Abstract
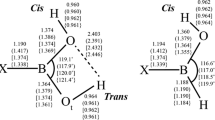
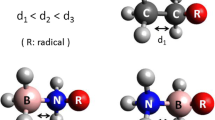
Boronic acids, R–B(OH)2, play an important role in synthetic, biological, medicinal, and materials chemistry. Borinic acids, R–BH(OH), find relevance in similar fields, although their properties, e.g., binding affinity to diols, can vary significantly. Dative boron–nitrogen bonds, N → B, are critical for protecting the boron atom in these acids from nucleophilic attack. In this article, we study the structure, bonding, and formation thermodynamics of the model donor–acceptor complexes H3N → BR(OH)2 and H3N → BRH(OH) (R = H; NH2, OH, and F). Geometry optimizations were performed using second-order Møller–Plesset perturbation theory (MP2) with the Dunning–Woon aug-cc-pVTZ basis set; single-point CCSD(FC)/aug-cc-pVTZ//MP2(FC)/aug-cc-pVTZ level calculations were used to generate a QCI density for analyses of the bonding. Extensive comparisons are made with results from density functional theory (DFT) optimizations with/without empirical dispersion corrections. The addition of an ammonia molecule dative bonded to the boron atom for these boronic and borinic acids results in the elongation of bonds to the boron atom, e.g., the boron–oxygen bond lengths increase in the range from ~ 4.5 to ~ 6.5%. The calculated values of ∆ \( {H}_{298}^0 \) for the ammoniation reactions, R–B(OH)2 + NH3 → H3N → BR(OH)2 are − 0.6, + 4.6, + 0.1, and − 6.0 kcal/mol for R = H, NH2, OH, and F, respectively, at the CCSD(FC)/aug-cc-pVTZ//MP2(FC)/aug-cc-pVTZ level; the corresponding values of ∆\( {H}_{298}^0 \) for the borinic acid reactions are − 8.9, + 2.1, − 1.5, and − 7.9 kcal/mol.


Similar content being viewed by others
References
Icli B, Sheepwash E, Riis-Johannessen T, Schenk K, Filinchuk Y, Scopelliti R, Severin K (2011) Dative boron-nitrogen bonds in structural supramolecular chemistry: multicomponent assembly of prismatic organic cages. Chem Sci 2(9):1719–1721
Dhara A, Beuerle F (2015) Reversible assembly of a supramolecular cage linked by boron-nitrogen dative bonds. Chem Eur J 21(48):17391–17396
Nunes M, Gois P, Rosa M, Martins S, Fernandes P, Ribeiro M (2016) Boronic acids as efficient cross linkers for PVA: synthesis and application of tunable hollow microspheres in biocatalysis. Tetrahedron 72(46):7293–7305
Reddy R, Srivastava A, Kumar A (2013) Monosaccharide-responsive phenylboronate-polyol cell scaffolds for cell sheet and tissue engineering applications. PLoS One. 8(10):1–10
James TD, Sandanayake KRA, Shinkai S (1994) A Glucose-Selective Molecular Fluorescence Sensor. Angewandtie Chemie 33(21):2207–2209
Larkin J, Fossey J, James T, Brooks B, Bock C (2010) A computational investigation of the nitrogen-boron interaction in o-(N,N-Dialkylaminomethyl)arylboronate systems. J Phys Chem A 114(47):12531–12539
Chapin B, Metola P, Vankayala S, Woodcock H, Mooibroek T, Lynch V, Larkin J, Anslyn E (2017) Disaggregation is a mechanism for emission turn-on of ortho-aminomethylphenylboronic acid-based saccharide sensors. J Am Chem Soc 139(15):5568–5578
Moller C, Plesset MS (1934) Note on an approximation treatment for manyelectron systems. Phys Rev 46:618–622
Dunning Jr TH (1989) Gaussian basis sets for use in correlated molecular calculations. I. the atoms boron through neon and hydrogen. J Chem Phys 90:1007–1023
Kendal RA, Dunning Jr TH, Harrison RJ (1992) Electron affinities of the first row atoms revisited. J Chem Phys 96:6796–6806
Woon DE, Dunning Jr TH (1993) Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J Chem Phys 98:1358–1371
Peterson KA, Woon DE, Dunning Jr TH (1994) Benchmark calculations with correlated molecular wave functions. IV. The classical barrier height of the H+H2--> H2 + H reaction. J Chem Phys 100:7410–7415
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts RE, Stratmann O, Yazyev AJ, Austin R, Cammi C, Pomelli JW, Ochterski R, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2003) G03; R B.02 ed. Gaussian Inc., Wallingford, CT
Frisch JM, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman RJ, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts RE, Stratmann O, Yazyev AJ, Austin R, Cammi C, Pomelli JW, Ochterski R, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) G09. Gaussian Inc., Wallingford, CT
Bartlett RJ, Purvis GD (1978) Many body perturbation theory, coupled pair many electron theory, and the importance of quadruple excitations for the correlation problem. Int J Quantum Chem 14:561–581
Grimme, S.; Ehrlich, S.; Goerigk, L., (2011)Effect of damping function in dispersion corrected density functional theory. J. Comput. Chem. 32(7):1456–1465
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate abinitio parameterization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132(15):154104
Becke AD (1993) A new mixing of Hartree-Fock and local density functional theories. J Chem Phys 98:1372–1377
Yanai T, Tew D, Handy N (2004) A new hybrid exchange-correlation functional using the coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51–57
Perdew JP, Burke K, Ernzerhoff M (1997) Errata: generalized gradient approximation made simple. Phys Rev Lett 78(7):1396
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Grimme S (2006) Semi-empirical hybrid density functional with perturbative second-order correlation. J Chem Phys 124(3):034108
Goerigk L, Grimme S (2011) Efficient and accurate double-hybrid-meta-GGA density functionals—evaluation with the extended GMTKN30 database for general main group thermochemistry, kinetics, and noncovalent interactions. J Chem Theory Comput 7:291–309
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Accounts 120:215–241
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735–746
Foster J, Weinhold F (1980) Natural hybrid orbitals. J Am Chem Soc 102:7211–7218
Carpenter J, Weinhold F (1988) Analysis of the geometry of the hydroxymethyl radical by the “different hybrids for different spins” natural bond orbital procedure. J Mol Struct THEOCHEM 169:41–62
Weinhold F, Glendening ED (2001) NBO 5.0 program manual: natural bond orbital analysis programs. Theoretical Chemistry Institute and Department of Chemistry, University of Wisconsin, Madison, WI, p 53706
Rao NZ, Larkin JD, Bock CW (2016) A comparison of the structure and bonding in the aliphatic boronic R-B(OH)2 and borinic R-BH(OH) acids (R=H, NH2, OH, and F): a computational investigation. Struct Chem 27:1081–1091
Demaison J, Lievin J, Csaszar AG, Gutle C (2008) Equilibrium structure and torsional barrier of BH3NH3. J Phys Chem A 112(19):4477–4482
Staubitz A, Robertson APM, Manners I (2010). Chem Rev 110:4079–4124
Klooster WT, Koetzle TF, Siegbhan PEM, Richards TB, Crabtree RH (1999) Study of the N-H---H-B dihydrogen bond including the crystal structure of BH3NH3 by neutron diffraction. J Am Chem Soc 121:6337–6343
Boese R, Niederprum N, Blaser D, Maksic ZB, Eckert-Masic M, Horwood E (1992) In molecules in natural science and medicine. Chichester
Thorne LR, Suenrum RD, Lovas FJ (1983) Microwave spectrum, torsional barrier, and structure of BH3NH3. J Chem Phys 78(1):167
Dixon DA, Gutkowski M (2005). J Phys Chem A 109:5129–5135
Horvath V, Kovacs A, Hargittai I (2003). J Phys Chem A 107:1197–1202
Bent HA (1961) An appraisal of valence-bond structures and hybridization in compounds of the first-row elements. Chem Rev 61:275–311
Acknowledgements
This research was supported in part by the National Science Foundation through XSEDE resources provided by the XSEDE Science Gateways program. The PQS Cluster Facility at Jefferson University was also used for the calculations described in this manuscript. J.D.L would like to thank the National Heart, Lung, and Blood Institute of the National Institutes of Health for generous support under Award Number K22HL113045. J.D.L. would also like to thank the National Science Foundation for support through grant CHE-1531590.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 170 kb)
Rights and permissions
About this article
Cite this article
Larkin, J.D., Bock, C.W. A comparison of the structure and bonding in the donor–acceptor complexes H3N → BR(OH)2 and H3N → BRH(OH) (R = H; NH2, OH, and F): a computational investigation. Struct Chem 30, 361–368 (2019). https://doi.org/10.1007/s11224-018-1205-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-018-1205-2