Abstract
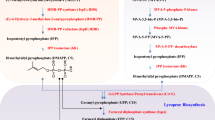
Carotenoids, a subfamily of terpenoids, are yellow- to red-colored pigments synthesized by plants, fungi, algae, and bacteria. They are ubiquitous in nature and take over crucial roles in many biological processes as for example photosynthesis, vision, and the quenching of free radicals and singlet oxygen. Due to their color and their potential beneficial effects on human health, carotenoids receive increasing attention. Carotenoids can be classified due to the length of their carbon backbone. Most carotenoids have a C40 backbone, but also C30 and C50 carotenoids are known. All carotenoids are derived from isopentenyl pyrophosphate (IPP) as a common precursor. Pathways leading to IPP as well as metabolic engineering of IPP synthesis and C40 carotenoid production have been reviewed expertly elsewhere. Since C50 carotenoids are synthesized from the C40 carotenoid lycopene, we will summarize common strategies for optimizing lycopene production and we will focus our review on the characteristics, biosynthesis, glycosylation, and overproduction of C50 carotenoids.





Similar content being viewed by others
References
Abbes M, Baati H, Guermazi S, Messina C, Santulli A, Gharsallah N, Ammar E (2013) Biological properties of carotenoids extracted from Halobacterium halobium isolated from a Tunisian solar saltern. BMC Complement Alternat Med 13:255. doi:10.1186/1472-6882-13-255
Alper H, Miyaoku K, Stephanopoulos G (2006) Characterization of lycopene-overproducing E. coli strains in high cell density fermentations. Appl Microbiol Biotechnol 72(5):968–974. doi:10.1007/s00253-006-0357-y
Andrewes AG, Starr MP (1976) (3R,3′R)-astaxanthin from the yeast Phaffia rhodozyma. Phytochemistry 15(6):1009–1011. doi:10.1016/S0031-9422(00)84391-5
Araya-Garay JM, Ageitos JM, Vallejo JA, Veiga-Crespo P, Sanchez-Perez A, Villa TG (2012) Construction of a novel Pichia pastoris strain for production of xanthophylls. AMB Express 2(1):24. doi:10.1186/2191-0855-2-24
Armstrong GA (1994) Eubacteria show their true colors: genetics of carotenoid pigment biosynthesis from microbes to plants. J Bacteriol 176(16):4795–4802
Armstrong GA, Hearst JE (1996) Carotenoids 2: genetics and molecular biology of carotenoid pigment biosynthesis. FASEB J 10(2):228–237
Arpin N, Liaaen-Jensen S, Trouilloud M (1972) Bacterial carotenoids. 38. C50-carotenoids. 9. Isolation of decaprenoxanthin mono- and diglucoside from an Arthrobacter sp. Acta Chem Scand 26(6):2524–2526
Aschoff S (1818) Beiträge zur Kenntnis des Saffrans. Berl Jb Pharm 19:142–157
Bari R, Jones JD (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69(4):473–488. doi:10.1007/s11103-008-9435-0
Beekwilder J, van der Meer IM, Simic A, Uitdewilligen J, van Arkel J, de Vos RC, Jonker H, Verstappen FW, Bouwmeester HJ, Sibbesen O, Qvist I, Mikkelsen JD, Hall RD (2008) Metabolism of carotenoids and apocarotenoids during ripening of raspberry fruit. Biofactors 34(1):57–66
Beuttler H, Hoffmann J, Jeske M, Hauer B, Schmid RD, Altenbuchner J, Urlacher VB (2011) Biosynthesis of zeaxanthin in recombinant Pseudomonas putida. Appl Microbiol Biotechnol 89(4):1137–1147. doi:10.1007/s00253-010-2961-0
Bhosale P, Bernstein PS (2005) Microbial xanthophylls. Appl Microbiol Biotechnol 68(4):445–455. doi:10.1007/s00253-005-0032-8
Boussiba S, Vonshak A, Cohen Z, Richmond A (2000) Procedure for large-scale production of astaxanthin from Haematococcus. US Patent 6022701
Bouvier F, Dogbo O, Camara B (2003) Biosynthesis of the food and cosmetic plant pigment bixin (annatto). Science 300(5628):2089–2091. doi:10.1126/science.1085162300/5628/2089
Brandi F, Bar E, Mourgues F, Horvath G, Turcsi E, Giuliano G, Liverani A, Tartarini S, Lewinsohn E, Rosati C (2011) Study of ‘Redhaven’ peach and its white-fleshed mutant suggests a key role of CCD4 carotenoid dioxygenase in carotenoid and norisoprenoid volatile metabolism. BMC Plant Biol 11:24. doi:10.1186/1471-2229-11-24
Britton G (2008) Functions of intact carotenoids. In: Britton, Liaaen-Jensen, Pfander (eds) Carotenoids: natural functions, vol 4. Birkhäuser Verlag, Basel, pp 189–212
Chae HS, Kim KH, Kim SC, Lee PC (2010) Strain-dependent carotenoid productions in metabolically engineered Escherichia coli. Appl Biochem Biotechnol 162(8):2333–2344. doi:10.1007/s12010-010-9006-0
Chen YY, Shen HJ, Cui YY, Chen SG, Weng ZM, Zhao M, Liu JZ (2013) Chromosomal evolution of Escherichia coli for the efficient production of lycopene. BMC Biotechnol 13:6. doi:10.1186/1472-6750-13-6
Cheng X, Ruyter-Spira C, Bouwmeester H (2013) The interaction between strigolactones and other plant hormones in the regulation of plant development. Front Plant Sci 4:199. doi:10.3389/fpls.2013.00199
Chumpolkulwong N, Kakizono T, Handa T, Nishio N (1997) Isolation and characterization of compactin resistant mutants of an astaxanthin synthesizing green alga Haematococcus pluvialis. Biotechnol Lett 19(3):299–302. doi:10.1023/A:1018330329357
Cooper DA, Eldridge AL, Peters JC (1999) Dietary carotenoids and certain cancers, heart disease, and age-related macular degeneration: a review of recent research. Nutr Rev 57(7):201–214
Coutinho PM, Deleury E, Davies GJ, Henrissat B (2003) An evolving hierarchical family classification for glycosyltransferases. J Mol Biol 328(2):307–317
Cutzu R, Coi A, Rosso F, Bardi L, Ciani M, Budroni M, Zara G, Zara S, Mannazzu I (2013) From crude glycerol to carotenoids by using a Rhodotorula glutinis mutant. World J Microbiol Biotechnol 29(6):1009–1017. doi:10.1007/s11274-013-1264-x
Das A, Yoon SH, Lee SH, Kim JY, Oh DK, Kim SW (2007) An update on microbial carotenoid production: application of recent metabolic engineering tools. Appl Microbiol Biotechnol 77(3):505–512. doi:10.1007/s00253-007-1206-3
Daum M, Herrmann S, Wilkinson B, Bechthold A (2009) Genes and enzymes involved in bacterial isoprenoid biosynthesis. Curr Opin Chem Biol 13(2):180–188. doi:10.1016/j.cbpa.2009.02.029
de Bont J (1998) Solvent-tolerant bacteria in biocatalysis. Trends Biotechnol 16(12):493–499. doi:10.1016/S0167-7799(98)01234-7
De Roos AL, van Dijk AA, Folkertsma B (2005) Bleaching of dairy products. International Patent WO 2005/004616
Dembitsky VM (2005) Astonishing diversity of natural surfactants: 3. Carotenoid glycosides and isoprenoid glycolipids. Lipids 40(6):535–557
Downham A, Collins P (2000) Colouring our foods in the last and next millennium. Int J Food Sci Tech 35(1):5–22. doi:10.1046/j.1365-2621.2000.00373.x
Fukuoka S, Ajiki Y, Ohga T, Kawanami Y, Izumori K (2004) Production of dihydroxy C50-carotenoid by Aureobacterium sp. FERM P-18698. Biosci Biotechnol Biochem 68(12):2646–2648
Gassel S, Schewe H, Schmidt I, Schrader J, Sandmann G (2013) Multiple improvement of astaxanthin biosynthesis in Xanthophyllomyces dendrorhous by a combination of conventional mutagenesis and metabolic pathway engineering. Biotechnol Lett 35(4):565–569. doi:10.1007/s10529-012-1103-4
Gopinath V, Meiswinkel TM, Wendisch VF, Nampoothiri KM (2011) Amino acid production from rice straw and wheat bran hydrolysates by recombinant pentose-utilizing Corynebacterium glutamicum. Appl Microbiol Biotechnol 92(5):985–996. doi:10.1007/s00253-011-3478-x
Graham JE, Bryant DA (2009) The biosynthetic pathway for myxol-2′ fucoside (myxoxanthophyll) in the cyanobacterium Synechococcus sp. strain PCC 7002. J Bacteriol 191(10):3292–3300. doi:10.1128/JB.00050-09
Harada H, Misawa N (2009) Novel approaches and achievements in biosynthesis of functional isoprenoids in Escherichia coli. Appl Microbiol Biotechnol 84(6):1021–1031. doi:10.1007/s00253-009-2166-6
Havaux M (2013) Carotenoid oxidation products as stress signals in plants. Plant J. doi:10.1111/tpj.12386
Heider SA, Peters-Wendisch P, Wendisch VF (2012) Carotenoid biosynthesis and overproduction in Corynebacterium glutamicum. BMC Microbiol 12(1):198. doi:10.1186/1471-2180-12-198
Heider SA, Peters-Wendisch P, Netzer R, Stafnes M, Brautaset T, Wendisch VF (2013) Production and glucosylation of C40 and C50 carotenoids by metabolically engineered Corynebacterium glutamicum. Appl Microbiol Biotechnol. doi:10.1007/s00253-013-5359-y
Hess BM, Xue J, Markillie LM, Taylor RC, Wiley HS, Ahring BK, Linggi B (2013) Coregulation of terpenoid pathway genes and prediction of isoprene production in using transcriptomics. PLoS One 8(6):e66104. doi:10.1371/journal.pone.0066104
Hundle BS, O'Brien DA, Alberti M, Beyer P, Hearst JE (1992) Functional expression of zeaxanthin glucosyltransferase from Erwinia herbicola and a proposed uridine diphosphate binding site. Proc Natl Acad Sci U S A 89(19):9321–9325
Jackson H, Braun CL, Ernst H (2008) The chemistry of novel xanthophyll carotenoids. Am J Cardiol 101(10A):50D–57D. doi:10.1016/j.amjcard.2008.02.008
Johnson EA, Schroeder WA (1996) Microbial carotenoids. Adv Biochem Eng Biotechnol 53:119–178
Julsing MK, Rijpkema M, Woerdenbag HJ, Quax WJ, Kayser O (2007) Functional analysis of genes involved in the biosynthesis of isoprene in Bacillus subtilis. Appl Microbiol Biotechnol 75(6):1377–1384. doi:10.1007/s00253-007-0953-5
Kelly M, Jensen SL (1967) Bacterial carotenoids. 26. C50-carotenoids. 2. Bacterioruberin. Acta Chem Scand 21(9):2578
Kim SH, Park YH, Schmidt-Dannert C, Lee PC (2010) Redesign, reconstruction, and directed extension of the Brevibacterium linens C40 carotenoid pathway in Escherichia coli. Appl Environ Microbiol 76(15):5199–5206. doi:10.1128/AEM.00263-10
Kirby J, Keasling JD (2009) Biosynthesis of plant isoprenoids: perspectives for microbial engineering. Annu Rev Plant Biol 60:335–355. doi:10.1146/annurev.arplant.043008.091955
Krinsky NI, Johnson EJ (2005) Carotenoid actions and their relation to health and disease. Mol Asp Med 26(6):459–516. doi:10.1016/j.mam.2005.10.001
Krubasik P, Kobayashi M, Sandmann G (2001a) Expression and functional analysis of a gene cluster involved in the synthesis of decaprenoxanthin reveals the mechanisms for C50 carotenoid formation. Eur J Biochem 268(13):3702–3708
Krubasik P, Takaichi S, Maoka T, Kobayashi M, Masamoto K, Sandmann G (2001b) Detailed biosynthetic pathway to decaprenoxanthin diglucoside in Corynebacterium glutamicum and identification of novel intermediates. Arch Microbiol 176(3):217–223
Lale R, Berg L, Stuttgen F, Netzer R, Stafsnes M, Brautaset T, Vee Aune TE, Valla S (2011) Continuous control of the flow in biochemical pathways through 5′ untranslated region sequence modifications in mRNA expressed from the broad-host-range promoter Pm. Appl Environ Microbiol 77(8):2648–2655. doi:10.1128/AEM.02091-10
Lange N, Steinbüchel A (2011) beta-Carotene production by Saccharomyces cerevisiae with regard to plasmid stability and culture media. Appl Microbiol Biotechnol 91(6):1611–1622. doi:10.1007/s00253-011-3315-2
Lange BM, Rujan T, Martin W, Croteau R (2000) Isoprenoid biosynthesis: the evolution of two ancient and distinct pathways across genomes. Proc Natl Acad Sci U S A 97(24):13172–13177
Lee PC, Schmidt-Dannert C (2002) Metabolic engineering towards biotechnological production of carotenoids in microorganisms. Appl Microbiol Biotechnol 60(1–2):1–11. doi:10.1007/s00253-002-1101-x
Li J, Zhu D, Niu J, Shen S, Wang G (2011) An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol Adv 29(6):568–574. doi:10.1016/j.biotechadv.2011.04.001
Liaaen-Jensen S, Hertzberg S, Weeks OB, Schwieter U (1968) Bacterial carotenoids XXVII. C50-carotenoids. 3. Structure determination of dehydrogenans-P439. Acta Chem Scand 22(4):1171–1186
Maresca JA, Bryant DA (2006) Two genes encoding new carotenoid-modifying enzymes in the green sulfur bacterium Chlorobium tepidum. J Bacteriol 188(17):6217–6223. doi:10.1128/JB.00766-06
Margalith PZ (1999) Production of ketocarotenoids by microalgae. Appl Microbiol Biotechnol 51(4):431–438
Martin VJ, Pitera DJ, Withers ST, Newman JD, Keasling JD (2003) Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat Biotechnol 21(7):796–802
Meijnen JP, Verhoef S, Briedjlal AA, de Winde JH, Ruijssenaars HJ (2011) Improved p-hydroxybenzoate production by engineered Pseudomonas putida S12 by using a mixed-substrate feeding strategy. Appl Microbiol Biotechnol 90(3):885–893. doi:10.1007/s00253-011-3089-6
Meiswinkel TM, Rittmann D, Lindner SN, Wendisch VF (2013) Crude glycerol-based production of amino acids and putrescine by Corynebacterium glutamicum. Bioresour Technol. doi:10.1016/j.biortech.2013.02.053
Miki W, Otaki N, Yokoyama A, Izumida H, Shimidzu N (1994) Okadaxanthin, a novel C50-narotenoid from a bacterium, Pseudomonas sp. KK10206c associated with marine sponge, Halichondria okadai. Experientia 50(7):684–686. doi:10.1007/Bf01952874
Miller NJ, Sampson J, Candeias LP, Bramley PM, Rice-Evans CA (1996) Antioxidant activities of carotenes and xanthophylls. FEBS Lett 384(3):240–242
Misawa N, Nakagawa M, Kobayashi K, Yamano S, Izawa Y, Nakamura K, Harashima K (1990) Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J Bacteriol 172(12):6704–6712
Miura Y, Kondo K, Saito T, Shimada H, Fraser PD, Misawa N (1998) Production of the carotenoids lycopene, beta-carotene, and astaxanthin in the food yeast Candida utilis. Appl Environ Microbiol 64(4):1226–1229
Naguib YM (2000) Antioxidant activities of astaxanthin and related carotenoids. J Agric Food Chem 48(4):1150–1154
Netzer R, Stafsnes MH, Andreassen T, Goksoyr A, Bruheim P, Brautaset T (2010) Biosynthetic pathway for gamma-cyclic sarcinaxanthin in Micrococcus luteus: heterologous expression and evidence for diverse and multiple catalytic functions of C(50) carotenoid cyclases. J Bacteriol 192(21):5688–5699. doi:10.1128/JB.00724-10
Nishizaki T, Tsuge K, Itaya M, Doi N, Yanagawa H (2007) Metabolic engineering of carotenoid biosynthesis in Escherichia coli by ordered gene assembly in Bacillus subtilis. Appl Environ Microbiol 73(4):1355–1361. doi:10.1128/AEM.02268-06
Norgård S, Aasen AJ, Liaaen-Jensen S (1970) Bacterial carotenoids. 32. C50-carotenoids 6. Carotenoids from Corynebacterium poinsettiae including four new C50-diols. Acta Chem Scand 24(6):2183–2197
Olson JA (1993) Molecular actions of carotenoids. Ann N Y Acad Sci 691:156–166
Osawa A, Ishii Y, Sasamura N, Morita M, Kasai H, Maoka T, Shindo K (2010) Characterization and antioxidative activities of rare C(50) carotenoids-sarcinaxanthin, sarcinaxanthin monoglucoside, and sarcinaxanthin diglucoside-obtained from Micrococcus yunnanensis. J Oleo Sci 59(12):653–659
Papagianni M (2012) Recent advances in engineering the central carbon metabolism of industrially important bacteria. Microb Cell Fact 11:50. doi:10.1186/1475-2859-11-50
Papp T, Velayos A, Bartok T, Eslava AP, Vagvolgyi C, Iturriaga EA (2006) Heterologous expression of astaxanthin biosynthesis genes in Mucor circinelloides. Appl Microbiol Biotechnol 69(5):526–531. doi:10.1007/s00253-005-0026-6
Pfander H (1994) C-45-carotenoids and C-50-carotenoids. Pure Appl Chem 66(10–11):2369–2374. doi:10.1351/pac199466102369
Pinnola A, Dall’Osto L, Gerotto C, Morosinotto T, Bassi R, Alboresi A (2013) Zeaxanthin binds to light-harvesting complex stress-related protein to enhance nonphotochemical quenching in Physcomitrella patens. Plant Cell 25(9):3519–3534. doi:10.1105/tpc.113.114538
Quinones MA, Zeiger E (1994) A putative role of the xanthophyll, zeaxanthin, in blue light photoreception of corn coleoptiles. Science 264(5158):558–561. doi:10.1126/science.264.5158.558
Rodrigues E, Mariutti LR, Mercadante AZ (2012) Scavenging capacity of marine carotenoids against reactive oxygen and nitrogen species in a membrane-mimicking system. Mar Drugs 10(8):1784–1798. doi:10.3390/md10081784
Rodriguez-Saiz M, de la Fuente JL, Barredo JL (2010) Xanthophyllomyces dendrorhous for the industrial production of astaxanthin. Appl Microbiol Biotechnol 88(3):645–658. doi:10.1007/s00253-010-2814-x
Rodriguez-Villalon A, Perez-Gil J, Rodriguez-Concepcion M (2008) Carotenoid accumulation in bacteria with enhanced supply of isoprenoid precursors by upregulation of exogenous or endogenous pathways. J Biotechnol 135(1):78–84. doi:10.1016/j.jbiotec.2008.02.023
Rohmer M (1999) The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep 16(5):565–574
Rohmer M, Bouvier P, Ourisson G (1979) Molecular evolution of biomembranes: structural equivalents and phylogenetic precursors of sterols. Proc Natl Acad Sci U S A 76(2):847–851
Rottem S, Markowitz O (1979) Carotenoids acts as reinforcers of the Acholeplasma laidlawii lipid bilayer. J Bacteriol 140(3):944–948
Saelices L, Youssar L, Holdermann I, Al-Babili S, Avalos J (2007) Identification of the gene responsible for torulene cleavage in the Neurospora carotenoid pathway. Mol Genet Genomics 278(5):527–537. doi:10.1007/s00438-007-0269-2
Sandmann G (2001) Carotenoid biosynthesis and biotechnological application. Arch Biochem Biophys 385(1):4–12. doi:10.1006/abbi.2000.2170
Scalcinati G, Partow S, Siewers V, Schalk M, Daviet L, Nielsen J (2012) Combined metabolic engineering of precursor and co-factor supply to increase alpha-santalene production by Saccharomyces cerevisiae. Microb Cell Fact 11:117. doi:10.1186/1475-2859-11-117
Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276(5320):1872–1874
Sedkova N, Tao L, Rouviere PE, Cheng Q (2005) Diversity of carotenoid synthesis gene clusters from environmental Enterobactetiaceae strains. Appl Environ Microbiol 71(12):8141–8146. doi:10.1128/aem.71.12.8141-8146.2005
Seibold G, Auchter M, Berens S, Kalinowski J, Eikmanns BJ (2006) Utilization of soluble starch by a recombinant Corynebacterium glutamicum strain: growth and lysine production. J Biotechnol 124(2):381–391. doi:10.1016/j.jbiotec.2005.12.027
Sivy TL, Fall R, Rosenstiel TN (2011) Evidence of isoprenoid precursor toxicity in Bacillus subtilis. Biosci Biotechnol Biochem 75(12):2376–2383
Song GH, Kim SH, Choi BH, Han SJ, Lee PC (2013) Heterologous carotenoid-biosynthetic enzymes: functional complementation and effects on carotenoid profiles in Escherichia coli. Appl Environ Microbiol 79(2):610–618. doi:10.1128/AEM.02556-12
Steinbrenner J, Sandmann G (2006) Transformation of the green alga Haematococcus pluvialis with a phytoene desaturase for accelerated astaxanthin biosynthesis. Appl Environ Microbiol 72(12):7477–7484. doi:10.1128/AEM.01461-06
Sui X, Kiser PD, Lintig J, Palczewski K (2013) Structural basis of carotenoid cleavage: from bacteria to mammals. Arch Biochem Biophys 539(2):203–213. doi:10.1016/j.abb.2013.06.012
Takaichi S, Maoka T, Masamoto K (2001) Myxoxanthophyll in Synechocystis sp. PCC 6803 is myxol 2′-dimethyl-fucoside, (3R,2′S)-myxol 2′-(2,4-di-O-methyl-alpha-L-fucoside), not rhamnoside. Plant Cell Physiol 42(7):756–762
Tao L, Schenzle A, Odom JM, Cheng Q (2005) Novel carotenoid oxidase involved in biosynthesis of 4,4′-diapolycopene dialdehyde. Appl Environ Microbiol 71(6):3294–3301. doi:10.1128/AEM.71.6.3294-3301.2005
Tao L, Yao H, Cheng Q (2007) Genes from a Dietzia sp. for synthesis of C40 and C50 beta-cyclic carotenoids. Gene 386(1–2):90–97. doi:10.1016/j.gene.2006.08.006
Tatituri RV, Illarionov PA, Dover LG, Nigou J, Gilleron M, Hitchen P, Krumbach K, Morris HR, Spencer N, Dell A, Eggeling L, Besra GS (2007) Inactivation of Corynebacterium glutamicum NCgl0452 and the role of MgtA in the biosynthesis of a novel mannosylated glycolipid involved in lipomannan biosynthesis. J Biol Chem 282(7):4561–4572. doi:10.1074/jbc.M608695200
Tjahjono AE, Kakizono T, Hayama Y, Nishio N, Nagai S (1994) Isolation of resistant mutants against carotenoid biosynthesis inhibitors for a green alga Haematococcus pluvialis, and their hybrid formation by protoplast fusion for breeding of higher astaxanthin producers. J Ferment Bioeng 77(4):352–357. doi:10.1016/0922-338x(94)90003-5
To KY, Lai EM, Lee LY, Lin TP, Hung CH, Chen CL, Chang YS, Liu ST (1994) Analysis of the gene cluster encoding carotenoid biosynthesis in Erwinia herbicola Eho13. Microbiology 140(Pt 2):331–339
Tobias AV, Arnold FH (2006) Biosynthesis of novel carotenoid families based on unnatural carbon backbones: a model for diversification of natural product pathways. Biochim Biophys Acta 1761(2):235–246. doi:10.1016/j.bbalip.2006.01.003
Tsuchidate T, Tateno T, Okai N, Tanaka T, Ogino C, Kondo A (2011) Glutamate production from beta-glucan using endoglucanase-secreting Corynebacterium glutamicum. Appl Microbiol Biotechnol 90(3):895–901. doi:10.1007/s00253-011-3116-7
Uhde A, Youn JW, Maeda T, Clermont L, Matano C, Kramer R, Wendisch VF, Seibold GM, Marin K (2013) Glucosamine as carbon source for amino acid-producing Corynebacterium glutamicum. Appl Microbiol Biotechnol 97(4):1679–1687. doi:10.1007/s00253-012-4313-8
Ukibe K, Hashida K, Yoshida N, Takagi H (2009) Metabolic engineering of Saccharomyces cerevisiae for astaxanthin production and oxidative stress tolerance. Appl Environ Microbiol 75(22):7205–7211. doi:10.1128/AEM.01249-09
Umeno D, Tobias AV, Arnold FH (2005) Diversifying carotenoid biosynthetic pathways by directed evolution. Microbiol Mol Biol Rev 69(1):51–78. doi:10.1128/MMBR.69.1.51-78.2005
Vershinin A (1999) Biological functions of carotenoids—diversity and evolution. Biofactors 10(2–3):99–104
Verwaal R, Wang J, Meijnen JP, Visser H, Sandmann G, van den Berg JA, van Ooyen AJ (2007) High-level production of beta-carotene in Saccharomyces cerevisiae by successive transformation with carotenogenic genes from Xanthophyllomyces dendrorhous. Appl Environ Microbiol 73(13):4342–4350. doi:10.1128/AEM.02759-06
Verwaal R, Jiang Y, Wang J, Daran JM, Sandmann G, van den Berg JA, van Ooyen AJ (2010) Heterologous carotenoid production in Saccharomyces cerevisiae induces the pleiotropic drug resistance stress response. Yeast 27(12):983–998. doi:10.1002/yea.1807
Wang F, Jiang JG, Chen Q (2007) Progress on molecular breeding and metabolic engineering of biosynthesis pathways of C30, C35, C40, C45, C50 carotenoids. Biotechnol Adv 25(3):211–222. doi:10.1016/j.biotechadv.2006.12.001
Westfall PJ, Pitera DJ, Lenihan JR, Eng D, Woolard FX, Regentin R, Horning T, Tsuruta H, Melis DJ, Owens A, Fickes S, Diola D, Benjamin KR, Keasling JD, Leavell MD, McPhee DJ, Renninger NS, Newman JD, Paddon CJ (2012) Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc Natl Acad Sci U S A 109(3):E111–E118. doi:10.1073/pnas.1110740109
Winterhalter P, Rouseff R (2001) Carotenoid-derived aroma compounds: an introduction carotenoid-derived aroma compounds. vol 802. ACS Symposium Series, Washington, DC, pp 1-17
Xue J, Ahring BK (2011) Enhancing isoprene production by genetic modification of the 1-deoxy-d-xylulose-5-phosphate pathway in Bacillus subtilis. Appl Environ Microbiol 77(7):2399–2405. doi:10.1128/AEM.02341-10
Ye VM, Bhatia SK (2012) Pathway engineering strategies for production of beneficial carotenoids in microbial hosts. Biotechnol Lett 34(8):1405–1414. doi:10.1007/s10529-012-0921-8
Yoshida K, Ueda S, Maeda I (2009) Carotenoid production in Bacillus subtilis achieved by metabolic engineering. Biotechnol Lett 31(11):1789–1793. doi:10.1007/s10529-009-0082-6
Zeiger E, Zhu JX (1998) Role of zeaxanthin in blue light photoreception and the modulation of light-CO2 interactions in guard cells. J Exp Bot 49:433–442. doi:10.1093/jexbot/49.suppl_1.433
Zhao J, Li Q, Sun T, Zhu X, Xu H, Tang J, Zhang X, Ma Y (2013) Engineering central metabolic modules of Escherichia coli for improving beta-carotene production. Metab Eng 17:42–50. doi:10.1016/j.ymben.2013.02.002
Zhou K, Zou R, Zhang C, Stephanopoulos G, Too HP (2013) Optimization of amorphadiene synthesis in Bacillus subtilis via transcriptional, translational, and media modulation. Biotechnol Bioeng 110(9):2556–2561. doi:10.1002/bit.24900
Acknowledgements
SAEH, PPW and VFW acknowledge the support in part by grants from BMBF project 0316017A and from EU project PROMYSE. JB acknowledges the “Platform Green Synthetic Biology” program (http://www.pgsb.nl/) funded by the Netherlands Genomics Initiative for financial support. TB acknowledges the support in part by EU project PROMYSE.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heider, S.A.E., Peters-Wendisch, P., Wendisch, V.F. et al. Metabolic engineering for the microbial production of carotenoids and related products with a focus on the rare C50 carotenoids. Appl Microbiol Biotechnol 98, 4355–4368 (2014). https://doi.org/10.1007/s00253-014-5693-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5693-8