Abstract
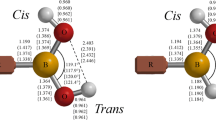
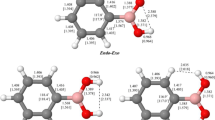
Boronic acids, R–B(OH)2, play an important role in synthetic, biological, medicinal, and materials chemistry. This investigation compares the structure and bonding surrounding the boron atoms in the simple aliphatic boronic acids, R–B(OH)2 (R=H; NH2, OH, and F), and the analogous borinic acids, R–BH(OH). Geometry optimizations were performed using second-order Møller–Plesset perturbation theory (MP2) with the Dunning–Woon aug-cc-pVTZ, aug-cc-pVQZ, and aug-cc-pV5Z basis sets; single-point CCSD(FC)/aug-cc-pVTZ//MP2(FC)/aug-cc-pVTZ level calculations were used to generate a QCI density for natural bond orbital analyses of the bonding. The optimized boron–oxygen bond lengths for the X–B–Ot–H trans-branch of the endo-exo form of the boronic acids and for the X–B–O–H cis-branch of the boronic and borinic acids (X=N, O, and F, respectively) decrease as the electronegativity of X increases. The boron–oxygen bond lengths are generally longer in the endo-exo or anti forms of the boronic acids than in the corresponding borinic acids. NBO analyses suggest the boron–oxygen bond in H2BOH is a double bond; the boron–oxygen bonding in the remaining boronic and borinic acids in this study has a significant contribution from dative pπ–pπ bonding. Values for Δ\({\text{H}}_{298}^{0}\) for the highly balanced reaction, R–B(OH)2 + R–BH2 → 2 R–BH(OH), suggest that the bonding surrounding the boron atom is stronger in the borinic acid than in the corresponding boronic acid.



Similar content being viewed by others
References
Arimori S, Bell ML, Oh CS, Frimat KA, James TD (2002) Modular fluorescence sensors for saccharides. J Chem Soc Perkin Trans 1(6):803–808
Arzt M, Seidler C, Ng DY, Weil T (2014) Reversible click reactions with boronic acids to build supramolecular architectures in water. Chem Asian J 9(8):1994–2003
Baker SJ, Tomsho JW, Benkovic SJ (2011) Boron-containing inhibitors of synthetases. Chem Soc Rev 40(8):4279–4285
Bull SD, Davidson MG, den Elsen Van, Jean MH, Fossey JS, Jenkins ATA, Jiang Y et al (2012) Exploiting the reversible covalent bonding of boronic acids: recognition, sensing, and assembly. Acc Chem Res 46(2):312–326
Cambre JN, Sumerlin BS (2011) Biomedical applications of boronic acid polymers. Polymer 52(21):4631–4643
Dembitsky VM, Quntar AA, Srebnik M (2004) Recent advances in the medicinal chemistry of α-Aminoboronic acids, amine-carboxyboranes and their derivatives. Mini Rev Med Chem 4(9):1001–1018
Deng CC, Brooks WL, Abboud KA, Sumerlin BS (2015) Boronic acid-based hydrogels undergo self-healing at neutral and acidic pH. ACS Macro Lett 4(2):220–224
Dienstmaier JF, Gigler AM, Goetz AJ, Knochel P, Bein T, Lyapin A et al (2011) Synthesis of well-ordered COF monolayers: surface growth of nanocrystalline precursors versus direct on-surface polycondensation. ACS Nano 5(12):9737–9745
Fossey JS, D’Hooge F, van den Elsen Jean MH, Pereira Morais MP, Pascu SI, Bull SD et al (2012) The development of boronic acids as sensors and separation tools. Chem Rec 12(5):464–478
Fu H, Fang H, Sun J, Wang H, Liu A, Sun J et al (2014) Boronic acid-based enzyme inhibitors: a review of recent progress. Curr Med Chem 21(28):3271–3280
Fujita N, Shinkai S, James TD (2008) Boronic acids in molecular self-assembly. Chem Asian J 3(7):1076–1091
Galbraith E, James TD (2010) Boron based anion receptors as sensors. Chem Soc Rev 39(10):3831–3842
Guan Y, Zhang Y (2013) Boronic acid-containing hydrogels: synthesis and their applications. Chem Soc Rev 42(20):8106–8121
Guo Z, Shin I, Yoon J (2012) Recognition and sensing of various species using boronic acid derivatives. Chem Commun 48(48):5956–5967
Hall DG (2006) Boronic acids: preparation, applications in organic synthesis and medicine, 1st edn. Wiley, New Jersey
Huang S, Jia M, Xie Y, Wang J, Xu W, Fang H (2012) The progress of selective fluorescent chemosensors by boronic acid. Curr Med Chem 19(16):2621–2637
James TD, Shinkai S (2002) Artificial receptors as chemosensors for carbohydrates. In: Penadés S (ed) Host-Guest Chemistry. Springer, Berlin Heidelberg, pp 159–200
James TD, Phillips MD, Shinkai S (2006) Boronic acids in saccharide recognition. Royal Society of Chemistry, Cambridge
Korich AL, Iovine PM (2010) Boroxine chemistry and applications: a perspective. Dalton Trans 39(6):1423–1431
Lacina K, Skládal P, James TD (2014) Boronic acids for sensing and other applications-a mini-review of papers published in 2013. Chem Cent J 8(1):60
Loveless D, Holtsclaw J, Weaver J, Ogle J, Saini R (2014) Multifunctional boronic acid crosslinker for fracturing fluids. IPTC 2014: International petroleum technology conference 2014
Martin AR, Vasseur J, Smietana M (2013) Boron and nucleic acid chemistries: merging the best of both worlds. Chem Soc Rev 42(13):5684–5713
Matsumoto A, Miyahara Y (2014) Current development status and perspectives of self-regulated insulin delivery systems: a review. Electron Commun Jpn 97(12):57–61
Nevalainen V (1996) HB, (OH)2 and H2 CO as probes for a study on binding of dialkoxyboranes and ketones to oxazaborolidines capable of catalyzing the enantioselective reduction of ketones. Tetrahedron Asymmetry 7(9):2655–2664
Nishiyabu R, Kubo Y, James TD, Fossey JS (2011) Boronic acid building blocks: tools for self assembly. Chem Commun 47(4):1124–1150
Nishiyabu R, Kubo Y, James TD, Fossey JS (2011) Boronic acid building blocks: tools for sensing and separation. Chem Commun 47(4):1106–1123
Petasis NA (2007) Expanding roles for organoboron compounds–Versatile and valuable molecules for synthetic, biological and medicinal chemistry. Aust J Chem 60(11):795–798
Scorei R (2012) Is boron a prebiotic element? A mini-review of the essentiality of boron for the appearance of life on Earth. Origins of Life and Evolution of Biospheres 42(1):3–17
Smoum R, Rubinstein A, Dembitsky VM, Srebnik M (2012) Boron containing compounds as protease inhibitors. Chem Rev 112(7):4156–4220
Touchet S, Carreaux F, Carboni B, Bouillon A, Boucher J (2011) Aminoboronic acids and esters: from synthetic challenges to the discovery of unique classes of enzyme inhibitors. Chem Soc Rev 40(7):3895–3914
Trippier PC, McGuigan C (2010) Boronic acids in medicinal chemistry: anticancer, antibacterial and antiviral applications. Med Chem Commun 1(3):183–198
Wang X, Xia N, Liu L (2013) Boronic acid-based approach for separation and immobilization of glycoproteins and its application in sensing. Int J Mol Sci 14(10):20890–20912
Collins BE, Metola P, Anslyn EV (2013) On the rate of boronate ester formation in ortho-aminomethyl-functionalised phenyl boronic acids. Supramol Chem 25(2):79–86
Lee D, Taylor MS (2011) Borinic acid-catalyzed regioselective acylation of carbohydrate derivatives. J Am Chem Soc 133(11):3724–3727
Chan L, Taylor MS (2011) Regioselective alkylation of carbohydrate derivatives catalyzed by a diarylborinic acid derivative. Org Lett 13(12):3090–3093
Lee D, Williamson CL, Chan L, Taylor MS (2012) Regioselective, borinic acid-catalyzed monoacylation, sulfonylation and alkylation of diols and carbohydrates: expansion of substrate scope and mechanistic studies. J Am Chem Soc 134(19):8260–8267
Bailey P, Cousins G, Snow G, White A (1980) Boron-containing antibacterial agents: effects on growth and morphology of bacteria under various culture conditions. Antimicrob Agents Chemother 17(4):549–553
Baker SJ, Akama T, Zhang Y, Sauro V, Pandit C, Singh R et al (2006) Identification of a novel boron-containing antibacterial agent (AN0128) with anti-inflammatory activity, for the potential treatment of cutaneous diseases. Bioorg Med Chem Lett 16(23):5963–5967
Benkovic S, Liu C, Tomsho JW (2015) Inventors; boronic and borinic acid compound as inhibitors of sulfenic acid-containing proteins. Patent 20,150,140,635, May 21, 2015
Lee V, Benkovic SJ (2004) Inventors; antibiotics containing borinic acid complexes and methods of use. Patent 20,040,224,923, Nov 11, 2004
Simpelkamp J, Jones JB (1992) Borinic acid inhibitors as probes of the factors involved in binding at the active sites of subtilisin carlsberg and α-chymotrypsin. Bioorg Med Chem Lett 2(11):1391–1394
Chudzinski MG, Chi Y, Taylor MS (2011) Borinic acids: a neglected class of organoboron compounds for recognition of diols in aqueous solution. Aust J Chem 64(11):1466–1469
Gouliaras C, Lee D, Chan L, Taylor MS (2011) Regioselective activation of glycosyl acceptors by a diarylborinic acid-derived catalyst. J Am Chem Soc 133(35):13926–13929
Lee D, Taylor MS (2013) Regioselective silylation of pyranosides using a boronic acid/Lewis base co-catalyst system. Org Biomol Chem 11(33):5409–5412
Dimitrijević E, Taylor MS (2013) 9-Hetero-10-boraanthracene-derived borinic acid catalysts for regioselective activation of polyols. Chem Sci 4(8):3298–3303
Bhat KL, Markham GD, Larkin JD, Bock CW (2011) Thermodynamics of boroxine formation from the aliphatic boronic acid monomers R–B (OH)2 (R=H, H3C, H2N, HO, and F): a computational investigation. J Phys Chem A 115(26):7785–7793
Bock CW, Larkin JD (2012) Heats of formation for the boronic acids R–B (OH)2 and boroxines R3 B3 O3 (R=H, Li, HBe, H2 B, H3C, H2N, HO, F, and Cl) calculated at the G2, G3, and G4 levels of theory. Comput Theor Chem 986:35–42
Rao NZ, Larkin JD, Bock CW (2015) A computational investigation of monosubstituted boroxines (RH2B3O3): structure and formation. Struct Chem 26:1151–1162
Møller C, Plesset MS (1934) Note on an approximation treatment for many-electron systems. Phys Rev 46(7):618
Dunning TH Jr (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90(2):1007–1023
Kendall RA, Dunning TH Jr, Harrison RJ (1992) Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J Chem Phys. 96(9):6796–6806
Peterson KA, Woon DE, Dunning TH Jr (1994) Benchmark calculations with correlated molecular wave functions. IV. The classical barrier height of the H H2 → H2 H reaction. J Chem Phys 100(10):7410–7415
Woon DE, Dunning TH Jr (1993) Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J Chem Phys. 98(2):1358–1371
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, et al (2008) Gaussian 03, revision C. 02
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, et al (2009) Gaussian 09, revision A. 1. Gaussian Inc., Wallingford, CT
Carpenter J, Weinhold F (1988) Analysis of the geometry of the hydroxymethyl radical by the “different hybrids for different spins” natural bond orbital procedure. J Mol Struct (Thoechem) 169:41–62
Foster J, Weinhold F (1980) Natural hybrid orbitals. J Am Chem Soc 102(24):7211–7218
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83(2):735–746
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor–acceptor viewpoint. Chem Rev 88(6):899–926
Weinhold F, Glendening ED (2001) NBO 5.0 Program Manual: Natural Bond Orbital Analysis Programs. Theoretical Chemistry Institute and Department of Chemistry, University of Wisconsin, Madison, WI 53706
Glendening E, Badenhoop J, Reed A, et al (2013) NBO 6.0. Theoretical Chemistry Institute and Department of Chemistry, University of Wisconsin, Madison, WI
Bader RF (1990) Atoms in molecules. John Wiley & Sons, Ltd
Cioslowski J, Mixon ST (1991) Covalent bond orders in the topological theory of atoms in molecules. J Am Chem Soc 113(11):4142–4145
Cioslowski J, Nanayakkara A (1994) A new robust algorithm for fully automated determination of attractor interaction lines in molecules. Chem Phys Lett 219(1):151–154
Boggs JE, Cordell FR (1981) Accurate ab initio gradient calculation of the structures and conformations of some boric and fluoroboric acids. Basis-set effects on angles around oxygen. J Mol Struct (Thoechem) 76(4):329–347
Duan X, Linder D, Page M, Soto M (1999) Structures and thermochemistry of BH l F m (OH) n and several XYBO compounds at the G-2 level of theory. J Mol Struct (Thoechem) 465(2):231–242
Larkin JD, Bhat KL, Markham GD, Brooks BR, Schaefer HF, Bock CW (2006) Structure of the boronic acid dimer and the relative stabilities of its conformers. J Phys Chem A 110(36):10633–10642
Larkin JD, Bhat KL, Markham GD, Brooks BR, Lai JH, Bock CW (2007) A computational investigation of the geometrical structure and protodeboronation of boroglycine, H2 N-CH2-B (OH) 2. J Phys Chem A 111(28):6489–6500
So S (1982) A theoretical study of the conformations of BF (OH)2. J Mol Struct (Thoechem) 89(3):255–258
Stefani D, Pashalidis I, Nicolaides AV (2008) A computational study of the conformations of the boric acid (B(OH)3), its conjugate base ((HO)2BO−) and borate anion. J Mol Struct (Thoechem) 853(1):33–38
Kawashima Y, Takeo H, Matsumura C (1978) Microwave spectroscopic detection of BFHOH and BH(OH)2. Chem Phys Lett 57(1):145–147
Kawashima Y, Takeo H, Matsumura C (1979) Microwave spectrum of fluorodihydroxy borane, BF(OH)2. J Mol Spectrosc 78(3):493–505
Gajhede M, Larsen S, Rettrup S (1986) Electron density of orthoboric acid determined by X-ray diffraction at 105 K and ab initio calculations. Acta Crystallogr B 42(6):545–552
Cyrański MK, Jezierska A, Klimentowska P, Panek JJ, Żukowska GZ, Sporzyński A (2008) Structural and spectroscopic properties of an aliphatic boronic acid studied by combination of experimental and theoretical methods. J Chem Phys. 128(12):124512
Private Communication with A. Sporzynski
Wells PR (1968) Group electronegativities. Prog Phys Org Chem 6:111–145
Kawashima Y, Takeo H, Matsumura C (1981) Microwave spectrum of borinic acid BH2OH. J Chem Phys 74(10):5430–5435
Straub DK (1995) Lewis structures of boron compounds involving multiple bonding. J Chem Educ 72(6):494
Mierzwa G, Gordon AJ, Latajka Z, Berski S (2015) On the multiple B O bonding using the topological analysis of electron localisation function (ELF). Comput Theor Chem 1053:130–141
Bent HA (1961) An appraisal of valence-bond structures and hybridization in compounds of the first-row elements. Chem Rev 61(3):275–311
Dill J (1975) Schleyer PvR, Pople J. Molecular orbital theory of the electron structure of organic compounds. XXIV. Geometries and energies of small boron compounds. Comparisons with carbocations. J Am Chem Soc 97(12):3402–3409
Cordell FR, Boggs JE (1980) The barriers to methyl group rotation in s-cis-and s-trans-methyl nitrite. J Mol Struct 64:57–65
Gropen O, Johansen R (1975) Barrier to internal rotation and π-bonding In hydroxyborane, H2BOH, studied by ab in1tio calculations. J Mol Struct 25(1):161–167
Carpenter JD, Ault BS (1992) Matrix isolation study of the mechanism of the reaction of diborane with ammonia: pyrolysis of the H3 B NH3 adduct. Chem Phys Lett 197(1):171–174
Kawashima Y, Takeo H, Matsumura C (1986) Microwave spectrum, structure, dipole moment, quadrupole coupling constants, and barrier to internal rotation of methoxyborane, CH3 OBH2. J Mol Spectrosc 116(1):23–32
Vidovic D, Moore JA, Jones JN, Cowley AH (2005) Synthesis and characterization of a coordinated oxoborane: Lewis acid stabilization of a boron–oxygen double bond. J Am Chem Soc 127(13):4566–4567
Kawashima Y, Endo Y, Hirota E (1989) Microwave spectrum, molecular structure, and force field of HBO. J Mol Spectrosc 133(1):116–127
Larkin JD, Bhat KL, Markham GD, James TD, Brooks BR, Bock CW (2008) A computational characterization of boron–oxygen multiple bonding in HN CH–CH CH–NH–BO. J Phys Chem A 112(36):8446–8454
Ortiz J (1989) An electron propagator study of bonding in aminoborane. Chem Phys Lett 156(5):489–493
Sugie M, Takeo H, Matsumura C (1987) Microwave spectrum and molecular structure of aminoborane, BH2 NH2. J Mol Spectrosc 123(2):286–292
Thorne L, Suenram R, Lovas F (1983) Microwave spectrum, torsional barrier, and structure of BH3NH3. J Chem Phys 78(1):167–171
Klooster WT, Koetzle TF, Siegbahn PE, Richardson TB, Crabtree RH (1999) Study of the NH HB dihydrogen bond including the crystal structure of BH3NH3 by neutron diffraction. J Am Chem Soc 121(27):6337–6343
Iminoboranes Paetzold P (1987) Adv Inorgan Chem Radiochem 31:123–170
Berski S, Latajka Z, Gordon AJ (2011) On the multiple B–N bonding in boron compounds using the topological analysis of electron localization function (ELF). New J Chem 35(1):89–96
Leroy G, Sana M, Wilante C (1993) Evaluation of the bond energy terms for the various types of boron–nitrogen bonds. Theoret Chim Acta 85(1–3):155–166
Niedenzu K, Dawson JW (1965) Boron nitride. In: Niedenzu K, Dawson JW (eds) Boron–nitrogen compounds, vol. 6. Springer, Berlin Heidelberg, pp 147–153
Takeo H, Curl R (1972) Microwave spectrum of BF2OH. J Chem Phys 56:4314–4317
Cazzoli G, Cludi L, Degli Esposti C, Dore L (1989) The millimeter and submillimeter-wave spectrum of boron monofluoride: equilibrium structure. J Mol Spectrosc 134(1):159–167
Takeo H, Sugie M, Matsumura C (1993) Microwave spectroscopic detection of fluoroborane, BH2 F. J Mol Spectrosc 158(1):201–207
Cox AP, Hubbard SD, Waterfield S (1986) The microwave spectrum and structure of CH3 BBr2. J Mol Spectrosc 118(2):459–470
Breidung J, Demaison J, D’Eu J, Margulès L, Collet D, Mkadmi E et al (2004) Ground-state constants, ab initio anharmonic force field, and equilibrium structure of F2 BOH. J Mol Spectrosc 228(1):7–22
George P, Trachtman M, Bock CW, Brett AM (1975) An alternative approach to the problem of assessing stabilization energies in cyclic conjugated hydrocarbons. Theoret Chim Acta 38(2):121–129
George P, Trachtman M, Bock CW, Brett AM (1976) An alternative approach to the problem of assessing destabilization energies (strain energies) in cyclic hydrocarbons. Tetrahedron 32(3):317–323
George P, Trachtman M, Bock CW, Brett AM (1976) Homodesmotic reactions for the assessment of stabilization energies in benzenoid and other conjugated cyclic hydrocarbons. J Chem Soc Perkin Trans 2(11):1222–1227
Wheeler SE, Houk KN, Schleyer PvR, Allen WD (2009) A hierarchy of homodesmotic reactions for thermochemistry. J Am Chem Soc 131(7):2547–2560
Acknowledgments
This research was supported in part by the National Science Foundation through XSEDE resources provided by the XSEDE Science Gateways program. The PQS Cluster Facility at Philadelphia University was also used for the calculations described in this manuscript. J.D.L. would like to thank the National Institutes of Health for research support (Grant: 5K22HL113045-04).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rao, N.Z., Larkin, J.D. & Bock, C.W. A comparison of the structure and bonding in the aliphatic boronic R–B(OH)2 and borinic R–BH(OH) acids (R=H; NH2, OH, and F): a computational investigation. Struct Chem 27, 1081–1091 (2016). https://doi.org/10.1007/s11224-015-0730-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-015-0730-5