevated antiTG levels (n = 7), sonographically suspicious cervical lymph nodes (n = 10), or combinations of these findings. Therefore, all enrolled patients were regarded as suffering from recurrent DTC. Correlative [131I]iodine imaging was present for 25 patients. The median interval between [18F]TFB-PET-CT and [131I]iodine dxWBS/SPECT-CT was 3.0 [7.3] days (range: 2–39 days). Correlative [18F]FDG-PET-CTs were present for 21 patients. The median interval between [18F]TFB-PET-CT and [18F]FDG-PET-CT was 6.9 [10.8] months. No local therapy was performed between [18F]TFB-PET-CT and [18F]FDG-PET-CT.
[18F]TFB detection rate and reference standards
The detection rate of [18F]TFB-PET-CT was 52.0% (n = 13). Details are given in Fig. 1. A total number of 27 lesions were detected by [18F]TFB-PET-CT. All lesions were delineated due to increased [18F]TFB-PET accumulation. Metastases or local recurrence were discovered in 10 additional patients compared to [131I]iodine dxWBS/SPECT-CT (Fig. 3). The detection rate of [18F]TFB-PET-CT was significantly higher compared to [131I]iodine dxWBS/SPECT-CT (recurrence detected in 13 vs. 3 patients; p = 0.002). A detailed list of findings is given in Table 2 (see also Figs. 4 and 5). Reference standard for [18F]TFB-PET-CT-positive patients (n = 13) was surgery together with histological examination (in 4 patients), a corresponding [131I]iodine accumulation (in 3 patients), [18F]FDG accumulation (in 5 patients), or morphological finding on CT (in 1 patient; lung metastasis). Therefore, the sensitivity of [18F]TFB-PET-CT was 52% (accuracy: 52%). The median [18F]TFB uptake of delineated lesions was 4.2 SUVmax [8.4].
Looking at patients who underwent both [18F]TFB-PET-CT and [18F]FDG-PET-CT, the detection rate of [18F]TFB-PET-CT was higher (51.9%) compared to [18F]FDG-PET-CT (47.6%), yet this difference was not statistically significant. In three patients, [18F]FDG-PET-CT discovered metabolically active metastases (median SUVmax: 11.9) with no increased [18F]TFB accumulation. In contrast, [18F]TFB-PET-CT discovered lesions in six patients (local recurrence, lymph node or lung metastases), with no [18F]FDG accumulation. Combining [18F]FDG-PET-CT and [18F]TFB-PET-CT for staging of suspected recurrent DTC resulted in the highest sensitivity (64%; positive predictive value, 100%; accuracy, 64%).
In 6 patients, cervical lymph node metastases were suspected due to moderately increased [18F]FDG uptake (median SUVmax: 4.5). Likewise, [18F]TFB-PET-CT revealed suspicious uptake of theses lymph nodes (median SUVmax: 4.2). [18F]TFB-PET-CT-guided surgery together with histological analysis was performed in two patients. In both cases, lymph node metastases could be confirmed.
[131I]iodine-SPECT-CT, [18F]FDG-PET-CT, and CT detection rates
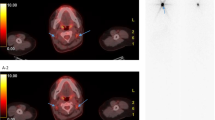
In three patients, [131I]iodine accumulating metastases were discovered by dxWBS and SPECT-CT (12.0%, one patient received a WBS with therapeutic dosage). In total, 11 lesions were detected by [131I]iodine imaging, which were significantly fewer compared to [18F]TFB-PET-CT (p = 0.001). All lesions were also detected by [18F]TFB-PET-CT, which additionally provided superior image quality (Fig. 2). Given histological CT or FDG-PET findings as reference standard, the sensitivity of iodine imaging was 12% (positive predictive value, 100%; accuracy, 12%). [18F]FDG accumulating local recurrence or metastases were delineated in 10 patients. The median uptake of these lesions was 5.2 SUVmax. Morphological findings without [18F]TFB, [18F]FDG, or [131I]iodine uptake were present in patients with lung metastases only (n = 3, 12%).
Superior image quality of [18F]TFB-PET-CT. In contrast to all other cases, patient #12 (92 years; TG, 5009 ng/ml; antiTG, 22.6 IU/ml; follicular DTC) received a therapeutic activity of [131I]iodine for imaging and therapy due to already known metastases. However, the delineation of local recurrence (dashed arrow) and a bone metastasis (arrow) is difficult in the SPECT-CT fusion (a). The transaxial SPECT reconstruction enables a better discrimination (b). However, [18F]TFB-PET-CT enabled superior delineation of metastases and local recurrence (c–e). Note additional bone metastases, e.g., skull
TG, TG-DT, and antiTG levels
Median TSH suppressed TG and antiTG levels for patients with [18F]TFB-positive findings were 2.46 [1566.9] ng/ml and 98.7 [144.7] IU/ml, respectively. For comparison, [18F]TFB-negative patients had median TG levels of 1.87 [4.4] ng/ml. Only one [18F]TFB-negative patient had elevated antiTG (3267 IU/ml). In six patients, no morphological or molecular findings were detected. Median TG level of these patients was 0.83 [1.2] ng/ml (no patient with elevated antiTG levels).
Discussion
The performance of [18F]TFB-PET-CT in the diagnostic workup of patients with suspected recurrence of DTC was evaluated in the present study. To this end, 25 patients with sonographically suspicious cervical lymph nodes or elevated blood tumor markers (TG or antiTG) were included in this retrospective analysis. [18F]TFB-PET-CT detected local recurrence or metastases of DTC in significantly more patients compared to [131I]iodine dxWBS/SPECT-CT. Moreover, the accuracy of [18F]TFB-PET-CT was greater compared to [131I]iodine dxWBS/SPECT-CT.
It was shown previously that [131I]iodine WBS/SPECT-CT is inferior to TG levels in detecting patients that develop metastases or local recurrence [18]. Gonzalez Carvalho et al. stated that up to 25% of patients with recurrent DTC had two subsequent negative [131I]iodine dxWBS image acquisitions [18]. This indicates that [131I]iodine dxWBS is not ideal for detecting recurrent DTC. However, evidence of recurrent DTC by elevated TG levels does often not enable a localized therapy. Therefore, molecular imaging strategies are needed to localize recurrent DTC more sensitively than [131I]iodine dxWBS.
It has been shown previously that [124I]iodine-PET is superior to [131I]iodine dxWBS in detecting recurrent DTC [4]. However, [124I]iodine is not available in many departments of nuclear medicine [2]. Moreover, PET imaging with [124I]iodine requires a tracer administration days before image acquisition, which causes patient management issues. Finally, the image quality of [124I]iodine-PET is not preferable due to low signal-to-noise ratios. In contrast, [18F]TFB-PET-CT can easily be produced by a cyclotron equipped radiochemistry department and delivers excellent image to noise ratios and the image acquisition can be initiated minutes after tracer administration. Interestingly, patients 1 and 10 showed similar blood parameters (TG 0.55 ng/ml, antiTG < 15); however, only patient 10 showed suspicious [18F]TFB accumulation, which was histologically confirmed to be a metastasis. Therefore, [18F]TFB-PET-CT might be advisable in patients with minimally elevated tumor marker levels.
[18F]FDG-PET-CT imaging in DTC has been proposed to overcome the limitations of diagnostic [131I]iodine WBS [3, 10, 19,20,21]. A large multicenter study could demonstrate that [18F]FDG-PET has a higher sensitivity than [131I]iodine dxWBS [3]. Riemann et al. argued that this is partly caused by the tumor biology of DTC [3]: In case of recurrent DTC, a dedifferentiation of tumor cells might occur that decreases hNIS expression but increases [18F]FDG metabolism in turn [9]. This constellation is known as TENIS or flip-flop phenomena [3, 8, 9]. However, [18F]FDG positiveness together with negative [131I]iodine dxWBS does not prove complete dedifferentiation. It has been shown that [124I]iodine PET and therapeutic [131I]iodine WBS have a greater sensitivity than [131I]iodine dxWBS [4]. Additionally, [18F]FDG uptake of metastases might at least partly be caused by tumor infiltrating immune cells [22]. Thus, the rate of TENIS could be overestimated utilizing [131I]iodine dxWBS together with [18F]FDG-PET-CT. Therefore, [18F]FDG-PET and hNIS targeting molecular imaging are not equivalent but complementary.
The advantage of combining [18F]FDG-PET-CT with high-quality hNIS targeting molecular imaging is corroborated by the present study. Cervical lymph node metastases with moderate [18F]FDG accumulation but negative diagnostic [131I]iodine WBS/SPECT-CT were discovered in six patients. Thus, TENIS was suspected in these cases. However, [18F]TFB-PET-CT was positive thus suggested only partly dedifferentiation. Moreover, [18F]TFB-PET enabled a clear delineation of the metastases that lead to successful surgery in two cases, which enabled the histological confirmation of DTC. Two different patients of this study showed strong [18F]FDG accumulation of DTC metastases without evidence of functional relevant hNIS expression (negative [131I]iodine WBS and [18F]TFB-PET). Therefore, dedifferentiation and TENIS were assumed. Taken together, the combination of [18F]FDG-PET and [18F]TFB-PET seems reasonable due to their complementary nature. The combination of both imaging modalities achieved the highest sensitivity and accuracy (both: 62%) by sensitively detecting both differentiated and dedifferentiated recurrence of DTC.
The present retrospective study faces some limitations. First, the enrollment of patients was done retrospectively and is therefore prone to selection biases. For instance, many enrolled patients had only minimally elevated TG levels or had undergone [131I]iodine dxWBS/SPECT-CT imaging several times with inconclusive findings. Therefore, the pretest probability of positive imaging findings might be low. This could affect the transferability of the results to the clinical routine and underestimate the diagnostic performance of [18F]TFB-PET-CT. Moreover, only a relatively small patient collective was included due to the limited application of [18F]TFB-PET-CT in the clinical routine.
Finally, [18F]TFB shows increased retention in the blood, which caused difficulties in the reading of head and neck acquisitions, especially when combined with low dose CT (note the blood pool in Fig. 3). Therefore, future studies should optimize the interval between injection of [18F]TFB and image acquisition. An increased interval between administration and image acquisition might result in lesser presence of [18F]TFB in the blood. Additionally, it might be warranted to combine [18F]TFB-PET with contrast enhanced morphology imaging to clearly delineate cervical blood vessels. This could facilitate the characterization of cervical [18F]TFB accumulating foci as either unspecific blood retention or cervical metastases.
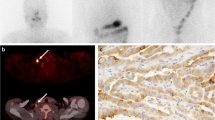
Detection of local recurrences and morphological correlates. No pathological accumulation of [131I]iodine is visible in the dxWBS (a), especially no cervical accumulation (dashed cycle). [18F]TFB-PET-CT enabled the detection of locally recurrent DTC at two sites (paralaryngeal, arrow, and retrosternal, dashed arrow (b, c, e)) in patient #2 (62 years; TG, 1.66 ng/ml; antiTG, 21.6 IU/ml; follicular DTC). Recurrence of DTC was histologically validated. Respectively, contrast enhanced foci were retrospectively detected in prior CT acquisitions (c, e)
Conclusion
[18F]TFB-PET-CT detected local recurrence or metastases of DTC in significantly more patients than [131I]iodine dxWBS/SPECT-CT. The combination of [18F]FDG and [18F]TFB PET-CT imaging achieved the highest diagnostic performance. Future studies evaluating [18F]TFB-PET-CT in recurrent DTC seem warranted.
References
Morari EC, Marcello MA, Guilhen ACT, Cunha LL, Latuff P, Soares FA, et al. Use of sodium iodide symporter expression in differentiated thyroid carcinomas. Clin Endocrinol (Oxf). 2011;75:247–54.
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133.
Riemann B, Uhrhan K, Dietlein M, Schmidt D, Kuwert T, Dorn R, et al. Diagnostic value and therapeutic impact of 18F-FDG-PET/CT in differentiated thyroid cancer. Nuklearmedizin. 2012;52:1–6.
Phan HTT, Jager PL, Paans AMJ, Plukker JTM, Sturkenboom MGG, Sluiter WJ, et al. The diagnostic value of 124I-PET in patients with differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008;35:958–65.
Arman R, Habib Z. PET versus SPECT: strength, limitations, and challenges. Nucl Med Commun. 2008;29:193–207.
Hicks RJ, Hofman MS. Is there still a role for SPECT-CT in oncology in the PET-CT era? Nat rev Clin Oncol. Nat Publ Group. 2012;9:712–20.
de Klerk JM. 131I therapy: inpatient or outpatient? J Nucl Med. 2000;41:1876–8.
Silberstein EB. The problem of the patient with thyroglobulin elevation but negative iodine scintigraphy: the TENIS syndrome. Semin Nucl Med. Elsevier Inc. 2011;41:113–20.
Basu S, Dandekar M, Joshi A, D’Cruz A. Defining a rational step-care algorithm for managing thyroid carcinoma patients with elevated thyroglobulin and negative on radioiodine scintigraphy (TENIS): considerations and challenges towards developing an appropriate roadmap. Eur J Nucl Med Mol Imaging. 2015;42:1167–71.
Vrachimis A, Burg MC, Wenning C, Allkemper T, Weckesser M, Schäfers M, et al. [18F]FDG PET/CT outperforms [18F]FDG PET/MRI in differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2016;43:212–20.
Samnick S, Al-Momani E, Schmid JS, Mottok A, Buck AK, Lapa C. Comparison initial clinical to investigation[124I]iodine PET/CT of [18F]Tetrafluoroborate for imaging thyroid PET/CT cancer in. Clin Nucl Med. 2018;43:162–7.
Jiang H, DeGrado TR. [18F]Tetrafluoroborate ([18F]TFB) and its analogs for PET imaging of the sodium/iodide symporter. Theranostics. 2018;8:3918–31.
Jiang H, Schmit NR, Koenen AR, Bansal A, Pandey MK, Glynn RB, et al. Safety, pharmacokinetics, metabolism and radiation dosimetry of 18F-tetrafluoroborate (18F-TFB) in healthy human subjects. EJNMMI Res. 2017;7.
Santhanam P, Solnes LB, Rowe SB. Molecular imaging of advanced thyroid cancer: iodinated radiotracers and beyond. Med Oncol Springer US. 2017;34:1–7.
O’Doherty J, Jauregui-Osoro M, Brothwood T, Szyszko T, Marsden PK, O’Doherty MJ, et al. 18F-tetrafluoroborate, a PET probe for imaging sodium/iodide symporter expression: whole-body biodistribution, safety, and radiation dosimetry in thyroid cancer patients. J Nucl Med. 2017;58:1666–71.
Jauregui-Osoro M, Sunassee K, Weeks AJ, Berry DJ, Paul RL, Cleij M, et al. Synthesis and biological evaluation of [ 18F]tetrafluoroborate: a PET imaging agent for thyroid disease and reporter gene imaging of the sodium/iodide symporter. Eur J Nucl Med Mol Imaging. 2010;37:2108–16.
Luster M, Clarke SE, Dietlein M, Lassmann M, Lind P, Oyen WJG, et al. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008;35:1941–59.
Gonzalez Carvalho JM, Görlich D, Schober O, Wenning C, Riemann B, Verburg FA, et al. Evaluation of131I scintigraphy and stimulated thyroglobulin levels in the follow up of patients with DTC: a retrospective analysis of 1420 patients. Eur J Nucl Med Mol Imaging. 2017;44:744–56.
Terroir M, Borget I, Bidault F, Ricard M, Deschamps F, Hartl D, et al. The intensity of 18FDG uptake does not predict tumor growth in patients with metastatic differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2017;44:638–46.
Manohar PM, Beesley LJ, Bellile EL, Worden FP, Avram AM. Prognostic value of FDG-PET/CT metabolic parameters in metastatic radioiodine-refractory differentiated thyroid cancer. Clin Nucl Med. 2018;43:641–7.
Giovanella L, Trimboli P, Verburg FA, Treglia G, Piccardo A, Foppiani L, et al. Thyroglobulin levels and thyroglobulin doubling time independently predict a positive 18F-FDG PET/CT scan in patients with biochemical recurrence of differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2013;40:874–80.
Kim MJ, Sun HJ, Song YS, Yoo S-K, Kim YA, Seo J-S, et al. CXCL16 positively correlated with M2-macrophage infiltration, enhanced angiogenesis, and poor prognosis in thyroid cancer. Sci Rep Springer US. 2019;9:1–10.
Funding
Open access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KR is a clinical consultant for ABX and has received consultant fees from Bayer and lectureship fees from Janssen Cilag, Amgen, AAA, and SIRTEX. The University of Muenster received consulting fees from ABX Advanced Biochemical Compounds, Radeberg, Germany, for KR. The authors declare that they have no conflict of interest regarding this study.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Imaging was performed in the clinical routine with informed consent. This retrospective analysis was approved by the local ethics committee (Ethik-Kommission der Ärztekammer Westfalen-Lippe und der Westfälischen Wilhelms-Universität Münster, 2019-615-f-S).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology – Head and Neck
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dittmann, M., Gonzalez Carvalho, J.M., Rahbar, K. et al. Incremental diagnostic value of [18F]tetrafluoroborate PET-CT compared to [131I]iodine scintigraphy in recurrent differentiated thyroid cancer. Eur J Nucl Med Mol Imaging 47, 2639–2646 (2020). https://doi.org/10.1007/s00259-020-04727-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04727-9