Abstract
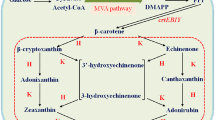
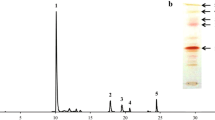
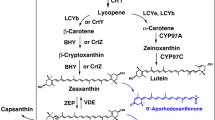
Corynebacterium glutamicum is a workhorse of industrial amino acid production employed for more than five decades for the million-ton-scale production of l-glutamate and l-lysine. This bacterium is pigmented due to the biosynthesis of the carotenoid decaprenoxanthin. Decaprenoxanthin is a carotenoid with 50 carbon atoms, and, thus, C. glutamicum belongs to the rare group of bacteria that produce long-chain C50 carotenoids. C50 carotenoids have been mainly isolated from extremely halophilic archaea (Kelly and Jensen, Acta Chem Scand 21:2578, 1967; Pfander, Pure Appl Chem 66:2369–2374, 1994) and from Gram-positive bacteria of the order Actinomycetales (Netzer et al., J Bacteriol 192:5688–5699, 2010). The characteristic yellow phenotype of C. glutamicum is due to the cyclic C50 carotenoid decaprenoxanthin and its glycosides. Decaprenoxanthin production has been improved by plasmid-borne overexpression of endogenous genes of carotenogenesis. Gene deletion resulted in the production of the C40 carotenoid lycopene, an intermediate of decaprenoxanthin biosynthesis. Heterologous gene expression was required to develop strains overproducing nonnative carotenoids and terpenes, such as astaxanthin (Henke et al., Mar Drugs 14:E124, 2016) and (+)-valencene (Frohwitter et al., J Biotechnol 191:205–213, 2014). Integration of additional copies of endogenous genes expressed from strong promoters improved isoprenoid biosynthesis. Here, we describe C. glutamicum strains, plasmids, and methods for overexpression of endogenous and heterologous genes, gene deletion, replacement, and genomic integration. Moreover, strain cultivation as well as extraction, identification, and quantitative determination of terpenes and carotenoids produced by C. glutamicum is detailed.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Kelly M, Jensen SL (1967) Bacterial carotenoids .26. C50-Carotenoids .2. Bacterioruberin. Acta Chem Scand 21(9):2578
Pfander H (1994) C-45-carotenoids and C-50-carotenoids. Pure Appl Chem 66(10–11):2369–2374
Netzer R et al (2010) Biosynthetic pathway for gamma-cyclic sarcinaxanthin in Micrococcus luteus: heterologous expression and evidence for diverse and multiple catalytic functions of C(50) carotenoid cyclases. J Bacteriol 192(21):5688–5699
Henke NA et al (2016) Production of the marine carotenoid astaxanthin by metabolically engineered Corynebacterium glutamicum. Mar Drugs 14(7):E124
Frohwitter J et al (2014) Production of the sesquiterpene (+)-valencene by metabolically engineered Corynebacterium glutamicum. J Biotechnol 191:205–213
Kinoshita S, Udaka S, Shimono M (1957) Studies on the amino acid fermentation. Production of L-glutamic acid by various microorganisms. J Gen Appl Microbiol 3:193–205
Zahoor A, Otten A, Wendisch VF (2014) Metabolic engineering of Corynebacterium glutamicum for glycolate production. J Biotechnol 192:366–375
Jorge JM et al (2016) Improved fermentative production of gamma-aminobutyric acid via the putrescine route: systems metabolic engineering for production from glucose, amino sugars and xylose. Biotechnol Bioeng 114(4):862–873
Jorge JM, Leggewie C, Wendisch VF (2016) A new metabolic route for the production of gamma-aminobutyric acid by Corynebacterium glutamicum from glucose. Amino Acids 48(11):2519–2531
Pérez-García F, Peters-Wendisch P, Wendisch VF (2016) Engineering Corynebacterium glutamicum for fast production of L-lysine and L-pipecolic acid. Appl Microbiol Biotechnol 100(18):8075–8090
Rohles CM et al (2016) Systems metabolic engineering of Corynebacterium glutamicum for the production of the carbon-5 platform chemicals 5-aminovalerate and glutarate. Microb Cell Fact 15(1):154
Schneider J, Wendisch VF (2010) Putrescine production by engineered Corynebacterium glutamicum. Appl Microbiol Biotechnol 88(4):859–868
Blombach B, Eikmanns BJ (2011) Current knowledge on isobutanol production with Escherichia coli, Bacillus subtilis and Corynebacterium glutamicum. Bioeng Bugs 2(6):346–350
Matsumoto K et al (2011) Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in recombinant Corynebacterium glutamicum using propionate as a precursor. J Biotechnol 152(4):144–146
Jo SJ et al (2007) Improvement of poly(3-hydroxybutyrate) [P(3HB)] production in Corynebacterium glutamicum by codon optimization, point mutation and gene dosage of P(3HB) biosynthetic genes. J Biosci Bioeng 104(6):457–463
Kinoshita S, Tanaka K (1972) Glutamic acid. In: Yamada K (ed) The microbial production of amino acids. Halsted Press, New York, NY, pp 263–324
Blombach B, Seibold GM (2010) Carbohydrate metabolism in Corynebacterium glutamicum and applications for the metabolic engineering of L-lysine production strains. Appl Microbiol Biotechnol 86(5):1313–1322
Meiswinkel TM et al (2013) Crude glycerol-based production of amino acids and putrescine by Corynebacterium glutamicum. Bioresour Technol 145:254–258
Gopinath V et al (2011) Amino acid production from rice straw and wheat bran hydrolysates by recombinant pentose-utilizing Corynebacterium glutamicum. Appl Microbiol Biotechnol 92(5):985–996
Uhde A et al (2013) Glucosamine as carbon source for amino acid-producing Corynebacterium glutamicum. Appl Microbiol Biotechnol 97(4):1679–1687
Matano C et al (2014) Engineering of Corynebacterium glutamicum for growth and L-lysine and lycopene production from N-acetyl-glucosamine. Appl Microbiol Biotechnol 98(12):5633–5643
Tsuchidate T et al (2011) Glutamate production from beta-glucan using endoglucanase-secreting Corynebacterium glutamicum. Appl Microbiol Biotechnol 90(3):895–901
Kim EM et al (2015) Engineering of Corynebacterium glutamicum for growth and succinate production from levoglucosan, a pyrolytic sugar substrate. FEMS Microbiol Lett 362(19)
Seibold G et al (2006) Utilization of soluble starch by a recombinant Corynebacterium glutamicum strain: growth and lysine production. J Biotechnol 124(2):381–391
Heider SA et al (2014) Metabolic engineering for the microbial production of carotenoids and related products with a focus on the rare C50 carotenoids. Appl Microbiol Biotechnol 98(10):4355–4368
Heider SA, Peters-Wendisch P, Wendisch VF (2012) Carotenoid biosynthesis and overproduction in Corynebacterium glutamicum. BMC Microbiol 12(1):198
Heider SA, Wendisch VF (2015) Engineering microbial cell factories: Metabolic engineering of Corynebacterium glutamicum with a focus on non-natural products. Biotechnol J 10(8):1170–1184
Binder D et al (2016) Light-controlled cell factories: employing photocaged isopropyl-beta-d-thiogalactopyranoside for light-mediated optimization of lac promoter-based gene expression and (+)-valencene biosynthesis in Corynebacterium glutamicum. Appl Environ Microbiol 82(20):6141–6149
Krubasik P, Kobayashi M, Sandmann G (2001) Expression and functional analysis of a gene cluster involved in the synthesis of decaprenoxanthin reveals the mechanisms for C50 carotenoid formation. Eur J Biochem 268(13):3702–3708
Heider SA et al (2014) Optimization of the IPP precursor supply for the production of lycopene, decaprenoxanthin and astaxanthin by Corynebacterium glutamicum. Front Bioeng Biotechnol 2:28
Heider SA et al (2014) IdsA is the major geranylgeranyl pyrophosphate synthase involved in carotenogenesis in Corynebacterium glutamicum. FEBS J 281(21):4906–4920
Stansen C et al (2005) Characterization of a Corynebacterium glutamicum lactate utilization operon induced during temperature-triggered glutamate production. Appl Environ Microbiol 71(10):5920–5928
Peters-Wendisch PG et al (2001) Pyruvate carboxylase is a major bottleneck for glutamate and lysine production by Corynebacterium glutamicum. J Mol Microbiol Biotechnol 3(2):295–300
Kirchner O, Tauch A (2003) Tools for genetic engineering in the amino acid-producing bacterium Corynebacterium glutamicum. J Biotechnol 104(1-3):287–299
Schäfer A et al (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145(1):69–73
Acknowledgments
We acknowledge Sabine A. E. Heider for her work on optimization of HPLC methods, Elizabeth Gingras-Lafleur for her help on carotenoid extraction, and Hironori Taniguchi for support during photometer measurements.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Henke, N.A., Frohwitter, J., Peters-Wendisch, P., Wendisch, V.F. (2018). Carotenoid Production by Recombinant Corynebacterium glutamicum: Strain Construction, Cultivation, Extraction, and Quantification of Carotenoids and Terpenes. In: Barreiro, C., Barredo, JL. (eds) Microbial Carotenoids. Methods in Molecular Biology, vol 1852. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-8742-9_8
Download citation
DOI: https://doi.org/10.1007/978-1-4939-8742-9_8
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-8741-2
Online ISBN: 978-1-4939-8742-9
eBook Packages: Springer Protocols