Abstract
Brain death is associated with complex physiologic changes that may impact the management of the potential organ donor. Medical management is critical to actualizing the individual or family’s intent to donate and maximizing the benefit of that intent. This interval of care in the PICU begins with brain death and consent to donation and culminates with surgical organ procurement. During this phase, risks for hemodynamic instability and compromise of end organ function are high. The brain dead organ donor is in a distinct and challenging pathophysiologic condition that culminates in multifactorial shock. The potential benefits of aggressive medical management of the organ donor may include increased number of donors providing transplantable organs and increased number of organs transplanted per donor. This may improve graft function, graft survival, and patient survival in those transplanted. In this chapter, pathophysiologic changes occurring after brain death are reviewed. General and organ specific donor management strategies and logistic considerations are discussed. There is a significant opportunity for enhancing donor multi-organ function and improving organ utilization with appropriate PICU management.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Successful medical management of the organ donor is critical to actualizing the individual or family’s intent to donate and maximizing the benefit of that intent. This interval of care in the PICU begins with brain death and consent to donation and culminates with surgical organ procurement. It generally ranges from 12 to 48 h or longer and is related to the time required for repeated brain death declarations, consent discussions with the family, procurement logistics of donor/organ evaluation, and donor/recipient matching. During this phase, risks for hemodynamic instability and compromise of end organ function are high (Table 38.1). There is a significant opportunity for enhancing donor multi-organ function and improving organ utilization with appropriate medical management [1].
The brain dead organ donor is in a distinct and challenging pathophysiological condition that culminates in a state of multifactorial shock. The current level of evidence supporting practices in pediatric donor management is limited by the inherent lack of prospective trial data, and based largely on adult human and animal studies; however, donor management practice is of increasing importance [2–5]. PICU care should be tailored by principles similar to the management of any patient with multifactorial shock. It is important to treat the donor as one would treat the transplant recipient. This can be accomplished by understanding the physiology of brain death coupled with aggressive and attentive PICU management.
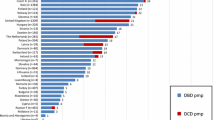
Figure 38.1 shows the Canadian experience of organ utilization across all age groups, comparable to international rates [6–8]. Utilization rates vary from region to region and transplant center to transplant center. Rates for heart and lung utilization have the greatest capacity for quantitative improvement. For the purposes of most international reports, a “donor” is one who has provided at least one organ that has been transplanted (Table 38.2). Initial interventions to increase transplantation focused on identification, referral, and consent of the donor, recent pursuit of organ yield has gained importance [9]. Pediatric investigators have reported rates of 3.6 organs per donor (of eight possible organs), but 22 % of consented pediatric donors failed to provide any transplantable organs primarily due to hemodynamic instability during the phase of PICU donor care [10].
Organ-specific utilization rates for deceased donors, Canada, 1993–2002 (Reprinted from Badovinac et al. [6]. With permission from Springer Science + Business Media.)
The goal of PICU based donor management is to improve the utilization of organs from consented donors to transplant recipients. The potential benefits of aggressive medical management of the organ donor may include increased number of donors providing transplantable organs and increased number of organs transplanted per donor. This may improve graft function, graft survival and patient survival in those transplanted [11].
The Physiology of Brain Death
The deterioration of cardiovascular and pulmonary function associated with intracranial hypertension will vary with the rapidity of rise of intracranial pressure (ICP) [12], time after herniation, and presence of coexisting forms of myocardial injury e.g., traumatic myocardial contusion, ischemia after cardiac arrest, shock, or hypoxemia [13, 14]. In the face of markedly elevated ICP, mean arterial pressure (MAP) rises in an effort to maintain cerebral perfusion pressure. As ICP rises further, cerebral herniation into the brainstem ensues, and brainstem ischemia is initiated in an orderly, rostral-caudal fashion. Initial apnea, bradycardia, hypotension and drop in cardiac output are mediated by vagal (parasympathetic) activation resulting from midbrain ischemia. Brainstem ischemia then progresses toward the pons, where sympathetic stimulation is superimposed on the initial vagal response, resulting in bradycardia and hypertension (the classic Cushing’s reflex) [15]. During this period, the ECG may be characterized by sinus bradycardia, junctional escape beats, and even complete heart block [16]. Further extension into the medulla oblongata occurs, at which point the vagal cardiomotor nucleus becomes ischemic, preventing tonic vagal stimuli. This results in unopposed sympathetic stimulation which may last for minutes to hours and manifests as arterial hypertension with elevated cardiac output with the potential for tachyarrhythymias [16]. This period of unopposed sympathetic stimulation is often termed the “autonomic” or “sympathetic storm” during which time cardiotoxicity occurs and severe vasoconstriction may compromise end organ perfusion [17, 18]. Subsequent changes occur in oxygen consumption and delivery [19]. Herniation triggers an inflammatory cascade that affects cardiorespiratory function, and hormonal regulation [20]. This cascade affects pituitary and hypothalamic function resulting in catecholamine, thyroid, and vasopressin abnormalities [21–24].
Neurogenic Myocardial Dysfunction
The sympathetic storm is responsible for potentially reversible myocardial injury and has been best studied in subarachnoid hemorrhage [25], where is called “neurogenically stunned myocardium” [26, 27]. Endogenous catecholamine-related increases in peripheral resistance may result in a sudden increase in myocardial work and oxygen consumption leading to myocardial ischemia or infarction and subsequent elevation of cardiac troponin I and T [15, 28]. Patients dying of acute intracranial events show scattered foci of transmural myocardial injury that are not seen in patients dying of noncerebral causes [29]. Myocardial necrosis after subarachnoid hemorrhage is a neurally mediated process that is dependent on the severity of neurological injury [30]. Brain dead cardiac donors with elevations in cardiac troponin I have been shown to have diffuse subendocardial myocytolysis and coagulative necrosis and a high incidence of graft failure after transplantation [31]. The magnitude of the rise of epinephrine after brain death and the extent of myocardial damage have also been shown to depend on the rate of rise in ICP in a canine model [12, 32]. Dogs given a sudden rise in ICP demonstrated a higher epinephrine surge and poorly functioning donor hearts. Surgical sympathectomy [33] or pharmacologic sympathetic blockade in humans [34] and animals [35, 36] effectively prevents the ICP-related catecholamine cardiotoxicity and the electrophysiologic, biochemical and pathologic changes characteristic of neurogenic injury in the heart. While the ICP-related sympathetic storm is characterized by myocardial injury and high systemic vascular resistance, it is soon followed by period of sympathetic depletion and a low SVR state. Brain dead patients become functionally decapitated and the sympathetic system is anatomically interrupted, similar to high spinal cord injuries [37].
Neurogenic Pulmonary Edema
This unopposed sympathetic stimulation mediates the myocardial injury and is also likely responsible for the neurogenic pulmonary edema often seen in the management of patients with acute elevations of ICP (Fig. 38.2) [38]. Practitioners should be aware of this fulminant presentation of sudden onset respiratory failure with large volume, frothy tracheal secretions. In primate models of acute intracranial hypertension, acute heart failure ensues with reversal of flow in the pulmonary circulation due to massive rises in left atrial pressure [33]. Rupture of pulmonary capillaries can occur from this retrograde increase in vascular hydrostatic pressure [39]. This hydrostatic pulmonary edema is responsive to high PEEP and is generally reversible with time [40, 41].
Inflammatory State
Brain death is also associated with the up-regulation and induction of the inflammatory response in all somatic organs [42], triggering a cascade of mediators that may affect graft function [43]. Transient focal cerebral ischemia upregulates the transcriptional levels of TNF-α, IL-6, and other markers [44, 45]. Rapid rises of ICP causes immune activation in peripheral organs resulting in enhanced immunogenicity [32]. In animal models, brain death has a detrimental effect on hepatic dysfunction related to immune activation and appears to be independent of hemodynamic instability [46] and magnified by longer ischemic times [47]. In comparison to living related kidney donors, kidneys from brain dead donors have significantly higher levels of pro-inflammatory mediators on biopsy (endothelial E-selectin and proximal tubular expression of HLA-DR antigens, intracellular adhesion molecule-1, and vascular cell adhesion molecule-1) [48, 49]. Delayed renal graft function and acute rejection in the recipient is correlated to higher indices of free radical mediated injury in the donor [50, 51]. Evidence that neurogenic pulmonary edema may be alleviated with glucocorticoids also suggests that an inflammatory component exists in this process [52, 53]. Recent animal work suggests that this inflammation is triggered by the acute hemodynamic effects of ICP-related neurogenic myocardial dysfunction, resulting in hydrostatic pressure based neurogenic pulmonary edema and rupture of the alveolar-capillary membrane [39, 54].
Brain death is an important risk factor itself and influences graft outcomes, mediated by postischemic reperfusion injury and other nonantigen-dependent inflammatory pathways [55, 56]. Deleterious processes such as inflammation and fibrosis occur in donor organs [57] potentiating graft immunogenicity and increases host alloresponsiveness organs, developing and contributing to reduced graft survival [58]. These findings may used to introduce specific cytoprotective interventions in the brain dead donor to reduce the immunogenicity or the pro-inflammatory status of the graft and better maintain or increase organ viability. Anti-inflammatory therapies may be beneficial on eventual graft status [45].
Donor Management: General
Cardiovascular Performance and Monitoring
The etiology of low cardiac output in brain dead patients is complex and time dependent. It may be characterized by low preload due to vascular volume depletion, contractile myocardial dysfunction, and variable SVR states ranging from extreme vasoconstriction from ICP-related sympathetic storm to vasodilation from sympathetic arrest. Resuscitation of the cardiopulmonary system benefits the function of all end organs in the brain dead donor. The variety of changes in volume status, cardiac inotropy, and peripheral vascular resistance that occur after brain death are similar to those in any pediatric critically ill patient with shock of diverse etiology. Intensivists should titrate cardiovascular therapy to clinical, biochemical and hemodynamic endpoints that ensure restoration of intravascular volume status, and appropriate support of the myocardium and vascular system to ensure optimal cardiac output for organ perfusion. Optimization with aggressive intensive care can optimize transplantation [59].
Evaluation of cardiocirculatory status is a global assessment of multiple variables. Traditional and vigilant hemodynamic assessments should be provided, based on physical findings, vital signs, central venous pressure, urine output, central or mixed venous oximetry and serial lactate measurements. Escalation of support should be accompanied by escalation of hemodynamic monitoring.
Echocardiographic parameters have also been demonstrated to be beneficial in predicting successful cardiac transplant outcomes [60, 61]. Echocardiographic systolic myocardial dysfunction is present in 42 % of adult brain death and associated with ventricular arrhythmias [60]. Diffuse wall motion abnormalities are a risk factor for 30-day heart transplant mortality [62]. Evaluation of left ventricular end diastolic diameter, ventricular wall thickness, and coronary flow are felt to influence transplantation [63]. Single echocardiographic evaluations may have limitations in detecting the reversible myocardial dysfunction often seen after brain injury [26]. Recent studies advocate pharmacologic stress evaluation for organ suitability [64]. The utility of serial echocardiograms to evaluate improvement in myocardial dysfunction in the brain dead donor and to better predict cardiac allograft survival has been reported in adults [65] and is evolving into routine practice. Studies show that serial echocardiogram lacked specificity but was particularly useful in showing improvements following aggressive donor management [66].
Right-sided pressures may underestimate left-sided pressures after brain death and may increase risk for elevated left-sided filling pressures and pulmonary edema [67]. Expert consensus supports pulmonary arterial catheterization (PAC) and cardiac output monitoring in adults, particularly if the donors are hemodynamically unstable or initial ejection fraction is less than 40–45 % [68, 69]. PAC and goal-directed hemodynamic therapy of initially unacceptable donors, in conjunction with hormonal therapy may improve the rate of organ procurement without compromising transplant outcomes [70]. A significant increase in heart recovery was seen with the use of PAC in adult studies [71]. The Transplantation Committee of the American College of Cardiology has recommended titrating volume infusions and dopamine to thermodilution indices [72]. Justifications for PAC are not limited to the precise titration of hemodynamic support but are also required for the evaluation of suitability for heart and lung transplantation. As the use of PAC in PICU care is limited, serial echocardiography at q6-12 hourly intervals has been recommended [69].
Newer non-invasive methods of monitoring cardiac output are becoming more common because of their ease of use and safety, but are still not well validated for use in donor management, especially in children [73, 74]. Though management of the donor is similar to other shock states, monitoring of central venous saturations has not been recommended because of the lack of normal values in the donor patient [75]. Monitoring of other biochemical markers such as acidosis, lactates, and electrolytes is essential [76].
Hemodynamic Targets and Supports
Following the sympathetic storm, a subsequent reduction in catecholamines and sympathetic hormones result in a normotensive or hypotensive phase. This stage is characterized by impaired cardiac inotropy and chronotropy, impaired vascular tone and a reduced cardiac output. Clinical deterioration (progressive hypotension, hypoxia, anuria ± cardiac arrest) during the interval from brain death to procurement is common without aggressive intervention [77]. Cardiovascular support should be based on rational physiology and should be preceded by volume resuscitation to normovolemia.
Preload
Significant volume depletion is anticipated in brain-injured patients after brain death due to fluid restriction, diuretics, hyperosmolar therapy, third space losses, hemorrhage and/or diabetes insipidus. In addition, a low SVR state may result in relative hypovolemia. In a Canadian study of 77 pediatric organ donors [2], 53 % suffered sustained hypotension and 35 % deteriorated to cardiac arrest. This was more common in patients treated with inotropic agents in the presence of a low central venous pressure and in those without anti-diuretic hormone replacement, emphasizing the importance adequate restoration of intravascular volume. Organ transplantation may be less favourable in preload dependent donors, possibly related to higher inflammatory response [78].
The optimal volume status of the brain dead patient is controversial and transplant-organ specific. Disparity exists between lung and kidney interests (“dry lungs” versus “wet kidneys”). In a study of crystalloid fluid management in 26 brain dead donors, a significant increase in the alveolar-arterial oxygen gradient was seen in those who achieved a central venous pressure (CVP) of 8–10 compared to those whose CVP was maintained at 4–6 mmHg [67]. Some authors advocate maintaining a CVP of 10–12 mmHg to volume replete those patients in whom only abdominal organs are to be procured, a CVP < 8 mmHg for potential lung donors and a CVP of 8–10 mmHg if both thoracic and abdominal organs are to be harvested [79]. This approach is impractical since all organs should initially be considered potentially transplantable. Effectively, euvolemia is the reasonable goal and the assessment of volume status should be based on experienced clinical evaluation [80–82].
Contractility
The preferred choice of contractility agents in PICU practice varies according to individual center. Traditionally, dopamine or dobutamine has been used as the initial inotrope of choice in the brain dead patient. However, no randomized trials exist comparing the hemodynamic effects of dopamine to other inotropes or vasopressors and their influence on graft survival. β-agonist therapy should be used with caution in potential heart donors given concerns about myocardial adenosine triphosphate (ATP) depletion and desensitization of β-receptors [83]. If the heart is being considered for donation, dopamine or its equivalent should not be escalated beyond 10 μg/kg/min due to risks of increases oxygen demand [69]. High dose dopamine has been related to poor graft survival for hearts but favourable for other organs such kidneys [84, 85]. Use of epinephrine alone or as an adjunct may be appropriate in these cases.
Systemic Vascular Resistance
The functional sympathectomy associated with brain death results in low SVR that often requires the use of vasoconstricting agents. The concern over the use of alpha-agonists such as norepinephrine or phenylephrine has arisen because of the fear of inducing central and peripheral vasoconstriction and subsequent ischemia in coronary and vascular beds supplying potentially transplantable organs. However, in studies of other causes of shock states with low SVR (septic patients), norepinephrine, as compared to dopamine, was demonstrated to increase mean perfusion pressures without adverse effects to renal and splanchnic blood flow [86–88]. Early use of vasoconstrictor agents alone or in adjunction with inotropes is suggested [69].
Vasopressin and Catecholamine Sparing in Brain Death
Arginine vasopressin (AVP) is a unique agent because it can be used for a variety of applications in donor management, e.g. hemodynamic vasopressor support, diabetes insipidus therapy, and hormonal therapy. Brain death and hypotension are often associated with vasopressin deficiency [89]. Low-dose AVP infusions have been shown to improve hemodynamic stability and spare catecholamine use [89, 90]. Prolonged hemodynamic stability can be maintained after brain death with low-dose AVP (1–2 units/h), permitting a significant decrease in epinephrine and extended preservation of renal function [91]. In a rigorous, randomized study of volume-resuscitated brain dead organ donors supported with dopamine, 0.30 mU/kg/min infusion of AVP significantly increased MAP and SVR and spared dopamine use compared to further fluid loading [92]. Pediatric donors given AVP (41 ± 69 mU/kg/h) respond by increasing MAP and weaning alpha-agonists (norepinephrine, epinephrine, phenylephrine) without significant differences in the quality of kidneys, livers and hearts recovered [21, 93]. Similar catecholamine-sparing effects of AVP have been demonstrated in septic shock patients with low SVR [94, 95].
Optimal dosing of AVP in relation to its effects on organ procurement and graft survival are unclear. Concern has been expressed regarding risks of splanchnic ischemia in vasodilatory shock [96, 97]. Practitioners should be cautioned regarding the multiple and confusing dosing units used throughout the literature. Although it is suggested that doses of AVP exceeding 0.04 U/min (approx. 40 mU/kg/h) may be associated with excessive vasoconstriction in sepsis [94] brain dead donors respond to AVP infusions of 0.04–0.1 U/min (40–100 mU/kg/h) [89] without histologic evidence of cardiac damage [98]. Recent evidence shows that systemic and SMA flow may be compromised with AVP versus dopamine [99]. Available literature suggests that the use of AVP at doses up to 0.04 U/min in adults (2.4 U/h) and 0.0003–0.0007 U/kg/min (0.3–0.7 mU/kg/min) in children can be recommended to support the MAP and spare catecholamines [69].
Oxygenation and Ventilation Strategies
Many potential donors have various etiologies of donor-related lung injury and dysfunction that may include neurogenic pulmonary edema, aspiration, atelectasis, pulmonary contusion, bronchopulmonary infection, alveolar-capillary inflammation, and diffuse alveolar damage [59]. Pulse oximetry, serial arterial blood gas monitoring, endotracheal tube suctioning, and serial chest x-rays are considered standard in donors [100]. Mechanical ventilation should be tailored to the following empirical recommendations: fraction of inspired oxygen (FiO2) titrated to keep oxygen saturation ≥ 95 %, partial pressure of arterial oxygen (PaO2) ≥ 80 mmHg, pH 7.35–7.45, PaCO2 35–45 mmHg, positive end expiratory pressure (PEEP) of 5 cm H2O [69, 101, 102]. A prospective, randomized control trial in potential adult donors, ARDS-type lung protective strategies with tidal volumes of 6–8 mL/kg and 8–10 cmH2O PEEP significantly increased lung utilization for transplantation [103].
Metabolic and Endocrine
Glycemia and Nutrition
Hyperglycemia is common in brain dead donors [77]. It may be secondary to insulin resistance as pancreatic function appears to be preserved [104], which may be aggravated by corticosteroid therapy and dextrose-based fluid replacements used for diabetes insipidus. Insulin is variably and inconsistently considered as part of hormonal resuscitation cocktails. The hypothesis that tight glycemic control in the brain dead donor improves graft survival has not been tested, but has been recommended by expert consensus [68, 69]. Hyperglycemia has been shown to be an independent risk factor for poor outcome after severe brain injury in children [105] and adults [106].
Dextrose infusions and nutrition are generally withheld in the acute PICU management after brain injury [107], a practice supported by animal models [108]. Malnutrition or depletion of cellular glycogen stores may be common during the phase of care leading to brain death [109]. The influence of donor nutrition on graft survival has been studied in several animal studies but not formally in humans. In a rabbit and porcine model, improved liver transplant survival was shown from donors receiving enteral nutrition versus fasting donors [110]. A significant improvement in hepatic sinusoidal lining cell viability has been demonstrated in rats with liver grafts from donors receiving enteral feeding and intraperitoneal glucose prior to liver procurement. Glycogen appears to protect the hepatic graft upon rewarming in rats [111].
The importance of nutritional support in the human multi-organ donor, however, is not clear but is of increased interest [112]. Studies of donor-specific predictors of graft function following liver transplantation suggested a length of stay in the ICU of greater than 3 days as a risk factor [113]. A contributing factor to this association may be the effect of starvation on the liver with depletion of glycogen stores. In a controlled prospective randomized study of 32 patients it was shown that an intraportal infusion of insulin (1 IU/kg/h) and glucose reglycogenates the liver, increases glycogen utilization during cold and rewarming periods, and improves transaminase levels [114]. However, the only human series of liver transplants that included donor nutritional status failed to identify an independent effect of donor nutrition on postoperative liver graft function [115]. As a general approach, intravenous dextrose infusions should be given routinely and routine enteral or parenteral feeding should be initiated or continued as tolerated [116].
Diabetes Insipidus and Hypernatremia
Dysfunction of the posterior pituitary in brain dead donors is common; anterior pituitary function is often preserved [117]. Histologic observations of the pituitary gland demonstrate various degrees of edema, hemorrhage, and tissue necrosis depending on the mechanism and site of traumatic or ischemic brain injury [118, 119]. This is likely to be a result of compromised blood supply to the cell bodies arising in the deep supraventricular and paraventricular nuclei of the hypothalamus, whose neurons supply the posterior pituitary and regulate AVP secretion. Anterior pituitary function is often preserved, implying that some blood supply via the hypophyseal arteries, which arise extradurally, is reaching the median eminence of the hypothalamus [117]. Undetectable levels of antidiuretic hormone (ADH) have been noted in 75 % of brain dead donors, and diabetes insipidus is present in up to 87 % [29, 30, 54, 55]. Diabetes insipidus may commonly appear prior to the diagnosis of brain death [118] and is associated with hemodynamic instability and the compromise of transplantable organ function [2, 23, 77].
Hypernatremia is frequently encountered, resulting from the preceding hyperosmolar therapy for initial brain injury or poorly controlled diabetes insipidus. Donor hypernatremia > 155 mmol/L at procurement has been shown to be independently associated with hepatic and renal dysfunction or graft loss after transplantation [115, 120–122], although new evidence may show that these concerns may be less significant [123]. A prospective study demonstrated the benefit of correcting donor sodium (Na) ≤ 155 mmol/L with equivalent graft success compared to donors who were never hypernatremic [124]. The mechanism of hepatic and kidney injury related to hypernatremia is unclear but may be related polyuria and dehydration and to the accumulation of idiogenic osmoles resulting in intracellular swelling after transplantation into the normonatremic recipient.
Ideal serum sodium (Na) target range is ≥130 ≤150 mmol/L [25]. A reasonable urine output target range is 0.5–3 ml/kg/h after brain death. Diabetes insipidus can be defined as a urine output > 4 ml/kg/h associated with rising serum Na ≥145 mmol/L and serum osmolarity ≥300 mosM and decreasing urine osmolarity ≤ 200 mosM [69].
DDAVP (analog 1-desamino-8-D-arginine vasopressin, or desmopressin) is commonly used for the treatment of diabetes insipidus in brain death without adverse effect on early or late graft function after renal transplantation [125]. It is highly selective for the vasopressin V2 receptor subtype found in the renal collecting duct and thus has a relatively pure antidiuretic effect with no significant vasopressor activity [126]. DDAVP has multiple potential routes of administration (iv, im, sc, intranasal, ETT) and corresponding variability of dose recommendations. In brain death, it is preferable to rely on the i.v. route with a recommended dosing range is 0.5–10 μg iv every 6–8 h [127]. Given its lack of vasopressor action, it can be safely titrated to the effect of ablating polyuria and normalizing serum sodium. Improved organ yield is associated with DDAVP use in donor management [11, 128].
Many authors have advocated the use of AVP for the treatment of diabetes insipidus in organ donors to modulate both diabetes insipidus and support cardiovascular system [68, 69, 74, 93, 129, 130]. In pediatric case series, doses of vasopressin between 0.25 and 2.7 mU/kg/h have been used to successfully treat hypothalamic diabetes insipidus [131–134]. Doses between 0.5 and 15 U/h of AVP have been advocated in adults, though there are concerns about high doses causing coronary, renal and splanchnic vasoconstriction, potentially jeopardizing cardiac, renal, pancreatic and hepatic function [99, 127]. The safety and efficacy of a combination of DDAVP (for its antidiuretic effect) with AVP as a vasopressor on cardiovascular and laboratory endpoints has been described [21, 92, 135]. Many protocols recommend separate dosing regimens for diabetes insipidus and support for perfusion. The upper limit of AVP recommended by the Transplantation Committee of the American College of Cardiology is 0.8–1.0 U/h (13–17 mU/kg/h) to treat diabetes insipidus [72].
Thyroid Hormone
Thyroid hormone increases cardiac output by improving both contractility and chronotropy, as well as by decreasing systemic vascular resistance [136]. The use of thyroid hormone therapy in brain dead donors is largely based on experimental animal models and human case series. Investigators describe variable levels of thyroid hormones after brain death and varying and conflicting effects of thyroid hormone administration. Thyroid-stimulating hormone (TSH), T4 and T3 levels were below normal in a majority of 22 brain dead donors [137]. Other studies have shown that these patients are suffering from “sick euthyroid syndrome” rather than TSH deficiency and do not require thyroid supplementation [54]. In the baboon model, T3 levels become depleted after brain death and the resulting transition to anaerobic metabolism is reversed with T3 replacement [138]. The positive effects on myocardial gene expression have been demonstrated [139].
In a comparative study in brain dead patients, T3, cortisol and insulin promoted aerobic metabolism, reduced the need for inotropic support and improved the rate of cardiac graft procurement [140, 141]. Other investigators were unable to demonstrate any improvement in echocardiographic function or organ retrieval rates with a similar hormone regimen [142]. Serum free T3 concentrations in organ donors may not correlate with hemodynamic stability [118] but replacement of thyroid hormone (T3) has shown to reduce need for vasopressor support and may improve the likelihood of heart transplantations [143–146]. There is equipoise for routine use since many other studies did not show improvement in cardiac and hemodynamic status [147, 148].
T4 infusion rather than T3 does not reduce vasopressor requirements or especially in pediatric donors [21] but this may be related to impaired peripheral conversion to T3. While there are numerous theoretical advantages of parenteral T3 over T4 (stability for iv infusion, does not require peripheral tissue conversion), it is extremely expensive in comparison to intravenous T4 and may not be commercially available in many countries. In those UNOS patients receiving hormone therapy, T4 was used in 93 % and T3 in 6.9 % of cases, with insufficient numbers to discriminate any benefit of T3 over T4 [Rosendale, Kauffman, personal communication]. Most studies for thyroid replacement in the context of the organ donor are of low quality with poor study design, thus limiting objective analysis [149].
Corticosteroids
Several publications have advocated the use of high-dose methylprednisolone in an effort to diminish inflammation thought to be present in donor lungs [102, 130, 150] and other organs. The initial evidence for this was largely based on a single retrospective analysis of 118 consecutive lung donors administered a non-uniform protocol of methylprednisolone (mean 14.5 mg/kg) compared with 38 donors not receiving methylprednisolone and demonstrating a significant improvement in donor oxygenation and lung procurement rate [151]. A recent analysis of the California Donor Network database demonstrated an independent effect of methylprednisolone on the successful procurement of lungs from the donor [152]. The UNOS database showed that heart graft survival benefit was also found in those donors receiving corticosteroids alone [150]. Recent studies do reveal a highly proinflammatory environment in donors but do not actually show benefit from methyprednisolone replacement [20]. Although the optimal dose and time effect (if any) of corticosteroids in brain dead donors are uncertain, guidelines recommend methylprednisolone 15 mg/kg q24h [69].
Combined Hormonal Therapy
Despite conflicting literature regarding use of hormones individually, there is strong evidence supporting the use of combined hormonal therapy in organ donors, defined as vasopressin, thyroid hormone, and methylprednisolone (insulin is inconsistently included in this strategy). The United Network for Organ Sharing (UNOS) database shows a 46 % reduced odds of post-transplant death within 30 days and a 48 % reduced odds of early cardiac graft dysfunction with the use of combined hormonal therapy in a large retrospective cohort [150]. Benefit was also found in those donors receiving corticosteroids alone or in combination with T3/T4 which is supported by independent studies [153, 154]. Recovery of organs was most beneficial to heart and lung transplantation [155]. Analysis of UNOS data suggests a substantial benefit from hormone therapy with minimal risk. A multivariate logistic regression analysis of 18,726 brain dead donors showed significant increases in kidney, liver and heart utilization from donors receiving three-drug hormonal therapy. Significant improvements in 1-year kidney graft survival and heart transplant patient survival were also demonstrated [147, 148, 150]. More recently, however, in a prospective randomized study T3 and methylprednisolone did not add to the effect on cardiac index shown by vasopressin and aggressive cardiovascular support [156]. Despite this recent finding, current expert consensus still strongly recommends the use of combined hormonal therapy for any donor with hemodynamic instability or reduced ejection fraction on echocardiography [68, 69].
Transfusion Thresholds
There are no rigorous studies that assess the role of red blood cell transfusions for short-term organ preservation during organ donor maintenance specifically. Prospective studies for transfusion thresholds suggest outcomes are similar with hemoglobin level at 7 g/dL in critically ill children [157]. However, consensus conferences recommend maintaining a hemoglobin level ≥ 10 g/dL or a hematocrit greater than 30 % [127, 130]. Large platelet transfusion requirements during liver transplant surgery are independently associated with more severe hepatic dysfunction after transplantation, but this is likely more indicative of a more technically complicated procedure and sicker recipient [115]. There is no literature identified to guide platelet or plasma factor replacement in the donor. Invasive procedures associated with bleeding risk may require correction of thrombocytopenia and coagulation status. Blood drawing for donor serology and tissue typing should occur prior to transfusions to minimize the risk of false results related to hemodilution. In regions where blood is routinely leukocyte depleted, and the risk of transmission of cytomegalovirus (CMV) is negligible and it may not be necessary to give CMV-negative blood to CMV-negative donors [69].
Invasive Bacterial Infections
Isolated cases of transmission of solid organ infection from donor to recipient may have significant consequences including graft infection, sepsis, and poor initial graft function [158–163]. While approximately 5 % of all donors will be bacteremic at the time of procurement, the routine use of broad spectrum antibiotics (vancomycin and ceftazidime/cefotaxime) in the recipient has been shown to prevent transmission of bacterial infection in organ recipients [164, 165]. Importantly, donor infections do not show differences in acute mortality or graft survival. The current expansion of potential marginal donors has increased the risk of infection. One study quoted bacteremia rates in the donor up to 21 % [166]. Donors with ICU stays greater than 3 days, rescue CPR, and inotropic agents are at increased risk [167]. Though rates of infections in donors may be up, organs obtained from donors with positive cultures continue to be transplanted safely, likely due to vigilant screening and polymicrobial therapy given to recipients [168, 169]. Other authors have described the successful transplantation of organs from donors declared brain dead from meningitis caused by Neisseria meningitides, Streptococcus pneumoniae and Escherichia coli without transmission to the recipient [170]. The finding of positive cultures does not preclude donation but may delay procurement until 24–48 h of treatment has been established. Prophylactic antibiotic therapy in the organ donor is generally not recommended.
Viral infections in the donor can affect recipients especially with post transplant immunosuppression. However, many chronic infections such as hepatitis B and C virus no longer preclude donation. Knowledge of potential risks is essential but often manageable in the appropriate recipient [171, 172].
Initial baseline blood, urine and endotracheal cultures should be obtained for all donors and repeated daily. PCR screens for common chronic viral infection are common. Positive blood cultures or presumed infections are not contraindications to organ donation but antibiotic therapy should be initiated early in cases of proven or presumed infection. Duration of therapy depends on the virulence of the organism and should be determined in consultation with the transplant team and infectious disease services.
Donor Management: Organ Specific
Heart
Wait list mortality among US children listed for heart transplant has decreased by two-thirds over the last 20 years [173]. Mostly this is related to extended donor and recipient criteria including ABO incompatible transplantation [174, 175]. This has not compromised clinical outcomes after transplantation [176]. Reasons for this are multifactorial but include improved donor management [177].
The majority of studies linking donor variables to heart transplant outcomes are in adults and related to known risk factors such as coronary artery disease, left ventricular hypertrophy, older age, diabetes mellitus, and chronic hypertension [62, 130, 178, 179]. While these variables may be indications for coronary angiography in the adult donor, they generally are not relevant to the pediatric population. Extrapolation from adult studies would suggest that myocardial dysfunction in the pediatric donor, as manifest by greater inotropic support [62, 180], pacemaker support [181], and reduced ejection fraction and/or wall motion abnormalities by echocardiography [62, 130] are important factors. Interestingly, donor CPR has not shown to be a negative factor for heart transplant survival [182, 183].
Potential heart donors should undergo routine screening by electrocardiogram (ECG) and 2D echocardiography. In children, initial echocardiography for heart donor evaluation should be performed only after hemodynamic resuscitation and repeat echocardiography should be considered after ≥6 h [69]. Intensive donor management has been show to improve function on serial echocardiography and may improve transplantability in up to 50 % [66, 184, 185]. Some advocate that the majority of echographic abnormalities in donor hearts resolve in the transplant recipient prior to discharge [186]. Reduced function should not preclude consideration for transplantation based on a single evaluation. Ejection fractions < 40–45 % do not necessarily translate into high transplant risk, as they may be related to related to inadequate cardiovascular resuscitation or neurogenic myocardial dysfunction that is reversible with time (see earlier sections). Adult data has shown significant improvements in echocardiographic function with time and conventional support [65], with up to 78 % of potential donors demonstrating clinically significant improvements [63, 184].
Serological markers such as donor troponin I and T have been linked to early cardiac graft failure [31, 187, 188] and should also be measured. However, these markers do not necessarily relate to graft dysfunction in recipients [189]. Though Tri-iodothyronine and methylprednisolone therapy is recommended, a recent study of 80 cardiac donors did not show acute improvement to cardiovascular function or donor yield [156].
Pulmonary artery catheterization (PAC) data has been linked to favorable transplant outcomes [70]. Reduced ejection fraction or hemodynamic instability has been recommended as an indication for PAC in adults to allow for both precision of hemodynamic support and evaluation of suitability for heart and lung transplantation especially in marginal donors [68, 190]. PAC use in pediatrics is still limited.
Lungs
Though lower yield than other organs donated, lung transplantation in pediatrics has increased with better techniques and improved management [191–193]. A relatively scarce donor pool has limited wider application for lung transplantation [194]. This has led to relaxation of donor criteria, specific donor management protocols that preserve lung function, and development of ex-vivo perfusion techniques to recondition suboptimal lungs, all of which have optimized transplantation [195, 196]. Outcomes for pediatric lung recipients are similar to adults but young children often do better due to a decreased incidence of rejection. Adolescent outcomes are poor mainly due to bronchiliolitis obliterans [197].
The ‘ideal’ lung donor has been previously defined but significant advances have been made in donor and recipient management allowing for increased use of marginal or ‘extended criteria’ donors [198–200]. There is some evidence that organs transplanted using extended donor criteria may have higher rates of early graft rejection [201, 202]. The quality of the lung donor and the subsequent recipient outcomes are related to the possibility of this primary graft dysfunction which is a result of multifactorial hemodynamic, metabolic, and inflammatory insults resulting from the brain dead donor [203]. Primary pulmonary allograft failure has pathological features of acute lung injury (ALI) and occurs in 12–50 % of transplanted patients [204–206]. This is often associated with inadequate lung preservation, ischemia-reperfusion injury and cellular rejection [207]. Despite this, many centers have adopted extended criteria in order to increase the number of potential donors [208, 209].
Traditional oxygenation criteria used as a threshold in the acceptance of donor lungs include a donor PaO2 > 300 mmHg on FiO2 of 100 % and PEEP of 5 cm H2O (P/F ratio > 300) [210]. However, recent and evolving efforts have improved the current criteria for donor selection [191, 211]. Physiological, microbiological and histological evaluation of rejected lungs from the California transplant registry show 41 % of rejected lungs were judged suitable for transplantation based on pulmonary edema, intact alveolar fluid clearance, and histology [212]. In a case series of 15 brain dead adults, lung grafts that did not meet the usual criteria for transplantation were found to have higher dynamic and static elastance measurements than donor lungs that met standard transplantation criteria [213]. The outcomes of 49 marginal donors (i.e., failing to meet one or more of the ideal criteria) showed no significant difference in duration of post-transplant mechanical ventilation or P/F ratio compared to ideal donors [211]. Investigators have also challenged donor PaO2 criteria by arguing that physiological donor factors influence peripheral arterial PaO2 independent of isolated individual lung function [214]. Despite poor global oxygenation, parenchymal abnormalities isolated to one lung may not preclude procurement of the contralateral lung [215].
The cause of brain death does not correlate with lung transplant outcomes, but there is improved outcomes with longer time interval before retrieval suggesting longer and specific donor management may reduce lung injury over time [101, 216]. Pulse oximetry, serial arterial blood gas monitoring, endotracheal tube suctioning, rotational positioning, chest x-ray, bronchoscopy and bronchoalveolar lavage are considered standard in the lung specific care of donor [100]. Mechanical ventilation should be tailored to the general targets (see previous section) [103]. Similar to the management of lung injury in general, alveolar recruitment and pressure limited ventilation strategies should be used in potential donors [59]. New strategies for the improvement of lung function in the donor such as airway pressure release ventilation have been utilized [217]. Excessive fluid administration deteriorates alveolar-arterial oxygenation gradients in potential donors [67] and may be an indication for diuresis. Steroid administration may also reduce progressive lung water accumulation [101]. Prolonged ventilation in the supine position results in loss of alveolar expansion and microatelectasis. In an experimental rat model, donor lungs develop microatelectasis despite PEEP and a relatively short ventilatory period before organ procurement [218]. Prevention of alveolar collapse enhances post mortem preservation of pulmonary grafts in a rabbit model [219]. Recruitment maneuvers in the form of high sustained PEEP for short durations may be a useful adjunct to prevent alveolar stress and collapse [220]. Lung donors failing traditional oxygenation criteria (P/F < 300) respond to aggressive bronchial toilet using bronchoscopy, physiotherapy, increasing tidal volume and increasing PEEP with improvements in P/F ratio > 300. Lungs were subsequently transplanted without differences in ICU length of stay or 30-day mortality compared to recipients of ideal donors [221]. Hemodynamic and reperfusion injury seem to play a significant role in donor lung injury [222]. The early use of norepinephrine or vasopressin may reduce lung injury [223].
Recent guidelines suggest that there should be no predefined lower limit for the P/F ratio that precludes consideration for transplantation. Timing of evaluation, temporal changes, response to alveolar recruitment and recipient status should be considered [69]. In cases of unilateral lung injury, pulmonary venous partial pressure of oxygen during intraoperative assessment is required to reliably evaluate contralateral lung function.
Bronchoscopy and Bronchopulmonary Infections
The consensus of expert opinion supports the use of bronchoscopy for the purposes of examining the tracheobronchial tree for abnormalities and collecting microbiological specimens [68, 129, 211]. Pathological studies of lungs rejected for donation have indicated that bronchopneumonia, diffuse alveolar damage, and diffuse lung consolidation are the three most common reasons for being deemed unsuitable [214]. Between 76 % and 97 % of bronchoalveolar lavages (BAL) will grow at least one organism [224, 225]. The most commonly identified organisms included Staphylococcus aureus and Enterobacter, and in 43 % of transplants, similar organisms were isolated from recipient bronchoscopy. Pulmonary infection in the graft recipient results in significantly lower survival compared with recipients who do not develop early graft infection [226]. Recipients with donor BAL cultures positive for either gram positive or gram negative bacteria had longer mean mechanical ventilation times and inferior 6-month to 4-year survival than those with negative bacterial BAL cultures [227]. Trauma donors (versus intracerebral hemorrhage) may be at higher risk for aspiration and for intubation under less sterile field conditions and were generally ventilated longer [228]. The etiology of donor death is not associated with lung transplant mortality [204] but may influence the type of organisms found on BAL and subsequent graft infection risk. The high rates of positive bacterial and fungal BAL results suggest the need for more aggressive critical care management and antibiotic therapy [229].
Liver
Liver transplantation from deceased donors has become accepted as standard of care for many children with liver failure. Advances in donor and recipient management has optimized graft survival with 80–90 % 5 year survival rates [230]. Whole liver transplants are still more successful with less morbidity and mortality than split liver grafts [231, 232]. Potential liver donors should be assessed by the following: aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin (direct and indirect where available), INR (or prothrombin time [PT]) (repeat q6h), serum electrolytes, creatinine, urea, Hepatitis B surface antigen (HBsAG), hepatitis B antibody (HBcAb), hepatitis C virus antibody positive (HCV Ab). There is no indication for routine liver imaging. The use of donor characteristics (donor risk index) and recipient matching using bicochemical models in end stage liver disease (MELD) are becoming more useful in predicting liver transplant outcomes [233–235].
There is variation in organ quality and recipient outcomes; larger volume centers tend to use higher risk organs but also have higher disease severity resulting in worse outcomes [236]. Predictors of early graft dysfunction or failure for whole or split liver transplantation include donor history of cardiac arrest, older donor age in adult transplantation (>40 years), [113, 237, 238], very young age in pediatric transplantation [113], reduced size livers, moderate to severe steatosis on liver biopsy, prolonged cold ischemia time (>6 h) [121, 239, 240] and donor hypernatremia (Na > 155 mmol/L). Donor hypernatremia is independently associated with death or retransplantation at 30 days [121] but this risk reverses with the correction of hypernatremia [124].
Although liver allograft dysfunction has been reported to be associated with prolonged ICU stay [113, 241], this was supported by univariate analysis but did not hold true by multivariate analysis [241]. In a cohort of 323 orthotopic liver transplants (OLT), longer donor hospitalization was not found to be associated with primary liver graft dysfunction [239]. Large platelet transfusion requirements during surgery are independently associated with more severe hepatic dysfunction after transplantation [115], although this may be indicative of a more technically complicated procedure, sicker recipient, or poor quality graft with subsequently greater sequestration of platelets within the donor liver [242]. As with other organs, the mechanisms of brain death itself impact the donor liver [243]. With the use of marginal livers for transplantation, studies are identifying more factors that may impact graft survival such as the liver’s gross appearance, the donor P/F ratio, and the donor hemoglobin [244].
The sinusoidal lining cells (SLC) of the liver are particularly vulnerable to the effects of preservation-reperfusion injury, the extent of which depends on the duration of cold ischemia rather than reperfusion. Cold preservation causes the SLC to become edematous and detach into the sinusoidal lumen [245]. While some authors recommend routine donor liver biopsies in all liver donors in an effort to decrease the rate of early graft dysfunction or failure [246, 247], the use of a biopsy in the decision making of liver suitability has generally been restricted to evaluating the amount of steatosis or in the presence of active hepatitis C in the appropriate risk groups.
Kidney
Donor age ≥ 40 or ≤10 years were thought to be independently associated with risk for graft failure [248, 249]. Now, recipients of kidneys from young donors < 5 years old have equivalent patient and graft survival [250]. En bloc kidneys from pediatric donors now show comparable outcomes with living kidney donation [251]. Older kidneys have a higher incidence of renovascular or parenchymal injury [249]. Adult donor characteristics that are independently associated with graft failure risk include creatinine > 133 μmol/L, history of hypertension independent of duration and cerebrovascular accident (CVA) as the cause of donor death [248]. During the past few years, there has been a renewed interest in the use of expanded criteria donors for kidney transplantation to increase number of donations with improving outcomes [252]. However, these kidneys have worse long-term survival and are only recommended for older recipients [253, 254].
A normal creatinine clearance (>80 ml/min/1.73 m2), as estimated by the Schwartz formula [255], defines the optimal function threshold for transplantation. However, an abnormal serum creatinine or calculated creatinine clearance in a donor does not necessarily preclude use of the kidneys [256]. Urinalysis is essential to rule out kidney abnormalities and serum creatinine and serum urea (blood urea nitrogen) measurements should be obtained q6h. Ultrasound with Doppler flow of renal vessels is often requested if creatinine levels are abnormal. If contrast angiography is performed (e.g. cerebral, coronary) N-acetylcysteine with hydration should be administered both before and after the angiographic procedure in order reduce the risk of contrast nephropathy [257] in potential donors, particularly in those with reduced renal function.
Delayed graft function predicts the development of adverse events such as decreased graft survival, decreased recipient survival and increased allograft nephropathy [258]. Most studies do not link a specific cause of brain death as a predictor of graft function in children [259]. The brain death process itself can affect acute rejection in renal transplantation [260, 261]. Greater sympathetic activity during the process produces endothelial damage, complement activation, and a proinflammatory state increases organ immunogenicity, then promoting rejection after transplant [262, 263]. Targeting this inflammatory state may improve outcomes of recipients [264, 265].
Other donor risk factors predicting kidney allograft dysfunction include hemodynamics, age, last creatinine level prior to donation, and cold ischemic time [266]. Donor hemodynamic instability is correlated with post-transplant acute tubular necrosis in adults [77, 267, 268] and children [2]. Reduced graft survival or acute tubular necrosis may occur in organs retrieved from donors receiving high-dose dopamine (>10 μg/kg/min) but these effects may be limited to donors who are hypotensive at the time of organ retrieval [268]. Hemodynamic resuscitation may improve outcome as donor use of dopamine and/or noradrenaline is independently associated with a lower risk of acute rejection [269], lower rate of delayed graft function [84, 270], and reduces the need for recipient dialysis [271]. In adults, donor hypertension is also a risk factor for inferior outcomes [272]. It is suggested that the time taken to optimize donor cardiovascular status may reduce renal ischemic injury and optimize donor yield [273, 274].
In an analysis of the Collaborative Transplant Study database of kidney transplants, cold ischemic preservation time > 12 h resulted in progressively worsening recipient graft survival, particularly once the cold ischemia time (CIT) was ≥48 h [275]. Other analyses have suggested that CIT is predictive of poorer graft survival [248] or function [267] if it was >24 h. Preservation incorporating pulsatile perfusion, rather than cold storage, may reduce the incidence of delayed graft function [276, 277].
Intestine
Small bowel transplantation has been become an increasingly feasible option for short bowel syndrome and liver failure [278]. Long term survival following intestinal transplant is above 60 %, but the incidence of morbidity and mortality is still significant [279, 280]. Because of this, many feel that intestinal transplantation as an option is still premature and remains unique to specialized centres only [281, 282]. For the brain dead donor, non-absorbable antibiotics for selective bowel decontamination are sometimes used for liver and intestine transplantation to prevent postoperative infections. Results are best if given >3 days prior to transplantation [283]. A meta-analysis showed an 84 % relative risk reduction in the incidence of gram negative infection following liver transplantation; however, the risk of antimicrobial resistance should be considered [284]. More recent studies have not duplicated these results. At this time, selective bowel decontamination is not routinely administered [285].
Logistics of Organ Donation
Donor Management Protocols and Education
One of the main reasons for insufficient organ procurement has been low organ yield due to poor multiorgan failure management in the potential donor [82]. Evidence has shown that multidisciplinary donor management protocols can improve donation outcomes [286, 287]. When these strategies are used, there are a significantly improved number of organs transplanted per donor [3, 4]. This is mostly attributed to the improvement in basic cardiovascular and respiratory monitoring and treatment [288–290]. Improved multimodal strategies aimed at preserving organ function specifically may increase numbers of potential donors, especially with the increasing use of “marginal” donors [81]. These protocols need to be supported with appropriate medical and nursing education [291–293] and influencing attitudinal changes for the role of donor [294]. Policies for organ donation and management should be developed with aim to change the culture at the bedside and with hospital administration [295, 296].
Optimal Time of Organ Procurement
In general, after brain death has been declared and consent to organ donation has been granted, all efforts are made to complete logistics and initiate procurement as quickly as possible. Expediting the interval from brain death to surgical procurement may allow grieving families to leave the hospital sooner and reduce ICU length of stay. This approach may also have been influenced by the misperception that brain dead patients are irretrievably unstable [77].
As a concept fundamental to ICU multiorgan support, resuscitation of the cardiopulmonary system benefits all end organs. Neurogenic myocardial injury related to primary brain injury is largely reversible with time and treatment [30, 65, 184]. Australian investigators advocate a delay in organ procurement until marginal donor lungs have been optimized with aggressive bronchial toilet using bronchoscopy, physiotherapy, increasing tidal volume and increasing (PEEP) [152, 221]. In a large cohort study of 1,106 renal transplant recipients, longer duration of brain death (time from declaration of brain death to onset of cold ischemia) was associated with improved initial graft function and graft survival, suggesting that the time taken to optimize donor cardiovascular status may reduce ischemic injury [273]. Despite early reports to the contrary [113], liver allograft dysfunction is not associated with prolonged ICU stay by multivariate analysis [239, 241]. A period of time may be needed to determine the trend of elevated AST or ALT, as generally accepted upper limits may be exceeded if the levels are falling rapidly (e.g., following a hypotensive episode with resuscitation).
Temporal changes in multi-organ function after brain death demand flexibility in identifying the optimal time of procurement. Recent consensus guidelines stress the importance of taking the necessary time in the ICU to optimize multi-organ function for the purposes of improving organ utilization and transplant outcomes [69]. Reversible organ dysfunction can be improved with resuscitation and re-evaluation and may include:
-
Myocardial/cardiovascular dysfunction
-
Oxygenation impairment related to potentially reversible lung injury
-
Invasive bacterial infections
-
Hypernatremia
-
The need to evaluate temporal trends in aspartate aminotransferase (AST) and alanine aminotransferase (ALT)
-
The need to evaluate temporal trends in creatinine
-
Any other potentially treatable situation.
This treatment period may be extended 24–48 or longer and should be accompanied by frequent re-evaluation to demonstrate improvement in organ function toward defined targets. Extending the interval of donor care in the ICU to optimize transplant outcomes should be factored into donation consent discussions and should be consistent with the wishes of the family or surrogate decision maker. Adequate PICU resource allocation should be anticipated.
Decisions Regarding Transplantability
End-of-life care in the ICU includes all efforts to actualize the opportunity and expressed intent to donate organs. Given the management of brain death and the organ donor is the exclusive domain of ICU practice, it is incumbent on critical care practitioners to assume leadership in this regard, in collaboration with organ procurement agencies and transplant programs. Table 38.3 provides an example of standing orders for pediatric donors to help guide practice [69].
It is important for ICU staff to know that individual programs may have different function thresholds for accepting organs, dependent on program experience and urgency of recipient need. Although the non-utilization of organs is most commonly related to organ dysfunction, it is also related to donor characteristics and/or flaws in the processes of transplant evaluation and decision making. A four-center Canadian review of heart and lung utilization identified deficits in the consent to individual organs, the offering of organs, and the utilization of offered organs unrelated to organ dysfunction [297]. Consent should be requested for all organs regardless of baseline function and all organs should be offered. Ideally, final decisions about transplantability should rest with the individual transplant programs represented by the organ-specific transplant doctors.
Management of marginal organs should include resuscitation and reevaluation to allow for potential organ rescue and utilization. Transplant programs should be accountable to the donor family and ICU donation efforts for the non-utilization of organs, to ensure that all useable organs are used. This evolving collaboration to establish best donor management practices in the ICU must be linked to ensuring optimal organ utilization, which in turn, must be linked to transplant graft and patient outcomes.
References
Smith M. Physiologic changes during brain stem death–lessons for management of the organ donor. J Heart Lung Transplant. 2004;23(9 Suppl):S217–22.
Finfer S, Bohn D, Colpitts D, Cox P, Fleming F, Barker G. Intensive care management of paediatric organ donors and its effect on post-transplant organ function. Intensive Care Med. 1996;22(12):1424–32.
Hagan ME, McClean D, Falcone CA, Arrington J, Matthews D, Summe C. Attaining specific donor management goals increases number of organs transplanted per donor: a quality improvement project. Prog Transplant. 2009;19(3):227–31.
Franklin GA, Santos AP, Smith JW, Galbraith S, Harbrecht BG, Garrison RN. Optimization of donor management goals yields increased organ use. Am Surg. 2010;76(6):587–94.
Malinoski DJ, Daly MC, Patel MS, Oley-Graybill C, Foster 3rd CE, Salim A. Achieving donor management goals before deceased donor procurement is associated with more organs transplanted per donor. J Trauma. 2011;71(4):990–5. discussion 996.
Badovinac K, Greig P, Ross H, Doig C, Shemie SD. Organ utilization among deceased donors in Canada, 1993-2002. Can J Anaesth. 2006;53(8):838–44.
Punch JD, Hayes DH, LaPorte FB, McBride V, Seely MS. Organ donation and utilization in the United States, 1996-2005. Am J Transplant. 2007;7(5 Pt 2):1327–38.
Klein AS, Messersmith EE, Ratner LE, Kochik R, Baliga PK, Ojo AO. Organ donation and utilization in the United States, 1999-2008. Am J Transplant. 2010;10(4 Pt 2):973–86.
Messersmith EE, Arrington C, Alexander C, Orlowski JP, Wolfe R. Development of donor yield models. Am J Transplant. 2011;11(10):2075–84.
Tsai E, Shemie SD, Hebert D, Furst S, Cox PN. Organ donation in children. The role of the pediatric intensive care unit. Pediatr Crit Care Med. 2000;1:156–60.
Selck FW, Deb P, Grossman EB. Deceased organ donor characteristics and clinical interventions associated with organ yield. Am J Transplant. 2008;8(5):965–74.
Shivalkar B, Van Loon J, Wieland W, Tjandra-Maga TB, Borgers M, Plets C, Fleming W. Variable effects of explosive or gradual increase of intracranial pressure on myocardial structure and function. Circulation. 1993;87(1):230–9.
Powner DJ, Bernstein IM. Extended somatic support for pregnant women after brain death. Crit Care Med. 2003;31(4):1241–9.
Wood KE, Becker BN, McCartney JG, et al. Care of the potential organ donor. N Engl J Med. 2004;351(26):2730–9.
Philip S, Udomphorn Y, Kirkham FJ, Vavilala MS. Cerebrovascular pathophysiology in pediatric traumatic brain injury. J Trauma. 2009;67(2 Suppl):S128–34.
Novitzky D. Detrimental effects of brain death on the potential organ donor. Transplant Proc. 1997;29:3770–2.
Novitzky D, Wicomb WN, Cooper DKC, Rose AG, Fraser RC, Barnard CW. Electrocardiographic, hemodynamic and endocrine changes occurring during experimental brain death in the chacma baboon. Heart Transplant. 1984;4:63–9.
Ferrera R, Hadour G, Tamion F, Henry JP, Mulder P, Richard V, Thuillez C, Ovize M, Derumeaux G. Brain death provokes very acute alteration in myocardial morphology detected by echocardiography: preventive effect of beta-blockers. Transpl Int. 2011;24(3):300–6.
Li J, Konstantinov IE, Cai S, Shimizu M, Redington AN. Systemic and myocardial oxygen transport responses to brain death in pigs. Transplant Proc. 2007;39(1):21–6.
Venkateswaran RV, Dronavalli V, Lambert PA, et al. The proinflammatory environment in potential heart and lung donors: prevalence and impact of donor management and hormonal therapy. Transplantation. 2009;88(4):582–8.
Katz K, Lawler J, Wax J, et al. Vasopressin pressor effects in critically ill children during evaluation for brain death and organ recovery. Resuscitation. 2000;47(1):33–40.
Dimopoulou I, Tsagarakis S, Anthi A, et al. High prevalence of decreased cortisol reserve in brain-dead potential organ donors. Crit Care Med. 2003;31(4):1113–7.
Salim A, Martin M, Brown C, et al. Complications of brain death: frequency and impact on organ retrieval. Am Surg. 2006;72(5):377–81.
Pérez López S, Otero Hernández J, Vázquez Moreno N, et al. Brain death effects on catecholamine levels and subsequent cardiac damage assessed in organ donors. J Heart Lung Transplant. 2009;28(8):815–20.
Mayer SA, Fink ME, Raps EC, et al. Cardiac injury associated with neurologic pulmonary edema following subarachnoid hemorrhage. Neurology. 1994;44:815–20.
Kono T, Morita H, Kuroiwa T, Onaka H, Takatsuka H, Fujiwara A. Left ventricular wall motion abnormalities in patients with subarachnoid hemorrhage: neurogenic stunned myocardium. J Am Coll Cardiol. 1994;24:636–40.
Temes RE, Tessitore E, Schmidt JM, Naidech AM, Fernandez A, Ostapkovich ND, Frontera JA, Wartenberg KE, Di Tullio MR, Badjatia N, Connolly ES, Mayer SA, Parra A. Left ventricular dysfunction and cerebral infarction from vasospasmafter subarachnoid hemorrhage. Neurocrit Care. 2010;13(3):359–65.
Macmillan CSA, Grant IS, Andrews PJD. Pulmonary and cardiac sequelae of subarachnoid haemorrhage: time for active management? Intensive Care Med. 2002;28:1012–23.
Kolin A, Norris JW. Myocardial damage from acute cerebral lesions. Stroke. 1984;15:990–3.
Tung P, Kopelnik A, Banki N, Ong K, Ko N, Lawton MT, Gress D, Drew B, Foster E, Parmley W, Zaroff J. Predictors of neurocardiogenic injury after subarachnoid hemorrhage. Stroke. 2004;35(2):548–51. Epub 2004 Jan 22.
Grant J, Canter CE, Spray TL, Landt YS, Jeffrey E, Ladenson JH, Jaffe AS. Elevated donor cardiac troponin I: a marker of acute graft failure in infant heart recipients. Circulation. 1994;90(6):2618–21.
Takada M, Nadeau KC, Hancock WW, Mackenzie HS, Shaw GD, Waaga AM, Chandraker A, Sayegh MH, Tilney NL. Effects of explosive brain death on cytokine activation of peripheral organs in the rat. Transplantation. 1998;65:1533–42.
Novitzky D, Wicomb WN, Rose AG, Cooper DK, Reichart B. Pathophysiology of pulmonary edema following experimental brain death in the chacma baboon. Ann Thorac Surg. 1987;43(3):288–94.
Cruickshank JM, Meil-Dwyer G, Degaute JP, Kuurne T, Kytta J, Carruthers ME, Patel S. Reduction of stress/catecholamine-induced cardiac necrosis by beta 1 selective blockade. Lancet. 1987;2:585–9.
Siaghy EM, Halejcio-Delophont P, Mertes PM, et al. Protective effects of labetalol on myocardial contractile function in brain-dead pigs. Transplant Proc. 1998;30:2842–3.
Hall SR, Wang L, Milne B, Ford S, Hong M. Intrathecal lidocaine prevents cardiovascular collapse and neurogenic pulmonary edema in a rat model of acute intracranial hypertension. Anesth Analg. 2002;94(4):948–53.
Baguley IJ. Autonomic complications following central nervous system injury. Semin Neurol. 2008;28(5):716–25. Epub 2008 Dec 29. Review.
Sedý J, Zicha J, Kunes J, Jendelová P, Syková E. Mechanisms of neurogenicpulmonary edema development. Physiol Res. 2008;57(4):499–506. Epub 2007 Nov 30.
Avlonitis VS, Wigfield CH, Kirby JA, Dark JH. The hemodynamic mechanisms of lung injury and systemic inflammatory response following brain death in the transplant donor. Am J Transplant. 2005;5(4 Pt 1):684–93.
Fontes RB, Aguiar PH, Zanetti MV, Andrade F, Mandel M, Teixeira MJ. Acute neurogenic pulmonary edema: case reports and literature review. J Neurosurg Anesthesiol. 2003;15(2):144–50.
Bruder N, Rabinstein A, The Participants in the International Multi-disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Cardiovascular and pulmonary complications of aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2011;15(2):257–69.
Pratschke J, Wilhelm MJ, Kusaka M, Hancock WW, Tilney NL. Activation of proinflammatory genes in somatic organs as a consequence of brain death. Transplant Proc. 1999;31:1003–5.
Barklin A. Systemic inflammation in the brain-dead organ donor. Acta Anaesthesiol Scand. 2009;53(4):425–35. Epub 2009 Feb 18. Review.
Amado JA, Lopez-Espadas F, Vazquez-Barquero A, Salas E, Riancho JA, Lopez-Cordovilla JJ, et al. Blood levels of cytokines in brain-dead patients: relationship with circulating hormones and acute phase reactants. Metabolism. 1995;44:812–6.
Kuecuek O, Mantouvalou L, Klemz R, Kotsch K, Volk HD, Jonas S, Wesslau C, Tullius S, Neuhaus P, Pratschke J. Significant reduction of proinflammatory cytokines by treatment of the brain-dead donor. Transplant Proc. 2005;37(1):387–8.
van der Hoeven JA, Ter Horst GJ, Molema G, de Vos P, Girbes AR, Postema F, Freund RL, Wiersema J, van Schilfgaarde R, Ploeg RJ. Effects of brain death and hemodynamic status on function and immunologic activation of the potential donor liver in the rat. Ann Surg. 2000;232:804–13.
van der Hoeven JA, Lindell S, van Schilfgaarde R, Molema G, Ter Horst GJ, Southard JH, Ploeg RJ. Donor brain death reduces survival after transplantation in rat livers preserved for 20 hr. Transplantation. 2001;72:1632–6.
Koo DDH, Welsh KI, McLaren AJ, Roake JA, Morris PJ, Fuggle SV. Cadaver versus living donor kidneys: impact of donor factors on antigen induction before transplantation. Kidney Int. 1999;56:1551–9.
Nijboer WN, Schuurs TA, van der Hoeven JA, Fekken S, Wiersema-Buist J, Leuvenink HG, Hofker S, Homan van der Heide JJ, van Son WJ, Ploeg RJ. Effect of brain death on gene expression and tissue activation in human donor kidneys. Transplantation. 2004;78(7):978–86.
Kosieradzki M, Kuczynska J, Piwowarska J, Wegrowicz-Rebandel I, Kwiatkowski A, Lisik W, et al. Prognostic significance of free radical mediated injury occurring in the kidney donor. Transplantation. 2003;75(8):1221–7.
Nijboer WN, Schuurs TA, van der Hoeven JA, Leuvenink HG, van der Heide JJ, van Goor H, Ploeg RJ. Effects of brain death on stress and inflammatory response in the human donor kidney. Transplant Proc. 2005;37(1):367–9.
Minnear FL, Connell RS. Prevention of aconitine-induced neurogenic pulmonary oedema with hypovolemia or methylprednisolone. J Trauma. 1982;22:121–8.
Edmonds HLJ, Cannon HCJ, Garretson HD, Dahlquist G. Effects of aerosolized methylprednisolone on experimental neurogenic pulmonary injury. Neurosurgery. 1986;19:36–40.
Zweers N, Petersen AH, van der Hoeven JA, de Haan A, Ploeg RJ, de Leij LF, Prop J. Donor brain death aggravates chronic rejection after lung transplantation in rats. Transplantation. 2004;78(9):1251–8.
Land WG. The role of postischemic reperfusion injury and other nonantigen-dependent inflammatory pathways in transplantation. Transplantation. 2005;79(5):505–14.
van der Hoeven JA, Molema G, Ter Horst GJ, Freund RL, Wiersema J, van Schilfgaarde R, Leuvenink HG, Ploeg RJ. Relationship between duration of brain death and hemodynamic (in)stability on progressive dysfunction and increased immunologic activation of donor kidneys. Kidney Int. 2003;64(5):1874–82.
Schuurs TA, Gerbens F, van der Hoeven JA, Ottens PJ, Kooi KA, Leuvenink HG, Hofstra RM, Ploeg RJ. Distinct transcriptional changes in donor kidneys upon brain death induction in rats: insights in the processes of brain death. Am J Transplant. 2004;4(12):1972–81.
Pratschke J, Neuhaus P, Tullius SG. What can be learned from brain-death models? Transpl Int. 2005;18(1):15–21.
Powner DJ, Hewitt MJ, Levine RL. Interventions during donor care before lung transplantation. Prog Transplant. 2005;15(2):141–8. Review.
Dujardin KS, McCully RB, Wijdicks EF, Tazelaar HD, Seward JB, McGregor CG, Olson LJ. Myocardial dysfunction associated with brain death: clinical, echocardiographic, and pathologic features. J Heart Lung Transplant. 2001;20:350–7.
Yokoyama Y, Cooper DKC, Sasaki H, Snow TR, Akutsu T, Zuhdi N. Donor-heart evaluation by monitoring the left ventricular pressure-volume relationship: clinical observations. J Heart Lung Transplant. 1992;11:685–92.
Young JB, Naftel DC, Bourge RC, Kirklin JK, Clemson BS, Porter CB, Rodeheffer RJ. Matching the heart donor and heart transplant recipient. Clues for successful expansion of the donor pool: a multivariable, multi-institutional report. J Heart Lung Transplant. 1994;13:353–65.
Hashimoto S, Kato TS, Komamura K, Hanatani A, Niwaya K, Funatsu T, Kobayashi J, Sumita Y, Tanaka N, Hashimura K, Asakura M, Kanzaki H, Kitakaze M. Utility of echocardiographic evaluation of donor hearts upon the organ procurement for heart transplantation. J Cardiol. 2011;57(2):215–22. Epub 2011 Jan 14.
Fine NM, Pellikka PA. Pharmacologic stress echocardiography for the assessment of organ suitability for heart transplantation: casting a broader net in search of donors. J Am Soc Echocardiogr. 2011;24(4):363–6.
Babcock WD, Menza RL, Zaroff JG. Increased donor heart utilization using serial echocardiography during donor management. J Heart Lung Transplant. 2003;22(1S):74.
Venkateswaran RV, Townend JN, Wilson IC, Mascaro JG, Bonser RS, Steeds RP. Echocardiography in the potential heart donor. Transplantation. 2010;89(7):894–901.
Pennefather S, Bullock RE, Dark JH. The effect of fluid therapy on alveolar arterial oxygen gradient in brain-dead organ donors. Transplantation. 1993;56(6):1418–22.
Rosengard BR, Feng S, Alfrey EJ, Zaroff JG, Emond JC, Henry ML, Garrity ER, Roberts JP, Wynn JJ, Metzger RA, Freeman RB, Port FK, Merion RM, Love RB, Busuttil RW, Delmonico FL. Report of the crystal city meeting to maximize the use of organs recovered from the cadaver donor. Am J Transplant. 2002;2:701–11.
Shemie SD, Ross H, Pagliarello J, Baker AJ, Greig PD, Brand T, Cockfield S, Keshavjee S, Nickerson P, Rao V, Guest C, Young K, Doig C, Pediatric Recommendations Group. Organ donor management in Canada: recommendations of the forum on Medical Management to Optimize Donor Organ Potential. CMAJ. 2006;174(6):S13–32.
Wheeldon DR, Potter CDO, Oduro A, Wallwork J, Large SR. Transforming the “unacceptable” donor: outcomes from the adoption of a standardized donor management technique. J Heart Lung Transplant. 1995;14:734–42.
Hadjizacharia P, Salim A, Brown C, Inaba K, Chan LS, Mascarenhas A, Margulies DR. Does the use of pulmonary artery catheters increase the number of organs available for transplantation? Clin Transplant. 2010;24(1):62–6. Epub 2009 Feb 14.
Hunt SA, Baldwin J, Baumgartner W, Bricker JT, Costanzo MR, Miller L, Mudge G, O’Connell JB. Cardiovascular management of a potential heart donor: a statement from the Transplantation Committee of the American College of Cardiology. Crit Care Med. 1996;24:1599–601.
Powner DJ, Hergenroeder GW. Measurement of cardiac output during adult donor care. Prog Transplant. 2011;21(2):144–50. quiz 151.
Su BC, Yu HP, Yang MW, Lin CC, Kao MC, Chang CH, Lee WC. Reliability of a new ultrasonic cardiac output monitor in recipients of living donor liver transplantation. Liver Transpl. 2008;14(7):1029–37.
Powner DJ, Doshi PB. Central venous oxygen saturation monitoring: role in adult donor care? Prog Transplant. 2010;20(4):401–5.
Huang J, Trinkaus K, Huddleston CB, Mendeloff EN, Spray TL, Canter CE. Risk factors for primary graft failure after pediatric cardiac transplantation: importance of recipient and donor characteristics. J Heart Lung Transplant. 2004;23(6):716–22.
Lagiewska B, Pacholczyk M, Szostek M, Walaszewski J, Rowinski W. Hemodynamic and metabolic disturbances observed in brain dead organ donors. Transplant Proc. 1996;28:165–6.
Murugan R, Venkataraman R, Wahed AS, Elder M, Carter M, Madden NJ, Kellum JA, HIDonOR Study Investigators. Preload responsiveness is associated with increased interleukin-6 and lower organ yield from brain-dead donors. Crit Care Med. 2009;37(8):2387–93.
Tuttle-Newhall JE, Collins BH, Kuo PC, Schroeder R. Organ donation and treatment of multi-organ donor. Curr Probl Surg. 2003;40(5):266–310.
Kutsogiannis DJ, Pagliarello G, Doig C, Ross H, Shemie SD. Medical management to optimize donor organ potential: review of the literature. Can J Anaesth. 2006;53(8):820–30.
Mascia L, Mastromauro I, Viberti S, Vincenzi M, Zanello M. Management to optimize organ procurement in brain dead donors. Minerva Anestesiol. 2009;75(3):125–33.
Dictus C, Vienenkoetter B, Esmaeilzadeh M, Unterberg A, Ahmadi R. Critical care management of potential organ donors: our current standard. Clin Transplant. 2009;23 Suppl 21:2–9.
D’Amico TA, Meyers CH, Koutlas TC, Peterseim DS, Sabiston DC, Van Trigt P, et al. Desensitization of myocardial β-adrenergic receptors and deterioration of left ventricular function after brain death. J Thorac Cardiovasc Surg. 1995;110:746–51.
Schnuelle P, Yard BA, Braun C, Dominguez-Fernandez E, Schaub M, Birck R, Sturm J, Post S, van der Woude FJ. Impact of donor dopamine on immediate graft function after kidney transplantation. Am J Transplant. 2004;4(3):419–26.
Santise G, D’Ancona G, Falletta C, Pirone F, Sciacca S, Turrisi M, Biondo D, Pilato M. Donor pharmacological hemodynamic support is associated with primary graft failure in human heart transplantation. Interact Cardiovasc Thorac Surg. 2009;9(3):476–9. Epub 2009 Jun 29.
Marik PE, Mohedin M. The contrasting effects of dopamine and norepinephrine on systemic and splanchnic oxygen utilization in hyperdynamic sepsis. JAMA. 1994;272:1354–7.
Martin C, Papazian L, Perrin G, Saux P, Gouin F. Norepinephrine or dopamine for the treatment of hyperdynamic septic shock? Chest. 1993;103:1826–31.
De Backer D, Creteur J, Silva E, Vincent JL. Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: which is best? Crit Care Med. 2003;31(6):1659–67.
Chen JM, Cullinane S, Spanier TB, Artrip JH, John R, Edwards NM, Oz MC, Landry DW. Vasopressin deficiency and pressor hypersensitivity in hemodynamically unstable organ donors. Circulation. 1999;100(suppl II):II-246.
Kinoshita Y, Yahata K, Yoshioka T, Onishi S, Sugimoto T. Long-term renal preservation after brain death maintained with vasopressin and epinephrine. Transplant Int. 1990;3:15–8.
Yoshioka T, Sugimoto H, Uenishi M. Prolonged haemodynamic maintenance by the combined administration of vasopressin and epinephrine in brain death: a clinical study. Neurosurgery. 1986;18:565–7.
Pennefather SH, Bullock RE, Mantle D, Dark JH. Use of low dose arginine vasopressin to support brain-dead organ donors. Transplantation. 1995;59(1):58–62.
Nakagawa K, Tang JF. Physiologic response of human brain death and the use of vasopressin for successful organ transplantation. J Clin Anesth. 2011;23(2):145–8.
Holmes CL, Patel BM, Russell JA, Walley KR. Physiology of vasopressin relevant to management of septic shock. Chest. 2001;120:989–1002.
Brierley J, Carcillo JA, Choong K, Cornell T, Decaen A, Deymann A, Doctor A, Davis A, Duff J, Dugas MA, Duncan A, Evans B, Feldman J, Felmet K, Fisher G, Frankel L, Jeffries H, Greenwald B, Gutierrez J, Hall M, Han YY, Hanson J, Hazelzet J, Hernan L, Kiff J, Kissoon N, Kon A, Irazuzta J, Lin J, Lorts A, Mariscalco M, Mehta R, Nadel S, Nguyen T, Nicholson C, Peters M, Okhuysen-Cawley R, Poulton T, Relves M, Rodriguez A, Rozenfeld R, Schnitzler E, Shanley T, Kache S, Skippen P, Torres A, von Dessauer B, Weingarten J, Yeh T, Zaritsky A, Stojadinovic B, Zimmerman J, Zuckerberg A. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37(2):666–88.
Guzman JA, Rosado AE, Kruse JA. Vasopressin vs norepinephrine in endotoxic shock: systemic, renal, and splanchnic hemodynamic and oxygen transport effects. J Appl Physiol. 2003;95(2):803–9.
Dunser MW, Mayr AJ, Ulmer H, Knotzer H, Sumann G, Pajk W, Friesenecker B, Hasibeder WR. Arginine vasopressin in advanced vasodilatory shock: a prospective, randomized, controlled study. Circulation. 2003;107(18):2313–9. Epub 2003 May 5.
Kinoshita Y, Okamoto K, Yahata K, Yoshioka T, Sugimoto T, Kawaguchi N, Onishi S. Clinical and pathological changes of the heart in brain death maintained with vasopressin and epinephrine. Pathol Res Pract. 1990;186:173–9.
Martikainen TJ, Kurola J, Kärjä V, Parviainen I, Ruokonen E. Vasopressor agents after experimental brain death: effects of dopamine and vasopressin on vitality of the small gut. Transplant Proc. 2010;42(7):2449–56.
Mascia L, Bosma K, Pasero D, Galli T, Cortese G, Donadio P, Bosco R. Ventilatory and hemodynamic management of potential organ donors: an observational survey. Crit Care Med. 2006;34(2):321–7.
Venkateswaran RV, Patchell VB, Wilson IC, Mascaro JG, Thompson RD, Quinn DW, Stockley RA, Coote JH, Bonser RS. Early donor management increases the retrieval rate of lungs for transplantation. Ann Thorac Surg. 2008;85(1):278–86.
Mallory Jr GB, Schecter MG, Elidemir O. Management of the pediatric organ donor to optimize lung donation. Pediatr Pulmonol. 2009;44(6):536–46. Review.
Mascia L, Pasero D, Slutsky AS, Arguis MJ, Berardino M, Grasso S, Munari M, Boifava S, Cornara G, Della Corte F, Vivaldi N, Malacarne P, Del Gaudio P, Livigni S, Zavala E, Filippini C, Martin EL, Donadio PP, Mastromauro I, Ranieri VM. Effect of a lung protective strategy for organ donors on eligibility and availability of lungs for transplantation: a randomized controlled trial. JAMA. 2010;304(23):2620–7.
Masson F, Thicoipe M, Gin H, De Mascarel A, Angibeau RM, Favarel-Garrigues JF, Erny P. The endocrine pancreas in brain-dead donors. Transplantation. 1993;56(2):363–7.
Cochran A, Scaife ER, Hansen KW, Downey EC. Hyperglycemia and outcomes from pediatric traumatic brain injury. J Trauma. 2003;55(6):1035–8.
Rovlias A, Kotsou S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery. 2000;46(2):335–42. discussion 342–3.
Kelly DF. Neurosurgical postoperative care. Neurosurg Clin North Am. 1994;5:789–810.
Cherian L, Goodman JC, Robertson CS. Effect of glucose administration on contusion volume after moderate cortical impact injury in rats. J Neurotrauma. 1998;15(12):1059–66.
Singer P, Cohen J, Cynober L. Effect of nutritional state of brain-dead organ donor on transplantation. Nutrition. 2001;17(11–12):948–52.
Boudjema K, Lindell SL, Southard JH, Belzer FO. The effects of fasting on the quality of liver preservation by simple cold storage. Transplantation. 1990;50:943–8.
Wakiyama S, Yanaga K, Soejima Y, Nishizaki T, Sugimachi K. Significance of donor nutritional status for rewarming injury of the hepatic graft in rats. Eur Surg Res. 1997;29(5):339–45.
Singer P, Shapiro H, Cohen J. Brain death and organ damage: the modulating effects of nutrition. Transplantation. 2005;80(10):1363–8.
Greig PD, Forster J, Superina RA, Strasberg SM, Mohamed M, Blendis LM, Taylor BR, Levy GA, Langer B. Donor-specific factors predict graft function following liver transplantation. Transplant Proc. 1990;22:2072–3.
Cywes R, Greig PD, Morgan GR, Sanabria JR, Clavien PA, Harvey PR, Strasberg SM. Rapid donor liver nutritional enhancement in a large animal model. Hepatology. 1992;16:1271–9.
Gonzalez FX, Rimola A, Grande L, Antolin M, Garcia-Valdecasas JC, Fuster J, Lacy AM, Cugat E, Visa J, Rodes J. Predictive factors of early postoperative graft function in human liver transplantation. Hepatology. 1994;20:565–73.
Shah VR. Aggressive management of multiorgan donor. Transplant Proc. 2008;40(4):1087–90.
Howlett TA, Keogh AM, Perry L, Touzel R, Rees LH. Anterior and posterior pituitary function in brain-stem-dead donors. Transplantation. 1989;47:828–34.
Gramm H, Meinhold H, Bickel U, Zimmermann J, Von Hammerstein B, Keller F, Dennhardt R, Voigt K. Acute endocrine failure after brain death. Transplantation. 1992;54(5):851–7.
Agha A, Phillips J, Thompson CJ. Hypopituitarism following traumatic brain injury (TBI). Br J Neurosurg. 2007;21(2):210–6.
Avolio AW, Agnes S, Magalini SC, Foco M, Castagneto M. Importance of donor blood chemistry data (AST, serum sodium) in predicting liver transplant outcome. Transplant Proc. 1991;23:2451–2.
Figueras J, Busquets J, Grande L, Jaurrieta E, Perez-Ferreiroa J, Mir J, Margarit C, Lopez P, Vazquez J, Casanova D, Bernardos A, De-Vicente E, Parrilla P, Ramon JM, Bou R. The deleterious effect of donor high plasma sodium and extended preservation in liver transplantation. A multivariate analysis. Transplantation. 1996;61:410–3.
Kazemeyni SM, Esfahani F. Influence of hypernatremia and polyuria of brain-dead donors before organ procurement on kidney allograft function. Urol J. 2008;5(3):173–7.
Mangus RS, Fridell JA, Vianna RM, Milgrom ML, Chestovich P, Vandenboom C, Tector AJ. Severe hypernatremia in deceased liver donors does not impact early transplant outcome. Transplantation. 2010;90(4):438–43.
Totsuka E, Dodson F, Urakami A, Moras N, Ishii T, Lee MC, Gutierrez J, Gerardo M, Molmenti E, Fung JJ. Influence of high donor serum sodium levels on early postoperative graft function in human liver transplantation: effect of correction of donor hypernatremia. Liver Transpl Surg. 1999;5:421–8.
Ourahma S, Guesde R, Leblanc I, Riou B, Goarin JP, Bitker MO, Coriat P. Administration of desmopressin in brain-dead donors does not modify renal function in kidney recipients. Transplant Proc. 1998;30:2844.
Richardson DW, Robinson AG. Desmopressin. Ann Intern Med. 1985;103:228–39.
Van Bakel AB. The cardiac transplant donor: identification, assessment, and management. Heart Transplant Symp. 1997;314(3):152–63.
Blasi-Ibanez A, Hirose R, Feiner J, Freise C, Stock PG, Roberts JP, Niemann CU. Predictors associated with terminal renal function in deceased organ donors in the intensive care unit. Anesthesiology. 2009;110(2):333–41.
Rosendale JD, Chabalewski FL, McBride MA, Garrity ER, Rosengard BR, Delmonico FL, Kauffman HM. Increased transplanted organs from the use of a standardized donor management protocol. Am J Transplant. 2002;2:761–8.
Zaroff JG, Rosengard BR, Armstrong WF, Babcock WD, D’Alessandro A, Dec GW, Edwards NM, Higgins RS, Jeevanandum V, Kauffman M, Kirklin JK, Large SR, Marelli D, Peterson TS, Ring WS, Robbins RC, Russell SD, Taylor DO, Van Bakel A, Wallwork J, Young JB. Consensus conference report maximizing use of organs recovered from the cadaver donor: cardiac recommendations. Circulation. 2002;106:836–41.
Lee YJ, Shen EY, Huang FY, Kao HA, Shyur SD. Continuous infusion of vasopressin in comatose children with neurogenic diabetes insipidus. J Pediatr Endocrinol Metab. 1995;8:257–62.
Lee YJ, Yang D, Shyur SD, Chiu NC. Neurogenic diabetes insipidus in a child with fatal Coxsackie virus B1 encephalitis. J Pediatr Endocrinol Metab. 1995;8:301–4.
Lugo N, Silver P, Nimkoff L, Caronia C, Sagy M. Diagnosis and management algorithm of acute onset of central diabetes insipidus in critically ill children. J Pediatr Endocrinol Metab. 1997;10:633–9.
Ralston C, Butt W. Continuous vasopressin replacement in diabetes insipidus. Arch Dis Child. 1990;65:896–7.
Meyer S, Gortner L, McGuire W, Baghai A, Gottschling S. Vasopressin incatecholamine-refractory shock in children. Anaesthesia. 2008;63(3):228–34. Epub 2007 Dec 13.
Klein I, Ojamaak K. Thyroid hormone and the cardiovascular system. NEJM. 2001;344(7):501.
Sazontseva IE, Kozlov IA, Moisuc YG, Ermolenko AE, Afonin VV, Illnitskiy VV. Hormonal response to brain death. Transplant Proc. 1991;23(5):2467.
Novitzky D, Cooper DKC, Morrell D, Isaacs S. Change from aerobic to anaerobic metabolism after brain death, and reversal following triiodothyronine therapy. Transplantation. 1988;45(1):32–6.
James SR, Ranasinghe AM, Venkateswaran R, McCabe CJ, Franklyn JA, Bonser RS. The effects of acute triiodothyronine therapy on myocardial gene expression in brain stem dead cardiac donors. J Clin Endocrinol Metab. 2010;95(3):1338–43.
Novitzky D, Cooper DKC, Reichart B. Hemodynamic and metabolic responses to hormonal therapy in brain-dead potential organ donors. Transplantation. 1987;43(2):852–4.
Novitzky D, Cooper DKC, Chaffin JS, Greer AE, Debault LE, Zuhdi N. Improved cardiac allograft function following triiodothyronine therapy to both donor and recipient. Transplantation. 1990;49(2):311–6.
Roels L, Pirenne J, Delooz H, Lauwers P, Vandermeersch E. Effect of triiodothyronine replacement therapy on maintenance characteristics and organ availability in hemodynamically unstable donors. Transplant Proc. 2000;32:1564–6.
Zuppa AF, Nadkarni V, Davis L, Adamson PC, Helfaer MA, Elliott MR, Abrams J, Durbin D. The effect of a thyroid hormone infusion on vasopressor support incritically ill children with cessation of neurologic function. Crit Care Med. 2004;32(11):2318–22.
Ranasinghe AM, Quinn DW, Pagano D, Edwards N, Faroqui M, Graham TR, Keogh BE, Mascaro J, Riddington DW, Rooney SJ, Townend JN, Wilson IC, Bonser RS. Glucose-insulin-potassium and tri-iodothyronine individually improve hemodynamic performance and are associated with reduced troponin I release after on-pumpcoronary artery bypass grafting. Circulation. 2006;114(1 Suppl):I245–50.
Cooper DK, Novitzky D, Wicomb WN, Basker M, Rosendale JD, Myron Kauffman H. A review of studies relating to thyroid hormone therapy in brain-dead organ donors. Front Biosci. 2009;14:3750–70. Review.
Pérez-Blanco A, Caturla-Such J, Cánovas-Robles J, Sanchez-Payá J. Efficiency of triiodothyronine treatment on organ donor hemodynamic management and adeninenucleotide concentration. Intensive Care Med. 2005;31(7):943–8.
Novitzky D, Cooper DK, Rosendale JD, Kauffman HM. Hormonal therapy of the brain-dead organ donor: experimental and clinical studies. Transplantation. 2006;82(11):1396–401. Review.
Rosendale JD, Kauffman HM, McBride MA, Skjei TC, Zaroff JG, Garrity ER, Delmonico FL, Rosengard BR. Hormonal resuscitation associated with more transplanted organs with no sacrifice in survival. Transplantation. 2004;78(Sup 2):17.
Powner DJ. Treatment goals during care of adult donors that can influence outcomes of heart transplantation. Prog Transplant. 2005;15(3):226–32.
Rosendale JD, Kauffman HM, McBride MA, Chabalewski FL, Zaroff JG, Garrity ER, Delmonico FL, Rosengard BR. Hormonal resuscitation yields more transplanted hearts, with improved early function. Transplantation. 2003;75(8):1336–41.
Follette DM, Rudich SM, Babcock WD. Improved oxygenation and increased lung donor recovery with high-dose steroid administration after brain death. J Heart Lung Transplant. 1998;17(4):423–9.
McElhinney DB, Khan JH, Babcock WD, Hall TS. Thoracic organ donor characteristics associated with successful lung procurement. Clin Transplant. 2001;15:68–71.
Abdelnour T, Rieke S. Relationship of hormonal resuscitation therapy and central venous pressure on increasing organs for transplant. J Heart Lung Transplant. 2009;28(5):480–5.
Nicolas-Robin A, Barouk JD, Amour J, Coriat P, Riou B, Langeron O. Hydrocortisone supplementation enhances hemodynamic stability in brain-dead patients. Anesthesiology. 2010;112(5):1204–10.
Nath DS, Ilias Basha H, Liu MH, Moazami N, Ewald GA. Increased recovery of thoracic organs after hormonal resuscitation therapy. J Heart Lung Transplant. 2010;29(5):594–6. Epub 2010 Mar 6.
Venkateswaran RV, Steeds RP, Quinn DW, Nightingale P, Wilson IC, Mascaro JG, Thompson RD, Townend JN, Bonser RS. The haemodynamic effects of adjunctive hormone therapy in potential heart donors: a prospective randomized double-blind factorially designed controlled trial. Eur Heart J. 2009;30(14):1771–80.
Lacroix J, Hébert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, Gauvin F, Collet JP, Toledano BJ, Robillard P, Joffe A, Biarent D, Meert K, Peters MJ, TRIPICU Investigators, Canadian Critical Care Trials Group, Pediatric Acute Lung Injury and Sepsis Investigators Network. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356(16):1609–19.
Ciancio G, Burke G, Roth D, Zucker K, Tzakis A, Miller J. The significance of infections in donor organs. Transpl Immunol. 1996;12:3–11. Abstract.
Doig RL, Boyd PJ, Eykyn S. Staphylococcus aureus transmitted in transplanted kidneys. Lancet. 1975;2:243–5.
Gottesdiener KM. Transplanted infections: donor-to-host transmission with the allograft. Ann Intern Med. 1989;110:1001–16.
Nery JR, Weppler D, Ketchum P, Olson L, Fragulidis GP, Khan MF, Webb MG, Miller J, Tzakis AG. Donor infection and primary nonfunction in liver transplantation. Transplant Proc. 1997;29:481–3.
Weber TR, Freier DT, Turcotte JG. Transplantation of infected kidneys: clinical and experimental results. Transplantation. 1979;27:63–5.
Powner DJ, Allison TA. Bacterial infection during adult donor care. Prog Transplant. 2007;17(4):266–74. Review.
Freeman RB, Giatras I, Falagas ME, Supran S, O’Connor K, Bradley J, Snydman DR, Delmonico FL. Outcome of transplantation of organs procured from bacteremic donors. Transplantation. 1999;68:1107–11.
Lumbreras C, Sanz F, Gonzalez A, Perez G, Ramos MJ, Aguado JM, Lizasoain M, Andres A, Moreno E, Gomez MA, Noriega AR. Clinical significance of donor-unrecognized bacteremia in the outcome of solid-organ transplant recipients. Clin Infect Dis. 2001;33:722–6.
Cerutti E, Stratta C, Romagnoli R, Serra R, Lepore M, Fop F, Mascia L, Lupo F, Franchello A, Panio A, Salizzoni M. Bacterial- and fungal-positive cultures in organ donors: clinical impact in liver transplantation. Liver Transpl. 2006;12(8):1253–9.
Wu TJ, Lee CF, Chou HS, Yu MC, Lee WC. Suspect the donor with potential infection in the adult deceased donor liver transplantation. Transplant Proc. 2008;40(8):2486–8.
Ruiz I, Gavaldà J, Monforte V, Len O, Román A, Bravo C, Ferrer A, Tenorio L, Román F, Maestre J, Molina I, Morell F, Pahissa A. Donor-to-host transmission of bacterial and fungal infections in lung transplantation. Am J Transplant. 2006;6(1):178–82.
Mattner F, Kola A, Fischer S, Becker T, Haverich A, Simon A, Suerbaum S, Gastmeier P, Weissbrodt H, Strüber M. Impact of bacterial and fungal donor organ contamination in lung, heart-lung, heart and liver transplantation. Infection. 2008;36(3):207–12. Epub 2008 May 9.
Lopez-Navidad A, Domingo P, Caballero F, Gonzalez C, Santiago C. Successful transplantation of organs retrieved from donors with bacterial meningitis. Transplantation. 1997;64:365–8.
Natov SN, Pereira BJ. Transmission of viral hepatitis by kidney transplantation: donor evaluation and transplant policies (Part 1: hepatitis B virus). Transpl Infect Dis. 2002;4(3):117–23.
Huskey J, Wiseman AC. Chronic viral hepatitis in kidney transplantation. Nat Rev Nephrol. 2011;7(3):156–65. Epub 2011 Feb 1. Review.
Singh TP, Almond CS, Piercey G, Gauvreau K. Trends in wait-list mortality in children listed for heart transplantation in the United States: era effect across racial/ethnic groups. Am J Transplant. 2011;11(12):2692–9.
Dipchand A, Cecere R, Delgado D, Dore A, Giannetti N, Haddad H, Howlett J, Leblanc MH, Leduc L, Marelli A, Perron J, Poirier N, Ross H. Canadian Consensus on cardiac transplantation in pediatric and adult congenital heart disease patients 2004: executive summary. Can J Cardiol. 2005;21(13):1145–7.
West LJ, Karamlou T, Dipchand AI, Pollock-BarZiv SM, Coles JG, McCrindle BW. Impact on outcomes after listing and transplantation, of a strategy to accept ABO blood group-incompatible donor hearts for neonates and infants. J Thorac Cardiovasc Surg. 2006;131(2):455–61.
Forni A, Luciani GB, Chiominto B, Pizzuti M, Mazzucco A, Faggian G. Results with expanded donor acceptance criteria in heart transplantation. Transplant Proc. 2011;43(4):953–9.
Tjang YS, Stenlund H, Tenderich G, Hornik L, Körfer R. Pediatric heart transplantation: current clinical review. J Card Surg. 2008;23(1):87–91.
McGiffin DC, Savunen T, Kirklin JK, Naftel DC, Bourge RC, Paine TD, White-Williams C, Sisto T, Early L. A multivariable analysis of pretransplantation risk factors for disease development and morbid events. J Thorac Cardiovasc Surg. 1995;109:1081–9.
Hong KN, Iribarne A, Worku B, Takayama H, Gelijns AC, Naka Y, Jeevanandam V, Russo MJ. Who is the high-risk recipient? Predicting mortality after heart transplant using pretransplant donor and recipient risk factors. Ann Thorac Surg. 2011;92(2):520–7. Epub 2011 Jun 17.
Sweeney MS, Lammermeier DE, Frazier OH, Burnett CM, Haupt HM, Duncan JM. Extension of donor criteria in cardiac transplantation: surgical risk versus supply-side economics. Ann Thorac Surg. 1990;50:7–11.
Michaelides A, Koen W. Effect of donor selection on the early outcome of heart transplant recipients. Prog Transplant. 2005;15(1):24–6.
Matsumoto CS, Kaufman SS, Girlanda R, Little CM, Rekhtman Y, Raofi V, Laurin JM, Shetty K, Fennelly EM, Johnson LB, Fishbein TM. Utilization of donors who have suffered cardiopulmonary arrest and resuscitation in intestinal transplantation. Transplantation. 2008;86(7):941–6.
L’ecuyer T, Sloan K, Tang L. Impact of donor cardiopulmonary resuscitation on pediatric heart transplant outcome. Pediatr Transplant. 2011;15(7):742–5.
Zaroff JG, Solinger LL, Babcock WD. Temporal changes in donor left ventricular function: results of serial echocardiography. J Heart Lung Transplant. 2003;22(4):383–8.
Paul JJ, Tani LY, Shaddy RE, Minich LL. Spectrum of left ventricular dysfunction in potential pediatric heart transplant donors. J Heart Lung Transplant. 2003;22(5):548–52.
Sopko N, Shea KJ, Ludrosky K, Smedira N, Hoercher K, Taylor DO, Starling RC, Gonzalez-Stawinski GV. Survival is not compromised in donor hearts with echocardiographic abnormalities. J Surg Res. 2007;143(1):141–4.
Potapov E, Ivanitskaia E, Loebe M, Mumlckel M, Mumller C, Sodian R, Meyer R, Hetzer R. Value of cardiac troponin I and T for selection of heart donors and as predictors of early graft failure. Transplantation. 2001;71(10):1394–400.
Venkateswaran RV, Ganesh JS, Thekkudan J, Steeds R, Wilson IC, Mascaro J, Thompson R, Bonser RS. Donor cardiac troponin-I: a biochemical surrogate of heart function. Eur J Cardiothorac Surg. 2009;36(2):286–92. discussion 292. Epub 2009 Apr 25.
Boccheciampe N, Audibert G, Rangeard O, Charpentier C, Perrier JF, Lalot JM, Voltz C, Strub P, Loos-Ayav C, Meistelman C, Mertes PM, Longrois D. Serum troponin Ic values in organ donors are related to donor myocardial dysfunction but not to graft dysfunction or rejection in the recipients. Int J Cardiol. 2009;133(1):80–6. Epub 2008 Feb 5.
Stoica SC, Satchithananda DK, Charman S, Sharples L, King R, Rozario C, Dunning J, Tsui SS, Wallwork J, Large SR. Swan-Ganz catheter assessment of donor hearts: outcome of organs with borderline hemodynamics. J Heart Lung Transplant. 2002;21(6):615–22.
Solomon M, Grasemann H, Keshavjee S. Pediatric lung transplantation. Pediatr Clin North Am. 2010;57(2):375–91.
LaRosa C, Baluarte HJ, Meyers KE. Outcomes in pediatric solid-organ transplantation. Pediatr Transplant. 2011;15(2):128–41.
Zafar F, Heinle JS, Schecter MG, Rossano JW, Mallory Jr GB, Elidemir O, Morales DL. Two decades of pediatric lung transplant in the United States: have we improved? J Thorac Cardiovasc Surg. 2011;141(3):828–32.
Yusen RD, Shearon TH, Qian Y, Kotloff R, Barr ML, Sweet S, Dyke DB, Murray S. Lung transplantation in the United States, 1999-2008. Am J Transplant. 2010;10(4 Pt 2):1047–68.
Cypel M, Yeung JC, Keshavjee S. Novel approaches to expanding the lung donor pool: donation after cardiac death and ex vivo conditioning. Clin Chest Med. 2011;32(2):233–44.
Kotloff RM, Thabut G. Lung transplantation. Am J Respir Crit Care Med. 2011;184(2):159–71.
Sweet SC. Pediatric lung transplantation. Proc Am Thorac Soc. 2009;6(1):122–7.
Orens JB, Boehler A, de Perrot M, Estenne M, Glanville AR, Keshavjee S, Kotloff R, Morton J, Studer SM, Van Raemdonck D, Waddel T, Snell GI, Pulmonary Council, International Society for Heart and Lung Transplantation. A review of lung transplant donor acceptability criteria. J Heart Lung Transplant. 2003;22:1183–200.
Snell GI, Westall GP. Selection and management of the lung donor. Clin Chest Med. 2011;32(2):223–32. Epub 2011 Mar 22.
Van Raemdonck D, Neyrinck A, Verleden GM, Dupont L, Coosemans W, Decaluwé H, Decker G, De Leyn P, Nafteux P, Lerut T. Lung donor selection and management. Proc Am Thorac Soc. 2009;6(1):28–38.
Botha P. Extended donor criteria in lung transplantation. Curr Opin Organ Transplant. 2009;14(2):206–10.
Mangi AA, Mason DP, Nowicki ER, Batizy LH, Murthy SC, Pidwell DJ, Avery RK, McCurry KR, Pettersson GB, Blackstone EH. Predictors of acute rejection after lung transplantation. Ann Thorac Surg. 2011;91(6):1754–62. Epub 2011 May 4.
Naik PM, Angel LF. Special issues in the management and selection of the donor for lung transplantation. Semin Immunopathol. 2011;33(2):201–10.
Christie JD, Kotloff RM, Pochettino A, Arcasoy SM, Rosengard BR, Landis JR, Kimmel SE. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124(4):1232–41.
Christie JD, Bavaria JE, Palevski HI, Litzky L, Blumenthal NP, Kaiser LR, Kotloff RM. Primary graft failure following lung transplantation. Chest. 1998;114:51–60.
Thabut G, Vinatier I, Stern J-B, Leseche G, Loirat P, Fournier M, Mal H. Primary graft failure following lung transplantation. Predictive factors of mortality. Chest. 2002;121:1876–82.
Jahania MS, Mullett TW, Sanchez JA, Narayan P, Lasley RD, Mentzer RM. Acute allograft failure in thoracic organ transplantation. J Card Surg. 2000;15:122–8.
Aigner C, Winkler G, Jaksch P, Seebacher G, Lang G, Taghavi S, Wisser W, Klepetko W. Extended donor criteria for lung transplantation–a clinical reality. Eur J Cardiothorac Surg. 2005;27(5):757–61.
Moretti MP, Betto C, Gambacorta M, Vesconi S, Scalamogna M, Benazzi E, Ravini M. Lung procurement for transplantation: new criteria for lung donor selection. Transplant Proc. 2010;42(4):1053–5.
Davis RD, Pasque MK. Pulmonary transplantation. Ann Surg. 1995;221(1):14–28.
Sundaresan S, Semenkovich J, Ochoa L, Richardson G, Trulock EP, Cooper JD, Patterson GA. Successful outcome of lung transplantation is not compromised by the use of marginal donor lungs. J Thorac Cardiovasc Surg. 1995;109:1075–80.
Ware R, Fang X, Wang Y, Sakuma T, Hall TS, Matthay MA. Lung edema clearance: 20 years of progress selected contribution: mechanisms that may stimulate the resolution of alveolar edema in the transplanted human lung. J Appl Physiol. 2002;93:1869–74.
Labrousse L, Sztark F, Kays C, Thicoipe M, Lassie P, Couraud L, Marthan R. Improvement of brain-dead lung assessment based on the mechanical properties of the respiratory system. Transplant Proc. 1996;28(1):354.
Aziz T, El-Gamel A, Saad RAG, Migliore M, Campbell CS, Yonan NA. Pulmonary vein gas analysis for assessing donor lung function. Ann Thorac Surg. 2002;73:1559–605.
Puskas JD, Winton TL, Miller JD, Scavuzzo M, Patterson GA. Unilateral donor lung function does not preclude successful contralateral single lung transplantation. J Thorac Cardiovasc Surg. 1992;103:1015–7.
Wauters S, Verleden GM, Belmans A, Coosemans W, De Leyn P, Nafteux P, Lerut T, Van Raemdonck D. Donor cause of brain death and related time intervals: does it affect outcome after lung transplantation? Eur J Cardiothorac Surg. 2011;39(4):e68–76.
Hanna K, Seder CW, Weinberger JB, Sills PA, Hagan M, Janczyk RJ. Airway pressure release ventilation and successful lung donation. Arch Surg. 2011;146(3):325–8.
Taskar V, John J, Evander E, Wollmer P, Robertson B, Johnson B. Healthy lungs tolerate repetitive collapse and reopening during short periods of mechanical ventilation. Acta Anaesthesiol Scand. 1995;39:370–6.
Van Raemdonck DEM, Jannis NCP, De Leyn PRJ, Flameng WJ, Lerut TE. Alveolar expansion itself but not continuous oxygen supply enhances postmortem preservation of pulmonary grafts. Eur J Cardiothorac Surg. 1998;13:431–41.
Moran I, Zavala E, Fernandez R, Blanch L, Mancebo J. Recruitment manoeuvres in acute lung injury/acute respiratory distress syndrome. Eur Respir J Suppl. 2003;42:37s–42.
Gabbay E, Williams TJ, Griffiths AP, Macfarlane LM, Kotsimbos TC, Esmore DS, Snell GI. Maximizing the utilization of donor organs offered for lung transplantation. Am J Respir Crit Care Med. 1999;160:265–71.
Avlonitis VS, Wigfield CH, Golledge HD, Kirby JA, Dark JH. Early hemodynamic injury during donor brain death determines the severity of primary graft dysfunction after lung transplantation. Am J Transplant. 2007;7(1):83–90.
Rostron AJ, Avlonitis VS, Cork DM, Grenade DS, Kirby JA, Dark JH. Hemodynamic resuscitation with arginine vasopressin reduces lung injury after brain death in the transplant donor. Transplantation. 2008;85(4):597–606.
Dowling RD, Zenati M, Yousem SA, Pasculle AW, Kormos RL, Armitage JA, Griffith BP, Hardesty RL. Donor-transmitted pneumonia in experimental lung allogafts. Successful prevention with donor antibiotic therapy. J Thorac Cardiovasc Surg. 1992;103:767–72.
Low DE, Kaiser LR, Haydock DA, Trulock E, Cooper JD. The donor lung: infectious and pathologic factors affecting outcome in lung transplantation. J Thorac Cardiovasc Surg. 1995;109:1263–4.
Zenati M, Dowling RD, Dummer JS, Paradis IL, Arena VC, Armitage JM, Kormos RL, Hardesty RL, Griffith BP. Influence of the donor lung on development of early infections in lung transplant recipients. J Heart Transplant. 1990;9:502–9.
Avlonitis VS, Krause A, Luzzi L, Powell H, Phillips J, Corris PA, et al. Bacterial colonization of the donor lower airways is a predictor of poor outcome in lung transplantation. Eur J Cardiothorac Surg. 2003;24:601–7.
Waller DA, Thompson AM, Wrightson WN, Gould FK, Corris PA, Hilton CJ, Forty J, Dark JH. Does the mode of donor death influence the early outcome of lung transplantation? A review of lung transplantation from donors involved in major trauma. J Heart Lung Transplant. 1995;14(2):318–21.
Shafaghi S, Dezfuli AA, Makki SS, Marjani M, Mobarhan M, Ghandchi G, Khoddami-Vishteh HR, Ghorbani F, Najafizadeh K. Microbial pattern of bronchoalveolar lavage in brain dead donors. Transplant Proc. 2011;43(2):422–3.
Tiao GM, Alonso MH, Ryckman FC. Pediatric liver transplantation. Semin Pediatr Surg. 2006;15(3):218–27.
Hong JC, Yersiz H, Farmer DG, et al. Longterm outcomes for whole and segmental liver grafts in adult and pediatric liver transplant recipients: a ten year outcome comparative analysis of 2,988 cases. J Am Coll Surg. 2009;208(5):682–9.
Vagefi PA, Parekh J, Ascher NL, Roberts JP, Freise CE. Outcomes with split liver transplantation in 106 recipients: the University of California, San Francisco, experience from 1993 to 2010. Arch Surg. 2011;146(9):1052–9.
Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6(4):783–90.
Bonney GK, Aldersley MA, Asthana S, Toogood GJ, Pollard SG, Lodge JP, Prasad KR. Donor risk index and MELD interactions in predicting long-term graft survival: a single-centre experience. Transplantation. 2009;87(12):1858–63.
Avolio AW, Cillo U, Salizzoni M, De Carlis L, Colledan M, Gerunda GE, Mazzaferro V, Tisone G, Romagnoli R, Caccamo L, Rossi M, Vitale A, Cucchetti A, Lupo L, Gruttadauria S, Nicolotti N, Burra P, Gasbarrini A, Agnes S, On behalf of the Donor-to-Recipient Italian Liver Transplant (D2R-ILTx) Study Group. Balancing donor and recipient risk factors in liver transplantation: the value of D-MELD with particular reference to HCV recipients. Am J Transplant. 2011;11(12):2724–36.
Volk ML, Reichert HA, Lok AS, Hayward RA. Variation in organ quality between liver transplant centers. Am J Transplant. 2011;11(5):958–64.
Alexander JW, Vaughn WK, Carey MA. The use of marginal donors for organ transplantation: the older and younger donors. Transplant Proc. 1991;23:905–9.
Lee KW, Cameron AM, Maley WR, Segev DL, Montgomery RA. Factors affecting graft survival after adult/child split-liver transplantation: analysis of the UNOS/OPTN data base. Am J Transplant. 2008;8(6):1186–96.
Ploeg RJ, D’Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M. Risk factors for primary dysfunction after liver transplantation—a multivariate analysis. Transplantation. 1993;55:807–13.
Porte RJ, Ploeg RJ, Hansen B, van Bockel JH, Thorogood J, Persijn GG, Terpstra OT. Long-term graft survival after liver transplantation in the UW era: late effects of cold ischemia and primary dysfunction. European Multicentre Study Group. Transpl Int. 1998;11 Suppl 1:S164–7.
Brokelman W, Stel AL, Ploeg RJ. Risk factors for primary dysfunction after liver transplantation in the University of Wisconsin solution era. Transplant Proc. 1999;31:2087–90.
Alper I, Ulukaya S. Anesthetic management in pediatric liver transplantation: a comparison of deceased or live donor liver transplantations. J Anesth. 2010;24(3):399–406.
Zhang SJ, Wang T. The influence of brain death on donor liver and the potential mechanisms of protective intervention. Front Med. 2011;5(1):8–14.
Nafidi O, Marleau D, Roy A, Bilodeau M. Identification of new donor variables associated with graft survival in a single-center liver transplant cohort. Liver Transpl. 2010;16(12):1393–9.
Clavien PA, Harvey PR, Strasberg SM. Preservation and reperfusion injuries in liver allografts. An overview and synthesis of current studies. Transplantation. 1992;53:957–78.
Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651–63.
Strasberg SM, Howard TK, Molmenti EP, Hertl M. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology. 1994;20:829–38.
Port FK, Bragg-Gresham JL, Metzger RA, Dykstra DM, Gillespie BW, Young EW, Delmonico FL, Wynn JJ, Merion RM, Wolfe RA, Held PJ. Donor characteristics associated with reduced graft survival: an approach to expanding the pool of kidney donors. Transplantation. 2002;74:1281–6.
Wigmore SJ, Seeney FM, Pleass HC, Praseedom RK, Forsythe JL. Kidney damage during organ retrieval: data from UK National Transplant Database. Kidney Advisory Group. Lancet. 1999;354:1143–6.
Moudgil A, Martz K, Stablein DM, Puliyanda DP. Good outcome of kidney transplants in recipients of young donors: a NAPRTCS data analysis. Pediatr Transplant. 2011;15(2):167–71.
Sharma A, Fisher RA, Cotterell AH, King AL, Maluf DG, Posner MP. En bloc kidney transplantation from pediatric donors: comparable outcomes with living donor kidney transplantation. Transplantation. 2011;92(5):564–9.
Kim JM, Kim SJ, Joh JW, Kwon CH, Song S, Shin M, Kim BN, Lee SK. Is it safe to use a kidney from an expanded criteria donor? Transplant Proc. 2011;43(6):2359–62.
Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, Ojo AO, Port FK. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294(21):2726–33.
Pascual J, Zamora J, Pirsch JD. A systematic review of kidney transplantation from expanded criteria donors. Am J Kidney Dis. 2008;52(3):553–86. Review.
Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34(3):571–90.
Lee S, Shin M, Kim E, Kim J, Moon J, Jung G, Choi G, Kwon C, Joh J, Lee S, Kim S. Donor characteristics associated with reduced survival of transplanted kidney grafts in Korea. Transplant Proc. 2010;42(3):778–81.
Birck R, Krzossok S, Markowetz F, Schnulle P, van der Woude FJ, Braun C. Acetylcysteine for prevention of contrast nephropathy: meta-analysis. Lancet. 2003;362:598–603.
Melk A, Gourishankar S, Halloran P. Long-term effects of nonimmune tissue injury in renal transplantation. Curr Opin Organ Transplant. 2002;7:171–7.
Marconi L, Moreira P, Parada B, Bastos C, Roseiro A, Mota A. Donor cause of brain death in renal transplantation: a predictive factor for graft function? Transplant Proc. 2011;43(1):74–6.
Morariu AM, Schuurs TA, Leuvenink HG, van Oeveren W, Rakhorst G, Ploeg RJ. Early events in kidney donation: progression of endothelial activation, oxidative stress and tubular injury after brain death. Am J Transplant. 2008;8(5):933–41.
Westendorp WH, Leuvenink HG, Ploeg RJ. Brain death induced renal injury. Curr Opin Organ Transplant. 2011;16(2):151–6.
Sánchez-Fructuoso A, Naranjo Garcia P, Calvo Romero N, Ridao N, Naranjo Gómez P, Conesa J, Barrientos A. Effect of the brain-death process on acute rejection in renal transplantation. Transplant Proc. 2007;39(7):2214–6.
de Vries DK, Lindeman JH, Ringers J, Reinders ME, Rabelink TJ, Schaapherder AF. Donor brain death predisposes human kidney grafts to a proinflammatory reaction after transplantation. Am J Transplant. 2011;11(5):1064–70.
Nijboer WN, Ottens PJ, van Dijk A, van Goor H, Ploeg RJ, Leuvenink HG. Donor pretreatment with carbamylated erythropoietin in a brain death model reduces inflammation more effectively than erythropoietin while preserving renal function. Crit Care Med. 2010;38(4):1155–61.
Damman J, Hoeger S, Boneschansker L, Theruvath A, Waldherr R, Leuvenink HG, Ploeg RJ, Yard BA, Seelen MA. Targeting complement activation in brain-dead donors improves renal function after transplantation. Transpl Immunol. 2011;24(4):233–7. Epub 2011 Apr 1.
Jung GO, Yoon MR, Kim SJ, Sin MJ, Kim EY, Moon JI, Kim JM, Choi GS, Kwon CH, Cho JW, Lee SK. The risk factors of delayed graft function and comparison of clinical outcomes after deceased donor kidney transplantation: single-center study. Transplant Proc. 2010;42(3):705–9.
Troppmann C, Gillingham KJ, Benedetti E, Almond S, Gruessner RWG, Najarian JS, Matas AJ. Delayed graft function, acute rejection, and outcome after cadaver renal transplantation. Transplantation. 1995;59(7):962–8.
Walaszewski J, Rowinski W, Pacholczyk M, Lagiewska B, Cajzner S, Chmura A, et al. Multiple risk factor analysis of delayed graft function (ATN) after cadaveric transplantation: positive effect of lidocaine donor pretreatment. Transplant Proc. 1991;23:2475–6.
Schnuelle P, Lorenz D, Mueller A, Trede M, Van Der Woude FJ. Donor catecholamine use reduces acute allograft rejection and improves graft survival after cadaveric renal transplantation. Kidney Int. 1999;56:738–46.
Domínguez J, Lira F, Troncoso P, Aravena C, Ortiz M, Gonzalez R. Factors that predict duration of delayed graft function in cadaveric kidney transplantation. Transplant Proc. 2009;41(6):2668–9.
Schnuelle P, Gottmann U, Hoeger S, Boesebeck D, Lauchart W, Weiss C, Fischereder M, Jauch KW, Heemann U, Zeier M, Hugo C, Pisarski P, Krämer BK, Lopau K, Rahmel A, Benck U, Birck R, Yard BA. Effects of donor pretreatment with dopamine on graft function after kidney transplantation: a randomized controlled trial. JAMA. 2009;302(10):1067–75.
Singh RP, Farney AC, Rogers J, Gautreaux M, Reeves-Daniel A, Hartmann E, Doares W, Iskandar S, Adams P, Stratta RJ. Hypertension in standard criteria deceased donors is associated with inferior outcomes following kidney transplantation. Clin Transplant. 2011;25(4):E437–46.
Kunzendorf U, Hohenstein B, Oberbarnscheid M, Muller E, Renders L, Schott GE, Offermann G. Duration of donor brain death and its influence on kidney graft function. Am J Transplant. 2002;2:282–94.
Nijboer WN, Moers C, Leuvenink HG, Ploeg RJ. How important is the duration of the brain death period for the outcome in kidney transplantation? Transpl Int. 2011;24(1):14–20.
Opelz G. Cadaver kidney graft outcome in relation to ischemia time and HLA match. Collaborative Transplant Study. Transplant Proc. 1998;30:4294–6.
Wight JP, Chilcott JB, Holmes MW, Brewer N. Pulsatile machine perfusion vs. cold storage of kidneys for transplantation: a rapid and systemic review. Clin Transplant. 2003;17:293–307.
Ojo AO, Hanson JA, Meier-Kriesche H, Okechukwu CN, Wolfe RA, Leichtman AB, Agodoa LY, Kaplan B, Port FK. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol. 2001;12:589–97.
Berg CL, Steffick DE, Edwards EB, Heimbach JK, Magee JC, Washburn WK, Mazariegos GV. Liver and intestine transplantation in the United States 1998-2007. Am J Transplant. 2009;9(4 Pt 2):907–31.
Kaufman SS, Atkinson JB, Bianchi A, Goulet OJ, Grant D, Langnas AN, McDiarmid SV, Mittal N, Reyes J, Tzakis AG, American Society of Transplantation. Indications for pediatric intestinal transplantation: a position paper of the American Society of Transplantation. Pediatr Transplant. 2001;5(2):80–7.
Gupte GL, Beath SV, Protheroe S, Murphy MS, Davies P, Sharif K, McKiernan PJ, de Ville de Goyet J, Booth IW, Kelly DA. Improved outcome of referrals for intestinal transplantation in the UK. Arch Dis Child. 2007;92(2):147–52.
Goulet O, Sauvat F. Short bowel syndrome and intestinal transplantation in children. Curr Opin Clin Nutr Metab Care. 2006;9(3):304–13.
Sudan D. Long-term outcomes and quality of life after intestine transplantation. Curr Opin Organ Transplant. 2010;15(3):357–60.
Arnow PM, Carandang GC, Zabner R, Irwin ME. Randomized controlled trial of selective bowel decontamination for prevention of infections following liver transplantation. Clin Infect Dis. 1996;22(6):997–1003.
Safdar N, Said A, Lucey MR. The role of selective digestive decontamination for reducing infection in patients undergoing liver transplantation: a systematic review and meta-analysis. Liver Transpl. 2004;10(7):817–27.
Hellinger WC, Yao JD, Alvarez S, Blair JE, Cawley JJ, Paya CV, O’Brien PC, Spivey JR, Dickson RC, Harnois DM, Douglas DD, Hughes CB, Nguyen JH, Mulligan DC, Steers JL. A randomized, prospective, double-blinded evaluation of selective bowel decontamination in liver transplantation. Transplantation. 2002;73(12):1904–9.
Arbour R. Clinical management of the organ donor. AACN Clin Issues. 2005;16(4):551–80.
Kong AP, Barrios C, Salim A, Willis L, Cinat ME, Dolich MO, Lekawa ME, Malinoski DJ. A multidisciplinary organ donor council and performance improvement initiative can improve donation outcomes. Am Surg. 2010;76(10):1059–62.
Salim A, Martin M, Brown C, Rhee P, Demetriades D, Belzberg H. The effect of a protocol of aggressive donor management: implications for the national organ donor shortage. J Trauma. 2006;61(2):429–33.
DuBose J, Salim A. Aggressive organ donor management protocol. J Intensive Care Med. 2008;23(6):367–75.
Kirschbaum CE, Hudson S. Increasing organ yield through a lung management protocol. Prog Transplant. 2010;20(1):28–32.
Bardell T, Hunter DJ, Kent WD, Jain MK. Do medical students have the knowledge needed to maximize organ donation rates? Can J Surg. 2003;46(6):453–7.
Cohen J, Ami SB, Ashkenazi T, Singer P. Attitude of health care professionals to brain death: influence on the organ donation process. Clin Transplant. 2008;22(2):211–5.
Bener A, El-Shoubaki H, Al-Maslamani Y. Do we need to maximize the knowledge and attitude level of physicians and nurses toward organ donation and transplant? Exp Clin Transplant. 2008;6(4):249–53.
Hobeika MJ, Simon R, Malik R, Pachter HL, Frangos S, Bholat O, Teperman S, Teperman L. U.S. surgeon and medical student attitudes toward organ donation. J Trauma. 2009;67(2):372–5.
Griffiths J, Verble M, Falvey S, Bell S, Logan L, Morgan K, Wellington F. Culture change initiatives in the procurement of organs in the United Kingdom. Transplant Proc. 2009;41(5):1459–62.
Committee on Hospital Care, Section on Surgery, and Section on Critical Care. Policy statement- -pediatric organ donation and transplantation. Pediatrics. 2010;125(4):822–8.
Hornby K, Ross H, Keshavjee S, Rao V, Shemie SD. Factors contributing to non-utilization of heart and lungs after consent for donation: a Canadian multicentre study. Can J Anaesth. in press, 2006.
Scheinkestel CD, Tuxen DV, Cooper DJ, Butt W. Medical management of the (potential) organ donor. Anaesth Intensive Care. 1995;23(1):51–9.
U.S. Department of Health & Human Services. Organ procurement and transplantation network. Donors : donation year (2009–2010) by donor type, age type, organ. Based on OPTN data as of October 7, 2011. http://optn.transplant.hrsa.gov. Accessed 10 Aug 2012.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Shemie, S.D., Dhanani, S. (2014). The Physiology of Brain Death and Organ Donor Management. In: Wheeler, D., Wong, H., Shanley, T. (eds) Pediatric Critical Care Medicine. Springer, London. https://doi.org/10.1007/978-1-4471-6362-6_38
Download citation
DOI: https://doi.org/10.1007/978-1-4471-6362-6_38
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-6361-9
Online ISBN: 978-1-4471-6362-6
eBook Packages: MedicineMedicine (R0)