Abstract
Background
Composting is one of the sustainable practices to convert sewage sludge into useful agricultural product because it is rich in organic matter, micro- and macronutrients, which is essential for plants growth and soil fauna to live. Therefore, the present study was to recycle the sewage sludge by rotary drum composter. Five sets of experiments (C/N 15, 20, 25, 30 including control) were carried out in the reactor, where sewage sludge was coupled with sawdust and cattle manure to attain the optimal feedstock. The process was monitored with respect to physico-chemical characteristics and stability parameters during 20 days of composting period. In addition, the kinetic parameters k m and r m have been evaluated in the present work.
Results
Higher reductions in CO2 evolution and oxygen uptake rate (OUR) observed in C/N 30 showed the stability, resulting in the total biodegradable ingredients to be stabilized. Similarly, higher percentage reduction in carbon-nitrogen ratio was observed in C/N 30 followed by C/N 20, C/N 25, C/N 15 and control experiments, respectively, indicates maximum degradation was achieved; as it corroborate higher temperature regime occurred in C/N 30. The composting parameters such as moisture content, volatile solids, CO2 evolution, OUR and C/N ratio were reduced significantly (p < 0.05). The kinetic model used illustrated the comparable variations in Michaelis constant (k m) and maximum rate constant (r m) under varying proportions of the initial feedstock. The overall assessment of kinetic data for C/N 30 experiment showed enhancement of degradation during the composting process.
Conclusions
The results suggested that composting of sewage sludge with optimal proportion of cattle manure and saw dust, especially in C/N 30, can produce stable compost within 20 days of composting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sewage sludge, a by-product generated in enormous quantity from various biological, chemical and physical treatment processes, may create environmental concerns in its disposal due to the presence of soil contaminants such as organic compounds, heavy metals and human pathogens (Singh and Agrawal 2008). The problem of efficient disposal and management of sewage sludge has become more rigorous owing to rapidly rising of population, economic growth and increasing numbers of treatment plants. Hence, it is necessary that we find approaches to effectively reuse the sewage sludge and reduce their impact on the environment. Composting is one of the most promising biological techniques to stabilize the organic matters contained in the sewage sludge (Doublet et al. 2010). During the composting process, pathogens may be killed due to the heat generated during the thermophilic phase and the organic fractions in sewage sludge will be converted into stabilized humic substances through mineralization and humification with a significant reduction in volume (Tuomela et al. 2000; Gouxue et al. 2001; Khwairakpam and Bhargava 2009). The reason is that compost made from sludge returns useful resources to the environment and also has a number of beneficial effects such as accelerating the plant growth, improving moisture retention capacity in the soil, increasing organic matter in the soil and improving erosion control (Liang et al. 2003; Cheng et al. 2007; Wang et al. 2014).
Rotary drum composting is an efficient and promising technology compared to conventional outdoor technologies such as windrows and static piles, as it provides adequate agitation, aeration and proper compost mixing to produce consistent and uniform end product without any nuisance odor or leachate-related problems (Kalamdhad et al. 2009; Rodríguez et al. 2012). Different types of wastes (cattle manure, swine manure, municipal biosolids, brewery sludge, chicken manure, animal mortalities and food residues) can be effectively composted in rotary drum reactors (Vuorinen and Saharinen 1997; Mohee and Mudhoo 2005; Aboulam et al. 2006; Kalamdhad and Kazmi 2008; Rodríguez et al. 2012). Until recently, reported data about the use of rotary drum in sewage sludge composting are rather scarce.
Many studies have reported that sewage sludge alone produces poor quality of compost due to its high moisture content and low air permeability. It is necessary to mix with other ingredients including bulking agents such as rice straw, sawdust, grass and leaves. These bulking agents are used to adjust moisture content and maintain inter-particle void dispersion, which provides adequate air and water exchange within the composting mass (Eftoda and McCartney 2004; Petric and Selimbasic 2008; Iqbal et al. 2010), and also provides optimal initial carbon-to-nitrogen (C/N) ratio to enhance the decomposition rate (Kalamdhad and Kazmi 2009). The fact that the application of immature and unstable compost may inhibit the growth of vegetables by competing for oxygen or causing phytotoxicity to plants, due to insufficient biodegradation of organic matter (Khwairakpam and Bhargava 2009). In addition, cattle manure having the tendency to bind the substrates together and also an important resource to supplement additional organic fraction, which improves the soil conditions. Furthermore, other composting factors such as temperature and nutritional requirements should also be controlled to attain good-quality compost (Kalamdhad and Kazmi 2009). Stability prevents nutrient from becoming tied up in rapid microbial growth, allowing them to be available for plant needs. In this regard, different techniques are widely used for measuring stability, based on physical (temperature, aeration demand, odor, color, etc.), chemical (organic matter loss, C/N ratio, chemical oxygen demand, etc.) and biological (respiration measured either as O2 consumption, CO2 production or heat generation, etc.) characteristics of composts (Wu et al. 2000; Brewer and Sullivan 2003; Matteson and Sullivan 2006; Gomez et al. 2006; Kalamdhad et al. 2008), but none has found universal acceptance. But, the respirometric indices (either oxygen uptake rate (OUR) or CO2 evolution) have been proven to be useful for the prediction of the stability of final compost products in the recent years (Barrena et al. 2011; Varma et al. 2014). Most of the literatures reported on compost stability are generally dealt with traditional composting techniques such as windrows and static pile types for various kinds of wastes. The study of stability analysis in rotary drum composter for sewage sludge in combination with optimal mixtures of cattle manure and saw dust is rarely present. Hence, it is essential to check the stability in the final compost which correlates with the degree of decomposition.
Besides the above facts, it is observed that most published information on the composting process is qualitative. Very few studies have been performed based on quantitative knowledge to design the operating conditions of the composting process, but they possessed less systematic. From the engineering point of view, kinetic study is essential to describe the controlling factors in the optimization of the process (Whang and Meenaghan 1980; Agamuthu et al. 2000; Bari et al. 2000). Keeping issues in mind, the scope of the present study was to evaluate the effect of process variables such as temperature, moisture content, ash content, volatile solids, total nitrogen, CO2 evolution, OUR and initial C/N ratio, during 20 days of composting period using pilot-scale rotary drum reactor. In addition, the dynamics of the composting process were also examined by simulating the results of organic matter degradation on the Monod equation, to determine the kinetic parameters like Michaelis–Menten constant k m and maximum velocity constant r m.
Materials and methods
Feedstock materials and its preparation
Undigested sewage sludge having high percentage of organic matter was procured from the sewage treatment plant of the IIT Guwahati campus. Fresh cattle manure and sawdust were obtained and/or purchased from nearby Amingaon village and saw mill, respectively. The initial characteristics of waste are detailed in Table 1. Prior to composting, sewage sludge (C/N = 11.2), cattle manure (C/N = 26.4) and sawdust (C/N = 135.9) were mixed with five different proportions (C/N = 15, 20, 25, 30 and control), as compiled in Table 2. To prepare the initial feedstock, C/N ratio of the compost mixture was calculated as given in Eqs. (1), 2 and (3) (CWMI 1996).
where Q n nis the mass of material n (wet weight basis); G the moisture goal (%); M n the moisture content (%) of material n.
where R is the C/N ratio of compost mixture; Q n the mass of material n (wet weight basis); C n the carbon (%) of material n; N n the nitrogen (%) of material n; M n the moisture content (%) of material n.
By simplifying and rearranging the above equation, the mass of the third material required would be Eq. (3):
Rotary drum composter design
Figure 1 shows the schematic diagram of a pilot-scale rotary drum composter of 550 L capacity operated by batch-mode operation. The composter is made up of 1.22 m in length and 0.76 m in diameter, fabricated by a 4-mm-thick metal sheet. The inner side of the drum is covered with anti-corrosive coating. The drum is mounted on four rubber rollers attached to a metal stand and is rotated manually with its handle. To ensure appropriate mixing and agitation of the wastes during rotation, 40 × 40 mm angles are welded longitudinally inside the drum, resulting in tumbling action inside the drum generally moves material through the drum for 20 days. In addition, two adjacent holes of 10 cm each are made on top of the drum to drain out the excess water. Rotary drum composter contained approximately 150 kg of different waste combinations and was manually turned on every 24 h through one complete rotation, to ensure that the material on the top portion moved to the central portion where it was subjected to higher temperature. Aerobic condition was maintained by opening the top half side doors of the two circular faces.
Sampling and parameter analysis
100 g of samples was collected through grab sampling from different locations, mainly from middle portion and terminal ends of the composter without disturbance of the adjacent materials. All the grab samples were thoroughly mixed together to make a homogenized sample. Triplicate homogenized samples were collected periodically and stored at 4 °C for stability analysis of the wet samples within 2 day. The sub-samples were air dried immediately, and pulverized to pass through 0.2 mm sieve and stored for physico-chemical analysis.
Temperature was monitored every 6 h using a digital thermometer throughout the composting period. Each air dried and grounded sample was analyzed for the following parameters: moisture content (105 °C for 24 h) and volatile solids/organic matter (loss ignition at 550 °C for 2 h). The total organic carbon (TOC) was calculated as follows in Eqs. (4), (5) and (6) (Mohee et al. 2008).
The percentage of nitrogen content was calculated using Kjeldahl method following Eq. (7) (Codell and Verderame 1954).
where R is the volume of H2SO4 to titrate boric acid (ml); S the volume of H2SO4 to titrate blank (ml), W the weight of sample (g); and N the normality of H2SO4.
Finally, the carbon-to-nitrogen ratio was determined by dividing the total organic carbon content to the total nitrogen content.
OUR was performed according to the method described by Kalamdhad et al. (2008). A liquid suspension of compost (8 g of compost in 500 mL of distilled water added with CaCl2, MgSO4, FeCl3 and phosphate buffer at pH 7.2) incubated at room temperature (24 ± 2 °C) was placed in the sample bottle. A DO sensor (Eutech instruments, ECD11001K) was placed in the sample bottle at a depth of 5–7 cm below the water surface. The suspension was continuously stirred by means of a magnetic stirrer. Then, O2 concentration (expressed in mg/L) was recorded every 30 s for approximately 15–20 min. Finally, OUR was calculated in terms of VS as mg O2/g VS/day.
CO2 evolution rate was measured using static measurement method (Knoepp and Vose 2002; Kalamdhad et al. 2008). Approximately, 10 g of sample was sealed in a 0.5 L vessel along with a beaker containing a known weight of oven-dried soda lime (105 °C, 1.5–2.0 sieve mesh) to trap CO2. The samples were incubated at room temperature (24 ± 2 °C). Blank experiment was performed without putting the sample in a vessel, needs for initial CO2 calculation. The soda lime traps were removed after 24 h, oven dried and reweighed to determine CO2 absorbed. The CO2 and OUR values were used to determine the Solvita maturity index on a scale of 1–8, which represents the maturity level of the compost samples.
All the results reported are the means of three replicates. The results were statistically analyzed at 0.05 levels using two-way analysis of variance (ANOVA) and Tukey’s HSD test was used as a post hoc analysis to compare the means (STATISTICA package).
Results and discussion
Physico-chemical characteristics
Temperature profiles for the four C/N ratios (15, 20, 25 and 30) including control are shown in Fig. 2. It justifies the optimal conditions at which the biological process proceeds and also plays an important role in evolution and succession of the microbiological communities (Hassen et al. 2001). Due to early decomposition of the soluble, readily degradable compounds (especially in C/N 30), the substrate passed from an initial mesophilic phase (<40 °C) to a thermophilic stage on 2nd day of the composting period caused by metabolic heat, indicating quick establishment of microbial activities (Nolan et al. 2011). Elevated temperatures (>50 °C) were maintained in the reactor for 12–18 h which is sufficient time for the sanitation of the substrate from potential pathogenic microorganisms that exists in sewage sludge (Chroni et al. 2009). Thereafter, the amount of degradable organic matter in the reactor decreased over the course of the composting process until the maturation process takes place. The fact that heat generation decreased leads to reduction of the temperature in the composter. In addition, lower temperature profiles observed in control, C/N 15 and 20 experiments compared to others may be due to higher amount of sewage sludge which reduces porosity between substrates, indicates that sewage sludge without appropriate amendments is less favorable for growth and biological activity of microorganisms (Nakasaki et al. 1987). All of the experiments accomplished ambient temperature at the end of 20 day show the reflective image of stability. Similar results have been observed by Yañez et al. (2009), where maximum temperature was recorded as 62 °C.
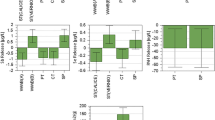
Moisture loss during the composting process can be examined as an index of decomposition rate, since the heat generation which accompanies decomposition drives vaporization (Liao et al. 1996). From the results obtained (Fig. 3), it is seen that the C/N 15 achieved higher initial moisture content (70.91 %) due to huge quantity of sewage sludge, which is further dropped to 51.01 % resulting in only 28.06 % moisture loss, whereas in C/N 30, moisture content at the beginning was 62.30 % which is reduced to 43.23 % within 20 days. On the other hand, moisture contents were reduced from 68.74, 64.93 and 68.10 % to 48.80, 47.18 and 55.16 % for C/N 20, 25 and control experiments, respectively. Higher moisture loss was observed in C/N 30 (30.61 %) followed by others, which is directly correlated with its evaporation rate of moisture leading to large heat generation. Leachate formation was not observed during the composting period. The reduction in the percentage of moisture content was significantly varied in all the experiments (p < 0.05).
The contents of TOC decreased with composting period especially in thermophilic phase, leading to higher microbial activity. The variation of organic carbon during composting is shown in Fig. 4. Thereafter, the degradation rate of OM was reduced gradually. TOC loss during composting is mainly attributed by mineralization of organic matter resulting in the evolution of CO2 and heat (Hassouneh et al. 1999). In addition, a large fraction (~30 %) of organic carbon was lost as utilized by microorganisms in the form of energy source (Kalamdhad and Kazmi 2008). Higher TOC reduction was observed in C/N 30 (39.97 %), followed by C/N 25 (37.08 %), C/N 20 (35.37 %), C/N 15 (33.81 %) and control (25.62 %), due to presence of readily degradable compounds as decomposed by microbes resulting in higher temperature regime in C/N 30. Significant variations in TOC were observed between all the reactors (p < 0.05).
Biodegradability was calculated from the initial and final organic matter contents, according to the following equation (Haug 1993; Külcu and Yaldiz 2004):
where OM i is the organic matter content at the beginning of the process and OM f is the organic matter content at the end of the process. The degradability coefficient (K b) values for all the reactors are shown in Table 5. Higher values of biodegradability were observed in C/N 30 (K b = 0.5887) and 25 (0.5478). As the added sewage sludge increases, lower values of biodegradability were observed in C/N 15 (0.4972) and C/N 20 (0.5199) including control (0.3841).
Figure 5 shows that total nitrogen was higher in final composts compared to initial substrate, as increased from 1.54 to 1.91 %, 1.20–1.69 %, 1.12–1.52 %, 1.05–1.60 % and 1.69–2.06 in C/N 15, 20, 25, 30 and control, respectively. Higher percentage in nitrogen content was observed in C/N 30 (34.38 %) followed by C/N 20 (28.99 %), C/N 25 (26.32 %), C/N 15 (19.37 %) and control (17.96 %) during the composting period. This enhancement was occurred due to the net loss of dry mass in terms of CO2 as well as water loss by evaporation during mineralization of OM by microorganisms (Yamada and Kawase 2006; Kalamdhad and Kazmi 2009). Alternatively, nitrogen fixing bacteria might also contribute to increase in total nitrogen during later phase of composting (Zucconi et al. 1985). ANOVA analysis showed significant changes in nitrogen content among the experiments (p < 0.05).
Stability analysis
Compost stability has been evaluated by CO2 evolution because it measures carbon derived directly from the compost being tested, caused by mineralization of the organic matter (Bernai et al. 1998; Kalamdhad et al. 2008). The CO2 evolution rates of the C/N 15, 20, 25, 30 and control decreased from initial values of 9.94, 10.03, 9.40, 9.54 and 10.46–2.12, 1.41, 1.35, 0.51 and 3.60 mg CO2/g VS/day, respectively (Table 3). The variation of CO2 evolution among the reactors was statistically significant (p < 0.05). Results suggested that higher reduction in CO2 evolution rate was observed in C/N 30 (94.7 %) followed by C/N 20 (85.9 %), C/N 25 (85.6 %), C/N 15 (78.7 %) and control (65.6 %). The reduction in CO2 evolution rate was very low after 16 days of composting in all the experiments, indicating the stability of finished compost. The reason is CO2 was mainly emitted during the thermophilic period because of the degradation of easily degradable carbon under vigorous bacterial and fungal activity. During the curing period, CO2 emissions are related to the degradation of complex organic molecules such as lignin and lignocelluloses by some fungi and actinomycetes. The Solvita® maturation index based on CO2 evolution increased from 3 to 8 for all the experiments. Solvita® results proved that the composts from all the reactors enter into the well stable condition after 20 days. Similarly, Wang et al. (2004) suggested that the composts become more stable when the respiration rates were below 1 mg CO2/g VS/day.
As composting begins, large organic molecules are broken down to smaller soluble structures and temporarily more substrate may become available. Furthermore, the microbial activity reduces with composting period as evidenced by the decrease in the values of OURs of all the experiments. It is observed that, a gradual decrease in OUR values from 19.98 to 3.96, 17.37–3.66, 13.12–1.77, 16.09–0.81 and 17.86–4.39 mg O2/g VS/day in C/N 15, C/N 20, C/N 25, C/N 30 and control, respectively, after 20 days of composting period in the rotary drum. OUR values attained stability after the initial reduction in all the experiments, while after the 14th day of composting the drop was moderate indicating the compost approaches the maturation period. Higher reduction observed in C/N 30 (94.9 %) followed by C/N 25 (86.5 %), C/N 15 (80.2 %), C/N 20 (78.9 %) and control (75.4 %), respectively (Table 3), might be due to optimal amount of cattle manure and sawdust which provided a favorable condition for the growth and biological activity of microorganisms (Gomez et al. 2006). The percentage reduction in OUR value was significantly varied among the reactors (p < 0.05). Results indicated that the composts produced from C/N 25 and 30 experiments are considered to be very stable and possessed a Solvita® index value of 8, which indicated that compost was ready for usage of soil conditioner (Cabanas-Vargas et al. 2005). In addition, C/N 15, 20 and control experiments have a Solvita® maturity index of 6 which can be considered as immature and unstable compost.
The changes in the C/N ratios reflect the organic matter decomposition and stabilization achieved during composting. The microorganisms which decomposed the waste mixture require a supply of nutrients, mainly carbon as a source of energy and nitrogen for building cell structures; however, these nutrients should be present in optimal amount to perform the composting process efficiently (Kalamdhad and Kazmi 2009). Moreover, if the C/N ratio of compost is more, the excess carbon tends to utilize nitrogen in the soil to build cell protoplasm resulting in loss of nitrogen which is known as robbing of nitrogen in the soil. While, if the C/N ratio is too low, the resultant product does not help improve the structure of the soil. In this study, the C/N ratio decreased rapidly from an initial value of 17.43–9.30, 23.02–10.52, 25.60–11.85, 28.33–11.19 and 14.04–8.53 in C/N 15, 20, 25, 30 and control, respectively, after 20 days of composting (Table 3). Hirai et al. (1983) and CPHEEO (2000) have reported that C/N ratio less than or equal to 20 is considered as a satisfactory value for maturity when the initial value is in between 25 and 30. Results suggested that higher reduction was observed in C/N 30 (60.5 %) followed by C/N 20 (54.3 %), C/N 25 (53.7 %), C/N 15 (46.6 %) and control (39.2 %) experiment, which led to rapid decrease in organic carbon due to the oxidation of the readily available organic matter. C/N ratio was reduced significantly (p < 0.05) in all experiments during the composting process.
Kinetic analysis
Composting is a biochemical process where enzyme kinetics concept could applicable with some theoretical considerations. In this regard, the enzyme kinetics approach is carried out by considering following assumptions: (1) each microbe in the rotary drum is uniformly dispersed in a solution of soluble organic matter to maintain homogeneity, (2) under controlled aerobic conditions, the microbes are amended with organic matters and the moisture required for microbial growth is optimized with respect to readily available organic carbon, (3) These biocomplex systems can be analyzed by Monod’s kinetics to describe its process. Various researchers have illustrated the comprehensive models for kinetic degradation (Finger et al. 1976; Whang and Meenaghan 1980; Haug 1993; Agamuthu et al. 2000; Kulcu and Yaldiz 2004; Yamada and Kawase 2006; Mason 2006). Among those models, the incorporation of organic matter or volatile solids’ decomposition to predict the kinetic degradation becomes a successful and easy way for sewage sludge composting (Whang and Meenaghan 1980). In this sense, the composting of organic ingredients or decomposition of organic matter is regarded as enzymatic-related microbe system (Haug 1993; Agamuthu et al. 2000; Bari et al. 2000; Nelson and Cox 2000; Kumar et al. 2009).
In enzyme kinetics concept, enzyme concentration forms an intermediate complex with the organic matter concentration leading to complex formation represented as EC. The decomposition of EC is carried out in two ways as given in Eq. (9):
where E is the microbial concentration (%); C is the limiting organic matter concentration (%); P is the by-product generated by endogenous reaction (%); k 1 , k 2 , k 3 are the specific reaction rates.
In equilibrium conditions,
By solving, EC is given by Eq. (10)
where E T is the total microbial concentration (%). On solving, we get
where r m = k 2 [E T], represented as maximum rate of enzymatic reaction (day−1).
Equation (12) is the projected kinetic rate equation for the composting process in accordance with Michaelis–Menten equation. To determine kinetic parameters, Eq. (12) is re-transformed into Lineweaver–Burk plot as follows:
The kinetic parameters k m and r m can be graphically determined from the slope and intercept of a Lineweaver–Burk plot (Whang and Meenaghan 1980) using Eq. (13) incorporating 1/r and 1/C data. It correlates the initial rate of reaction (r) and the organic matter concentration (C) results into a linear relationship. In the Michaelis–Menten equation, the value of k m (i.e., dissociation constant) is inversely proportional to the chemical affinity of the enzyme for the utilization of organic matter. The value of r m (i.e., maximum or limiting velocity) gives the extent of reaction rate which directly correlated with the operational parameters of the process such as temperature, moisture, aeration and chemical conditions. Higher the value of r m, the faster is the rate of degradation of organic matter.
The organic matter was evaluated over the 20 days of composting period (Fig. 6) and the consumption rate of organic matter due to microbial activities (i.e., r) can be obtained on each sampling day by plotting the tangent of the curve organic matter (on ash basis) vs. time, as shown in Table 4. The reciprocals of reaction rate (consumption rate, i.e., 1/r) and organic matter content (1/C) were compiled by plotting a Lineweaver–Burk plot (1/r vs. 1/C), to determine the kinetic constants k m and r m for all sets of experiments as summarized in Table 5. It is observed that C/N 30 shows the least k m value (0.4482) compared to other reactors, which reveals that 100 % ingredients act as feedstock to be satisfied for the enzyme–substrate affinity. Subsequently, C/N 30 yielded highest r m value (114.9425) concludes the maximum rate of degradation which might be due to regular aeration and optimal nutrients balance occurred. Results showed that the reduction in temperature was constant after maintaining the peak value may caused due to lack of biodegradable organic matter, results the microbial deactivation. It is seen that C/N 30 showed higher R 2 value as compared to other experiments, which is well suited for the Lineweaver–Burk plot (Fig. 7).
While highest k m value (98.5349) was observed in C/N 25 compared to other reactors, showed that the microorganisms does not decomposed the organic matter efficiently, might be due to insufficient oxygen as the air could not penetrate inside the waste mixture. It also correlated with lesser degradability coefficient (K b = 0.5478) and limiting constant (r m = 1.1609) values observed in C/N 25. The comparative analysis between C/N 15 (k m = 68.1105), C/N 20 (k m = 80.1805) and control (k m = 62.4675) experiments revealed that cattle manure which contains readily available biodegradable materials, when coupled with sewage sludge and sawdust in the desired proportions; it does not satisfied the affinity of the compost mixture for microbial activities, and was further as evidenced with lesser r m values (C/N 15: 0.5214; C/N 20: 0.8297; control: 0.3330). As a result, these waste proportions do not suit well for the degradation process due to lesser availability of the carbon source as compared to C/N 25 and 30. It also might be due to insufficient aeration which resembles lesser r m values observed in C/N 15 (0.5214), C/N 20 (0.8297) and control (0.3330) experiments. The results concluded that higher degradation was achieved in C/N 30 as compared to other experiments, due to their optimal nutrient balance for the excellent microbial activities, which further correlated with its higher temperature regime.
Conclusions
Stability studies carried out in rotary drum composter for various C/N ratios reveal that C/N 30 produced more stable compost as compared to others after 20 days, implying that rigorous decomposition was occurred. Higher percentage reductions in CO2 evolution (94.7 %), OUR (94.9 %) and C/N ratio (60.5 %) were observed in C/N 30 experiment, which showed that total biodegradable ingredients are stabilized. In addition, the degradation became decreased with increasing the amount of sewage sludge, caused by insufficient free spaces between the substrate mass for feasible microbial activities. Moreover, the decomposition process was analyzed by Monod kinetics which resembles Michaelis–Menten equation, and the following results (i.e., k m = 0.4482 and r m = 114.9425) suggested that the optimal nutrient balance has been occurred in C/N 30 experiment. Lower k m value concluded that 100 % ingredients act as feeding material was to be satisfied for the enzyme–substrate affinity. Hence, it is concluded that the rotary drum composting of sewage sludge with initial C/N ratio of 30 can produce mature and stable compost after 20 days of the composting period.
References
Aboulam S, Morvan B, Revel JC (2006) Use of a rotating-drum pilot plant to model the composting of household waste on an industrial scale. Compost Sci Util 14(3):184–190
Agamuthu P, Choong LC, Hasan S, Praven VV (2000) Kinetic evaluation of composting of agricultural wastes. Environ Technol 21(2):185–192
Bari QH, Koenig A, Guihe T (2000) Kinetic analysis of forced aeration composting. I. Reaction rates and temperature. Waste Manag Res 18:303–312
Barrena R, Turet J, Busquets A, Farrés M, Font X, Sánchez A (2011) Respirometric screening of several types of manure and mixtures intended for composting. Bioresour Technol 102(2):1367–1377
Bernai MP, Paredes C, Sanchez-Monedero MA, Cegarra J (1998) Maturity and stability parameters of composts prepared with a wide range of organic wastes. Bioresour Technol 63(1):91–99
Brewer LJ, Sullivan DM (2003) Maturity and stability evolution of composted yard trimmings. Compost Sci Util 11(2):96–112
Cabanas-Vargas DD, Sanchez-Monedero MA, Urpilainen ST, Kamilaki A, Stentiford EI (2005) Assessing the stability and maturity of compost at large-scale plants. Ingenieria 9(2):25–30
Cheng H, Xu W, Liu J, Zhao Q, He Y, Chen G (2007) Application of composted sewage sludge (CSS) as a soil amendment for turfgrass growth. Ecol Eng 29(1):96–104
Chroni C, Kyriacou A, Manios T, Lasaridi KE (2009) Investigation of the microbial community structure and activity as indicators of compost stability and composting process evolution. Bioresour Technol 100(15):3745–3750
Codell M, Verderame FD (1954) The determination of nitrogen in copper–titanium alloys. Anal Chim Acta 11(1):40–47
CPHEEO (2000) Manual on municipal solid waste management. Central public health and environmental engineering organization, New Delhi
CWMI (Cornell Waste Management Institute) (1996) Calculate C/N ratio for three materials. Cornell Waste Management Institute, Ithaca
Doublet J, Francou C, Poitrenaud M, Houot S (2010) Sewage sludge composting: influence of initial mixtures on organic matter evolution and N availability in the final composts. Waste Manag 30(10):1922–1930
Eftoda G, McCartney D (2004) Determining the critical bulking agent requirement for municipal biosolids composting. Compost Sci Util 12(3):208–218
Finger SM, Hatch RT, Regan TM (1976) Aerobic microbial growth in semisolid matrices: heat and mass transfer limitation. Biotechnol Bioeng 18:1193–1218
Gomez RB, Lima FV, Ferrer AS (2006) The use of respiration indices in the composting process: a review. Waste Manag Res 24(1):37–47
Gouxue L, Zhang F, Sun Y, Wong JWC, Fang M (2001) Chemical evaluation of sewage sludge composting as a mature indicator for composting process. Water Air Soil Pollut 132:333–345
Hassen A, Belguith K, Jedidi N, Cherif A, Cherif M, Boudabous A (2001) Microbial characterization during composting of municipal solid waste. Bioresour Technol 80(3):217–225
Hassouneh O, Jamrah A, Qaisi K (1999) Sludge stabilization by composting: a Jordanian case study. Bioprocess Eng 20:413–421
Haug RT (1993) The practical handbook of compost engineering. Lewis Publishers, Boca Raton
Hirai MF, Chanyasak V, Kubota H (1983) A standard method for measurement of compost maturity. Bio Cycles 24:54–56
Iqbal MK, Shafiq T, Ahmed K (2010) Characterization of bulking agents and its effects on physical properties of compost. Bioresour Technol 101(6):1913–1919
Kalamdhad AS, Kazmi AA (2008) Mixed organic waste composting using rotary drum composter. Int J Environ Waste Manag 2(1–2):24–36
Kalamdhad AS, Kazmi AA (2009) Effects of C/N ratio on mixed organic waste composting in a rotary drum composter. Int J Environ Eng 1(2):187–207
Kalamdhad AS, Pasha M, Kazmi AA (2008) Stability evaluation of compost by respiration techniques in a rotary drum composter. Resour Conserv Recycl 52(5):829–834
Kalamdhad AS, Singh YK, Ali M, Khwairakpam M, Kazmi AA (2009) Rotary drum composting of vegetable waste and tree leaves. Bioresour Technol 100(24):6442–6450
Khwairakpam M, Bhargava R (2009) Vermitechnology for sewage sludge recycling. J Hazard Mater 161(2–3):948–954
Knoepp JD, Vose JM (2002) Quantitative comparison of in situ soil CO2 flux measurement method, USDA Forest Service, Southern Research Station, Research Paper SRS-28
Kulcu R, Yaldiz O (2004) Determination of aeration rate and kinetics of composting some agricultural wastes. Bioresour Technol 93(1):49–57
Kumar S, Sakhale A, Mukherjee S (2009) Simplified kinetic analysis for composting of municipal solid waste. Pract Period Hazard Toxic Radioact Waste Manag 13(3):179–186
Liang C, Das K, McClendon C (2003) The influence of temperature and moisture contents regimes on the aerobic microbial activity of a biosolids composting blend. Bioresour Technol 86:131–137
Liao PH, Jones L, Lau AK, Walkemeyer S, Egan B, Holbek N (1996) Composting of fish wastes in a full-scale in-vessel system. Bioresour Technol 59:163–168
Mason IG (2006) Mathematical modelling of the composting process: a review. Waste Manage 26(1):3–21
Matteson TL, Sullivan DM (2006) Stability evolution of mixed food waste composts. Compost Sci Util 14(3):170–177
Mohee R, Mudhoo A (2005) Analysis of the physical properties of an in-vessel composting matrix. Powder Technol 155(1):92–99
Mohee R, Driver MF, Sobratee N (2008) Transformation of spent broiler litter from exogenous matter to compost in a sub-tropical context. Bioresour Technol 99(1):128–136
Nakasaki K, Nakano Y, Akiyama T, Shoda M, Kubota H (1987) Oxygen diffusion and microbial activity in the composting of dehydrated sewage sludge cakes. J Ferment Technol 65(1):43–48
Nelson DL, Cox MM (2000) Lehninger principles of biochemistry, 3rd edn. Worth Publishers, New York
Nolan T, Troy SM, Healy MG, Kwapinski W, Leahy JJ, Lawlor PG (2011) Charaterization of compost produced from separated pig manure and a variety of bulking agents at low initial C/N ratios. Bioresour Technol 102(14):7131–7138
Petric I, Selimbasic V (2008) Composting of poultry manure and wheat straw in a closed reactor: optimum mixture ratio and evolution of parameters. Biodegradation 19(1):53–63
Rodríguez L, Cerrillo MI, García-Albiach V, Villaseñor J (2012) Domestic sewage sludge composting in a rotary drum reactor: optimizing the thermophilic stage. J Environ Manag 112:284–291
Singh RP, Agrawal M (2008) Potential benefits and risks of land application of sewage sludge. Waste Manag 28(2):347–358
Tuomela M, Vikman M, Hatakka A, Itavaara M (2000) Biodegradation of lignin in a compost environment: a review. Bioresour Technol 72(2):169–183
Varma VS, Chatuphale M, Kalamdhad A (2014) Effects of bulking agent in composting of vegetable waste and leachate control using rotary drum composter. Sustain Environ Res 24(4):245–256
Vuorinen AH, Saharinen MH (1997) Evolution of microbiological and chemical parameters during manure and straw co-composting in a drum composting system. Agr Ecosyst Environ 66(1):19–29
Wang P, Changa CM, Watson ME, Dick WA, Chen Y, Hoitink HAJ (2004) Maturity indices for composted dairy and pig manures. Soil Biol Biochem 36:767–776
Wang K, Li X, He C, Chen C, Bai J, Ren N, Wang J (2014) Transformation of dissolved organic matters in swine, cow and chicken manures during composting. Bioresour Technol 168:222–228
Whang DS, Meenaghan GF (1980) Kinetic model of composting process. Compost Sci 21(3):44–46
Wu L, Ma LQ, Martinez GA (2000) Comparison of methods for evaluating stability and maturity of biosolids compost. J Environ Qual 29:424–429
Yamada Y, Kawase Y (2006) Aerobic composting of waste activated sludge: kinetic analysis for microbiological reaction and oxygen consumption. Waste Manag 26(1):49–61
Yañez R, Alonso JL, Díaz MJ (2009) Influence of bulking agent on sewage sludge composting process. Bioresour Technol 100:5827–5833
Zucconi F, Monaco A, Forte M (1985) Phytotoxins during the stabilization of organic matter. In: Grasser JKR (ed) Composting of agricultural and other wastes. Elsevier, New York, pp 73–88
Acknowledgments
The authors are thankful to Indian Institute of Technology, Guwahati, India for the financial support to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nayak, A.K., Kalamdhad, A.S. Sewage sludge composting in a rotary drum reactor: stability and kinetic analysis. Int J Recycl Org Waste Agricult 4, 249–259 (2015). https://doi.org/10.1007/s40093-015-0104-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-015-0104-4