Abstract
This study consists of the theoretical analysis of some organic molecules and their inorganic similar compounds, through substitution of two carbon atoms by boron and nitrogen atoms. The methods DFT/B3-LYP/TZVPP and CC2/TZVPP were considered. Firstly, ethane, ethene, and ethyne molecules (based on C atoms and their BN/NB analogs) were studied. These molecules were considered as a reference for the analysis of other molecules with functional groups. These molecules with functional groups are: ethanol, ethanal (and its isomer ethenol), ethanoic acid (and its isomer ethenediol), ethylamine, ethylbenzene, propane, and fluoroethane. We studied the energies, bond length, population analysis, and bond order. The dative bonds (BN) are bigger and weaker than that covalent based on C atoms. The dative bond has π character when the BN bond is double and triple. It is possible to distinguish two different behaviors for BN bonds, one when the functional group is bounded to the B atom, and the other to the N atom. When the functional group is bounded to the B atom, the BN bond is weaker and lengthier than that when the same group is bounded to the N atom. However, the isomer with weaker BN bond is the most stable one.
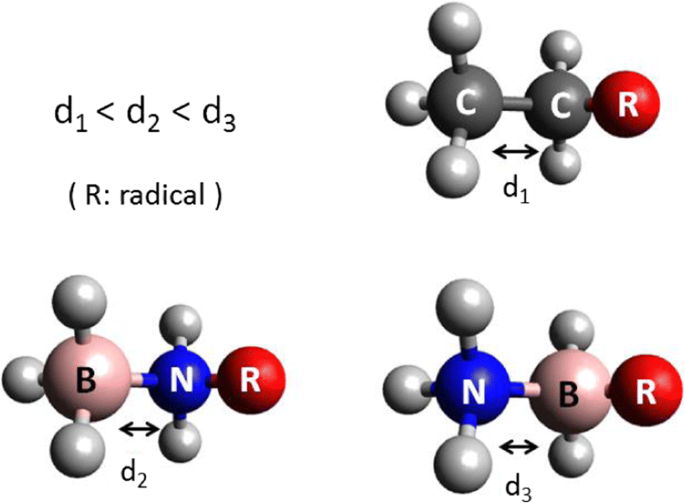
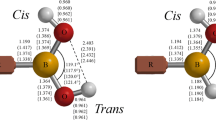
Comparative studies of dative bonds among substituted inorganic molecules, e.g., BN-ethanol, show important differences in terms of length and energy in comparison to organic analogous. There is also a difference when comparing BN or NB molecules (according to witch atom the functional group is bonded to, B or N); bond length, for example, is bigger for BN molecules.








Similar content being viewed by others
References
Frenking G (2015) Inorganic chemistry: peculiar boron startles again. Nature 522(7556):297–298. https://doi.org/10.1038/522297a
Borggaard OK, Gimsing AL (2008) Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: a review. Pest Manag Sci. 64(4):441–456. https://doi.org/10.1002/ps.1512
Haaland A (1989) Covalent versus dative bonds to main group metals, a useful distinction. Angew Chem Int Ed. Engl. 28(8):992–1007. https://doi.org/10.1002/anie.198909921
López-Castillo A (2012) Prediction of boron–phosphorous nanographene-like material. Int J Quantum Chem 112(19):3152–3157. https://doi.org/10.1002/qua.24096
Skancke A, Skancke PN (1996) Density functional theory and perturbation calculations on some Lewis acid−base complexes. A systematic study of substitution effects. J Phys Chem 100(37):15079–15082. https://doi.org/10.1021/jp9609444
Catao AJL, Lopez-Castillo A (2017) Stability and molecular properties of the boron-nitrogen alternating analogs of azulene and naphthalene: a computational study. J Mol Model 23(4):119. https://doi.org/10.1007/s00894-017-3279-y
Niedenzu K, Dawson JW (1959) Boron-nitrogen compounds. I. Syntheses of B-aminoborazines1. J Am Chem Soc 81(14):3561–3564. https://doi.org/10.1021/ja01523a019
Niedenzu K, Dawson JW (1959) Boron-nitrogen compounds. II.1,2 Aminoboranes, part 1: the preparation of organic substituted aminoboranes through a Grignard reaction. J Am Chem Soc 81(21):5553–5555. https://doi.org/10.1021/ja01530a010
Niedenzu K, Dawson JW (1960) Boron-nitrogen compounds. III.1,2 Aminoboranes, part 2: the B–N bond character in substituted aminoboranes. J Am Chem Soc 82(16):4223–4228. https://doi.org/10.1021/ja01501a028
Dahl GH, Schaeffer R (1961) Studies of boron-nitrogen compounds. IV. Isotopic exchange reactions of borazole. J Am Chem Soc 83(14):3034–3037. https://doi.org/10.1021/ja01475a015
Niedenzu K, Beyer H, Dawson JW (1962) Boron-nitrogen compounds. VI. Amino-phenylboranes. Inorg Chem 1(4):738–742. https://doi.org/10.1021/ic50004a004
Niedenzu K, Dawson JW (1965) Boron-nitrogen compounds. 1st edn. Springer-Verlag, Berlin-Heidelberg. https://doi.org/10.1007/978-3-642-85826-0
Schaefer A, Huber C, Ahlrichs R (1994) Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J Chem Phys 100:5829. https://doi.org/10.1063/1.467146
Ahlrichs R, Bar M, Haser M, Horn H, Kolmel C (1989) Electronic structure calculations on workstation computers: the program system Turbomole. Chem Phys Lett 162:165. https://doi.org/10.1016/0009-2614(89)85118-8
Becke AD (1993) Density-functional thermochemistry III the role of exact exchange. J Chem Phys 98:5648. https://doi.org/10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785. https://doi.org/10.1103/PhysRevB.37.785
Christiansen O, Koch H, Jørgensen P (1995) The second-order approximate coupled cluster singles and doubles model CC2. Chem Phys Lett 243:409. https://doi.org/10.1016/0009-2614(95)00841-Q
Hättig C, Weigend F (2000) CC2 excitation energy calculations on large molecules using the resolution of the identity approximation. J Chem Phys 113:5154. https://doi.org/10.1063/1.1290013
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297. https://doi.org/10.1039/B508541A
Pupim CF, Morgon NH, Lopez-Castillo A (2015) Spurious phosphorus pyramidalization induced by some DFT functionals. J Braz Chem Soc 26:1648–1655. https://doi.org/10.5935/0103-5053.20150138
Blanksby SJ, Ellison GB (2003) Bond dissociation energies of organic molecules. Acc Chem Res 36(4):255–263. https://doi.org/10.1021/ar020230d
Rowsell BD, Gillespie RJ, Heard GL (1999) Ligand close-packing and the Lewis acidity of BF3 and BCl3. Inorg Chem 38(21):4659–4662. https://doi.org/10.1021/ic990713m
Bessac F, Frenking G (2003) Why is BCl3 a stronger Lewis acid with respect to strong bases than BF3? Inorg Chem 42(24):7990–7994. https://doi.org/10.1021/ic034141o
Acknowledgements
This work was supported in part by the FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo), grants: 2010/11385-2; CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), grants: 131879/2014-6; and by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior). We also wish to thank professors Rose Maria Carlos, Nelson H. Morgon, and Jean Marcel R. Gallo for discussions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 220 kb)
Rights and permissions
About this article
Cite this article
Pupim, C.F., Catão, A.J.L. & López-Castillo, A. Boron–nitrogen dative bond. J Mol Model 24, 283 (2018). https://doi.org/10.1007/s00894-018-3810-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3810-9