Abstract
Carcinoscorpius rotundicauda is one of the four extant species of horseshoe crab, occurring only in Asia: around India, Indonesia, Malaysia, Philippines, Singapore, Thailand and Hong Kong. Virtually nothing is known about the physiology of this Asian species. In the present study, the respiratory physiology in terms of oxygen consumption rate of the trilobite larvae of C. rotundicauda was evaluated under laboratory conditions. The trilobite larvae were exposed to different levels of temperature (10, 20, 30 and 40 °C), salinity (10, 20, 30 and 40 ppt), pH (5, 6, 7, 8 and 9) and dark–light conditions for a period of 12 h. Effect of temperature on oxygen consumption in trilobite larvae was not so significant among all the temperatures tested. However, both 10 and 40 °C temperature showed comparatively high rate of oxygen consumption at the initial 4 h of experiment. More or less consistent oxygen consumption pattern observed at 20 and 30 °C temperature suggested 20–30 °C as the best temperature range for rearing trilobite larvae. Trilobite larvae revealed enormous tolerance to temperature and indicated that the temperature is not critical in terms of respiration. The trend in oxygen consumption was more or less uniform at all salinities tested indicating the insignificant influence of salinity on respiration. Though not significant, the oxygen consumption trend was more consistent at 20 ppt as compared to other salinities and thus could be considered as the most suitable salinity for rearing trilobite larvae. There was a positive relationship between oxygen consumption rate and the various seawater water pH tested with trilobite larvae. Maximum oxygen consumption was recorded between pH 7 and 9 at the initial hours of the experiment and the overall trend suggests that the larvae preferred slightly acidic conditions. Although the larvae showed higher oxygen consumption under light, in the overall analysis of the data the light–dark condition did not have a statistically significant influence. However, more consistent oxygen consumption pattern and swimming activity in the dark condition as compared to light condition during the 12 h of experiment revealed that the larvae have active nocturnal behaviour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Over the past century, the degradation of habitat, especially the loss of spawning and nursery grounds, marine pollution, human exploitation for food and bio-medical production, has led to a decline in population of horseshoe crabs in many parts of the world (Widener and Barlow 1999; Shin et al. 2009; Mishra and Mishra 2011). Recently, all four species of horseshoe crabs (Limulus polyphemus, Carcinoscorpius rotundicauda, Tachypleus gigas, Tachypleus tridentatus) have been listed on the IUCN red list and among them three are categorized as data deficient (IUCN 2010). Although there is a lot of anecdotal knowledge on the C. rotundicauda, only a little scientific work has been published. Very recently this species has been listed as vulnerable under Singapore Red Data book (Davidson et al. 2008).
The horseshoe crab, C. rotundicauda is found in the inshore waters of the Indo-West Pacific region (Shuster 1990). This is the smallest horseshoe crab species (Brockman and Smith 2009; Zaleha et al. 2011) and the only species, which visits the extreme physiochemically challenging habitats like mangrove for their spawning (Cartwright-Taylor et al. 2009). Therefore, movement and spawning patterns of C. rotundicauda may be very different from those of other species in higher latitudes and different habitats (Cartwright-Taylor et al. 2012). Adult C. rotundicauda migrates from the offshore continental shelf to the inter-tidal mudflat areas for spawning during the full and new moon phases (Sekiguchi 1988). The fertilization in horseshoe crab is external and is accomplished when the amplexed males, along with or without any satellite males, releases sperm as the female extrudes her eggs (Brockmann 2003). Fecundity of C. rotundicauda ranges from 4,217 to 10,982 with an average of 7,438 and females deposit their eggs in clutches at 10–20 cm deep mostly within the upper parts of the mangroves (Chatterji and Parulerkar 1992). These buried eggs remain in their breeding ground for a period of 5 to 6 weeks before they hatch out as swimming “trilobite” (first instar) larvae.
Hatching phenomenon in horseshoe crab is suggested to be under endogenous control (Rudloe 1979) and many other environmental and ecological factors also have an influence over the whole event during its egg development (Ehlinger et al. 2003, Jackson et al. 2005; Ehlinger and Tankersley 2006; Hu et al. 2009). The newly hatched larvae do not need to feed as they can subsist on yolk of the embryo for several months (Botton et al. 1992) and only at the 2nd instars they start active feeding (Sekiguchi 1988). Larval and juvenile stages of this species live near the breeding grounds in the silty mudflat of the mangroves or near the shores, while the juveniles live offshore (Itow et al. 2004; Cartwright-Taylor and Hsu 2012). Trilobite larvae of other horseshoe crab species also have a strong tendency to remain close to the beach (100–200 m offshore) and they are mostly nocturnally active with a planktonic life (Botton and Loveland 2003). Compared to the available information regarding the food preference in the larval stages of horseshoe crab species, only very limited information so far prevails for C. rotundicauda. Accordingly in the wild, C. rotundicauda scavenge on small fish, oligochaetes, small crabs and thin-shelled bivalves (Zhou and Morton 2004). Trilobite larvae have the capability of altering their swimming period and can remain quiescent during daylight by settling or burrowing into sediments even while the current is moving (Botton and Loveland 2003).
Although early life stages of horseshoe crabs resemble that of their adult, the early developmental stage is morphologically more specialized and unlike than all other subsequent stages (Rudloe 1979). There is a paucity of information regarding the ecology of horseshoe crab larvae in their habitat (Botton and Loveland 2003). The early life stages of horseshoe crabs (egg/larvae/juveniles) are encountered with extreme physiological stresses associated with diurnal, tidal fluctuations, marine pollution and anthropogenic activities (Shuster 1982; Walls et al. 2002; Srijaya et al. 2010a, b). However, horseshoe crab life stages are known to be relatively tolerant to unpredictable osmotic and temperature stresses (Sugita 1988; Shuster and Sekiguchi 2003; Ehlinger and Tankersley 2004; Botton et al. 2006; Greene et al. 2011), and contaminants such as heavy metals (Botton et al. 1998a, b; Botton 2000). This ability of horseshoe crabs to tolerate a wide range of environmental conditions may explain their persistence over geological time (Anderson and Shuster 2003; Towle and Henry 2003). To prevent this unique species from global extinction proper conservation or management measures have to be taken. For such effort, knowledge of the biology and behaviour of the horseshoe crab is essential. It is rather critical to note that once a horseshoe crab population is extirpated locally, re-establishment takes a long time, even if the habitat is restored (Cartwright-Taylor and Hsu 2012). Considering their declining population and the increasing applications in biomedical area have prompted an increasing interest in rearing and breeding of horseshoe crabs under controlled conditions (Carmichael and Brush 2012), which again brings out the importance on the knowledge of their optimal environmental conditions. Aquaculture of horseshoe crabs could eventually serve as an alternative approach to augment and restore the over-exploited population in the wild, while continuing to support other useful activities (Smith et al. 2011; Carmichael and Brush 2012).
Knowledge of the oxygen consumption of an organism has significant importance in aquaculture, since it gives a good indication of the overall metabolic state of an animal and also gives a sensitive measure of daily expenditure of energy in an organism to maintain their vital functions through an aerobic metabolism (Salvato et al. 2001; Daoud et al. 2007). Thus a change in the oxygen consumption is taken as a valuable indicator for metabolic stress. Moreover, this parameter is the most used tool for understanding the physiological action of toxicant in aquatic life (Ketpadung and Tangkrockolan 2006; Mukke and Chinte 2012). In crustaceans, rate of oxygen consumption can be so sensitive that it is modified by a great variety of factors, both internal (body size, activity level, starvation, moulting cycle and biological rhythm) and external (salinity, light intensity, oxygen concentration, light–dark cycle and temperature) (Cockcroft and Wooldridge 1985; Ali et al. 2000; Crear and Forteath 2000; Valverde et al. 2009). The manner in which the organism responds to these factors ultimately delineates its suitability to a given habitat (Stern et al. 1984).
Horseshoe crabs have well-developed external gills in the form of appendages which are known as gill lamellae and these can be opened and moved easily, which helps the larvae of horseshoe crabs to swim upside down (Srijaya 2011). The gill lamellae have well-developed circulating system with blood vessels that helps the animal in respiration (Farley 2010). Except one study by Suniza et al. (2011), the respiratory physiology of larval horseshoe crabs has not been evaluated until now. This study has evaluated the effects of salinity, pH and temperature on the respiratory metabolism of trilobite larvae of Tachypleus gigas. Most other research works on horseshoe crab larvae regarding the influence of extrinsic factors (temperature and salinity) were based on growth, moulting and survival (Sekiguchi et al. 1988a, b; Chatterji et al. 2004; Ehlinger and Tankersley 2004; Zaleha et al. 2011). The aim of the present study is to evaluate the effect of various extrinsic factors such as temperature, salinity, pH and dark–light condition on oxygen consumption in the trilobite larvae of C. rotundicauda, using a range of values for the selected variables to find out the best parameters for culture.
Methods
Fertilized eggs of the Malaysian horseshoe crab (C. rotundicauda) were collected directly from the nest made on the breeding grounds of Setiu at Terengganu (Eastern coast of Peninsular Malaysia; Lat 5°42′60″N; Long 102°42′0″E, see Srijaya et al. 2010b for details of location). All the experiments were carried out in the Akuatrop Plankton Laboratory of University Malaysia Terengganu. The fertilized eggs collected from the nest were kept for incubation at a constant temperature of 26 ± 1 °C, pH of 7 ± 0.5 and salinity of 25 ± 1 ppt. In this condition, the hatching of the trilobite larvae occurred between 42 and 45 days during the incubation. Newly hatched first-stage larvae resemble adult horseshoe crabs except for the lack of a telson, which shows up after the first moult (75 days of the incubation period). The trilobite larvae of same age (second instar) collected from different nests were randomly mixed and divided into several groups immediately after the first moulting for performing the experiment.
Experimental setup
Acclimatization
The oxygen consumption of trilobite larvae of C. rotundicauda under four different conditions (temperature, salinity, pH and dark–light conditions) was investigated. The trilobite larvae were exposed to different levels of temperature (10, 20, 30 and 40 °C), salinity (10, 20, 30 and 40 ppt), pH (5, 6, 7, 8 and 9) and dark–light conditions for a period of 12 h. Seawater of different salinities was prepared by diluting 32 ppt filtered seawater with sterilized freshwater, whereas salinity of 40 ppt was prepared by adding NaCl salt (Merck, Germany). A refractometer (Atago, S/Mill-E, Japan) was used to determine the salinity of the water. The pH of the seawater was adjusted from 5 to 7 with 1 M HCL (Merck, Germany) and pH 8 and pH 9 by 1 M NaOH (BDH, England). A thermal compact heater (Askoll, Italy) was used to control temperature of the experimental tank at 30 °C, whereas temperatures 10 and 20 °C were maintained using microchillers (Resun L-650, 1,800 l h−1 capacity). A thermo regulated water bath of 45 L capacity (Memmert, Ltd) was used for controlling the temperature at 40 °C.
A preparation tank of (50 L) capacity was used to prepare the desired physiochemical seawater parameters for each experiment. The experiment was carried out only after the acclimatization of trilobite larvae for 36 h to the desired temperatures, pH and salinity prior to the commencement of the study. During the acclimatization and whole experimental period no feeding was administered to the trilobite larvae to avoid any potential increases in metabolic (and thus respiratory) rates associated with specific dynamic action. Initially the physiochemical parameter of the preparation tanks (4 tanks) was maintained at a temperature of 25 °C, salinity of 25 ppt and pH 7. A total of 360 larvae each were maintained in the preparation tanks of temperature and salinity. Meanwhile 450 larvae were maintained in preparation tank for pH and 180 larvae in dark–light preparation tank. In order to evaluate the effect of different temperature and salinity on the oxygen consumption pattern, a slow acclimatization procedure was followed before taking the measurement. A gradient of 5 °C and 5 ppt was either increased or decreased from the previously maintained temperature (25 °C) and 25 ppt salinity of trilobite larvae, whereas for pH experiment, a gradient of 1 pH was considered. The larvae were maintained in the newly introduced physiochemical parameters over a period of 24 h. An extended 12 h of acclimatization was only provided for the trilobite larvae which were taken for experimentation at that particular parameter and thus a total of 36 h was provided in total for acclimatization. Rest of the larvae were shifted to the new environmental parameters but before further modifying that specific parameter, the trilobite larvae were maintained for another 24 h. In general, after reaching the desired temperature the larvae were kept undisturbed for the next 36 h before commencing the experiment and after this a batch of 30 larvae was introduced into each respective respiratory chamber. For measuring the effect of different temperatures ranging from 10 to 40 °C on the oxygen consumption of trilobite larvae, pH of the water was maintained at 7 and salinity at 25 ppt for all different temperatures. Similarly, for measuring the effect of various salinities (10, 20, 30 and 40 ppt), on oxygen consumption rate in trilobite larvae, pH of the water was maintained at 7 and temperature at 25 °C. Meanwhile, for evaluating the effect of various pH (5, 6, 7, 8 and 9) on oxygen consumption, a constant salinity (25 ppt) and temperature (25 °C) was maintained. In the fourth set of experiment, the oxygen consumption by the trilobite larvae was assessed under light and dark conditions. The larvae were kept under complete light and dark conditions for 36 h before starting the experiment. The light condition was obtained with two fluorescent tube lights (10 W) and the dark condition was obtained by covering the respiratory chambers with black plastic sheets. This prevented any penetration of light into the respiratory chambers. Experiment to determine the effect of light and dark condition on oxygen consumption of trilobite larvae was conducted at a constant salinity (25 ppt), temperature (25 °C) and pH (7).
Oxygen consumption rate
For each experiment triplicate treatment measures were taken. For studying the oxygen consumption, we specially designed a rectangular shaped glass respiratory chamber of 4 L capacity having 20 cm length, 12 cm width and 15 cm height. A small PVC pipe (45 mm diameter) was attached to one end of the respiratory chamber and was fixed 5 cm above from the bottom of the respiratory chamber for inserting the pinpoint dissolved oxygen monitor (YSI 556 MPS; Yellow springs, Ohio 45387, USA) to record the dissolved oxygen (DO) measurement. To reduce microbial activity, the chamber was sterilized before trials. Before transferring the larvae from the preparation tank to the respiratory chambers, proper aeration was provided in the experimental chambers for a period of 2 h. A total of 30 trilobite larvae were introduced in each respiratory chamber after recording the weight of larvae to the nearest 0.01 g. Larvae were blotted using a dry soft tissue paper before weighing and weight of the larvae used for the present study ranged from 0.060 to 0.0746 g. During the blotting process, the larvae were handled very gently to avoid any possible damage to the gill tissues and the whole process was done as quickly as possible to avoid any stress to the animal. Extreme care was taken to avoid variation in total biomass of the larvae in each replicated tanks and also for the entire experimental sets. The initial dissolved oxygen in the water was measured immediately after the introduction of weighed larvae in each tank in all set of experiment. This was followed by sealing the water surface with a thin layer of liquid paraffin wax (Gene Chem., Canada) to avoid any diffusion of oxygen from the atmosphere. The probe reached almost to the bottom of the chamber while taking the measurement; however, it did not harm larvae in anyway as they were found swimming away from the location during the oxygen recording.
In all experiments, the oxygen content of the respiratory chamber was recorded at an interval of 4 h, for 12 h. The larval movement and activities allowed the mixing of the water inside the chambers, which did not allow any oxygen gradient [The mass-specific oxygen consumption rate of the trilobite larvae during the experimental period was expressed as mg of oxygen consumed per gramme body weight per 4 h (mg O2 g−1 h−1), Table 1]. The rate of oxygen consumption at each time interval was calculated as (Initial DO value–Final successive DO value)/Initial DO value × 100. Respiration chambers (n = 3) of the same volume, but with no trilobite larvae, were used as a control (Temp 26°, pH 7 and salinity 25 ppt), and exactly the same procedure was followed. The difference between the oxygen content of the control and experimental chambers was used to determine the rate of oxygen consumption of trilobite larvae. In this way, the possible contribution of bacterial respiration was taken into account.
Statistical analysis
Using ‘IBM SPSS Statistics 20′ software, a one-way repeated measures analysis of variance (RM-ANOVA) was run to determine if oxygen consumption at different time points differed significantly between different temperatures, salinities, pHs and light conditions. The data were initially analysed for sphericity using the Mauchly’s test of sphericity. Repeated measures ANOVA is very sensitive to the violations of the assumption of sphericity. If the assumption is violated, the P value will be too low. Hence, data violating the prerequisite of sphericity were interpreted according to P values calculated using the Greenhouse-Geisser correction. All means are reported as ±SE, and the level of significance for all tests was 0.05.
Results
Trilobite larvae showed remarkably high tolerance to all the values for the extrinsic factors (temperature, salinity, pH and light–dark cycle) during acclimatization and also the whole experimental period. Details regarding the oxygen consumption by trilobite larvae of C. rotundicauda at different environmental condition are presented in Table 1. The oxygen consumption in the control treatment (without any larvae) was negligible (0.0014 ± 0.0002 mg O2 g−1 h−1 at 4 h, 0.0021 ± 0.0007 mg O2 g−1 h−1 at 8 h and 0.0025 ± 0.009 mg O2 g−1 h−1 at 12 h).
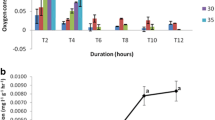
Even though the statistical test did not indicate an effect of temperature [F(3,3.136) = 1.778; p > 0.05), there may be a trend for increased oxygen consumption at 10 and 40 °C compared to the values at 20 and 30 °C at the initial 4 h. The mean oxygen consumption by the trilobite larvae was highest for 40 °C (1.98 mg O2 g−1 h−1) followed by 10 °C (1.34 mg O2 g−1 h−1) at initial 4 h. The least mean oxygen consumption was recorded for 20 °C (0.13 mg O2 g−1 h−1) at initial 4 h, while 30 °C had 0.36 mg O2 g−1 h−1 (Table 1). After the initial high rates of oxygen consumption, temperatures 10 and 40 °C showed a decline in consumption rate at 8 h, 0.17 and 0.40 mg O2 g−1 h−1, respectively, which were more or less comparable to the oxygen consumption values obtained at 20 and 30 °C. This is suggestive of the ability of trilobite larvae to acclimatize and survive under harsh environmental conditions. This trend was continued with a gradual decrease in the dissolved oxygen value at both these temperatures until the termination of the experiment during 12 h. Meanwhile an increasing trend of oxygen consumption was recorded for temperatures 20 and 30 °C during 12 h (Fig. 1). The sphericity assumption was not violated (χ2(5) = 10.135, p = 0.113).
Figure 2 shows oxygen consumption recordings over a 12-h period in the trilobite larvae, which was maintained at various salinities. The sphericity was violated (χ2(5) = 12.998, p = 0.045). Though no significance for mean oxygen consumption under different salinities was recorded (F(3, 9) = 2.184, p > 0.05), a trend for increased oxygen consumption was recorded at a salinity of 30 ppt (0.60 mg O2 g−1 h−1) at the initial 4 h compared with other salinities. Thereafter, the oxygen consumption started showing a sudden decline at 8 h and after this duration, the rate of consumption did not vary much (Table 1). For 10 and 40 ppt salinity, however, a consistent declining trend in oxygen consumption rate was observed (Table 1).
Statistical analysis revealed a positive correlation between pH and the oxygen consumption pattern in trilobite larvae. Mauchly’s test of sphericity for pH indicated that the assumption of sphericity was violated (χ2(5) = 20.376, p = 0.001). RM-ANOVA using the Greenhouse–Geisser correction showed that the mean scores for oxygen concentration were significantly different (F(1.108, 6.645) = 4.050, p < 0.05). During the first 4 h of the experiment, maximal oxygen consumption was recorded between pH 7 and 9 (Table 1). Meanwhile, the pH 5 and pH 6 showed relatively lesser amount of oxygen consumption at the initial 4 h of the experiment (Fig. 3). A sudden drop in oxygen consumption was observed between pH 7 and 9 at 8 h and the oxygen consumption rate showed further declining trend in all these pH until 12 h. Lower pH concentrations (pH 5 and 6) showed that the oxygen consumption rate was more or less consistent than higher pH concentrations during the 12-h experiment.
Even though the statistical test did not indicate an effect of light–dark conditions on oxygen consumption (F(1.000, 1.000) = 11.284, p = 0.184), there may be a trend for increased oxygen consumption under light (2.12 mg O2 g−1 h−1) compared to dark conditions (1.40 mg O2 g−1 h−1) within 4 h of the experiment (Table 1). A decreasing pattern in oxygen consumption was recorded for both light and dark conditions after 4 h until 12 h (Fig. 4). Using RM-ANOVA with a Greenhouse–Geisser correction, the mean scores for oxygen consumption were found not significantly different (p > 0.05). Though not significant, dark condition showed more consistent oxygen consumption pattern at 8 and 12 h than light condition (Table 1).
Discussion
Respiratory metabolism is considered to be an essential biological activity of aquatic organisms. Respiration rate measured in terms of oxygen consumption has been commonly used as a proxy for metabolic rate in aquatic and terrestrial animals (Daoud et al. 2007). Measurements of this parameter are necessary to examine the physiological behaviour of the animal, which in turn also helps in assessing the favourable and unfavourable conditions of the environment. A number of factors (both extrinsic and intrinsic) can influence the rate of oxygen consumption in marine organisms. In the current experiment, the trilobite larvae of horseshoe crab (C. rotundicauda) showed tolerance to a wide range of environmental parameters, which indicated the resistance capability of the animal. The eggs and larval stages of horseshoe crabs are known for their capability to withstand even the harsh environmental conditions in their intertidal habitat (Jegla and Costlow 1982; Palumbi and Johnson 1982; Laughlin 1983; Sugita 1988; Shuster and Sekiguchi 2003; Ehlinger and Tankersley 2004). In order to overcome these unfavourable conditions, horseshoe crabs have unique abilities to adapt and acclimatize rapidly to the environmental conditions (Ehlinger and Tankersley 2009; Botton et al. 2006). Indeed, physiologically tolerant early stages horseshoe crabs, which are capable of surviving extreme environmental regimes, may be a factor that has contributed to the evolutionary success of horseshoe crabs (Ehlinger and Tankersley 2009).
As trilobite larvae are inhabitant of the intertidal zone, they are constantly exposed to tide fluctuation and there by availability of oxygen availability. In order to cope with this hypoxic condition, the larvae may tend to respond by reducing their oxygen consumption. A study on Limulus polyphemus showed that the rate of respiratory gill movement is found to be proportional to the logarithm of ambient oxygen concentration, which completely ceased in oxygen-depleted water (Towle and Henry 2003). However, the information regarding the possibility of trilobite larvae being oxyconformers is still lacking. If these animals are oxyconformers, this may have led to an underestimation of oxygen consumption in the current study. In fact, we have observed that air saturation in some of the treatments dropped below 80 % (please refer to the respective figures). Therefore, we recommend for further studies on such respiratory metabolism in trilobite larvae of horseshoe crabs.
Many authors have verified the impact of handling stress on the respiration of crustaceans (Aldrich 1975; Carvalho and Phan 1997). Although an acclimation period of 1–2 h is suggested to be sufficient (Villarreal et al. 2003), the impact of stress duration can be varied greatly depending on the species and size (Dall 1986; Crear and Forteath 2000; Romero et al. 2006; Daoud et al. 2007). There is possibility of argument that the high rate of oxygen consumption in the initial 4 h of experiment under various parameters could be because of the artefacts of experimental procedures such as insufficient acclimation time. However, such arguments cannot be valid as it could be realized that the high consumption pattern at the initial phase was found only at the unfavourable environmental variables. Although our experiment was carried out only after the proper acclimatization, perhaps handling stress encountered while transferring the larvae from the acclimatization tank to the respiratory chambers along with the sub-optimal environmental conditions could have made the larvae to consume more oxygen at the initial hours. Meanwhile, at optimal environmental parameters the larvae might have quickly recovered from such stress level.
Temperature is the most important extrinsic controlling factor influencing metabolic rate in aquatic organisms (Fry 1971). Temperature stress can have deleterious impacts on health; affecting fecundity, immune competence, metabolite equilibrium and survival in many invertebrates (Le Moullac and Haffner 2000; Harper and Wolf 2009). Even under a similar temperature regime, standard rates of oxygen consumption can vary widely with species and different life stages (Crear and Forteath 2000; Daoud et al. 2007). Therefore, species-specific and life stage-specific information regarding the oxygen consumption is essential and currently such information is lacking in many marine organisms. Even though not so significant with the effect of temperature on the oxygen consumption, an increased patter of oxygen consumption was observed in the initial 4 h of the experiment when trilobite larvae were held at lower temperature (10 °C) and high temperature (40 °C). Comparatively lower oxygen consumption rate was observed at 20 and 30 °C temperatures during first 4 h. However, in the course of experimentation hours, the consumption rates increased mildly until 12 h indicating the most favourable conditions for the larvae, which were reflected by their increased swimming activities as compared to the activities of the larvae at 10 and 40 °C. It is a fact that under unfavourable environmental conditions, marine organism will respond to overcome the physiological stress in some ways. Thus for short-term adjustments they utilize additional energy for various metabolic activities to stabilize the homeostatic equilibrium, which can be measured by an increase in respiration rate (Suniza et al. 2011). This could probably be the reason behind the high oxygen consumption at 10 and 40 °C at the initial 4 h of the experiment.
We have observed that the trilobite larvae were less active mostly at 10 °C temperature compared to higher temperatures. After the initial 4 h, the rest of the time, the larvae of 10 °C were found showing less swimming activity and mostly found swimming near the bottom of the test chamber. This could be the reason for the least mean consumption rate at this temperature till the termination of the experiment. A similar trend was noticed for the larvae at 40 °C, where the oxygen consumption rate reduced consistently over the experimental duration, but the larvae were seen not as inactive as at 10 °C. Under sub-optimal conditions some marine organisms have been observed to respond by reducing their metabolic rates (Gaudy and Sloane 1981; Crear and Forteath 2000). During extreme temperatures in the hot summer and cold winter, the horseshoe crabs in the wild have been known to become inactive, bury into deep sediments or mud and even diapause in winter (Sekiguchi 1988; Chiu and Morton 2004). The effect of temperature on the respiratory physiology of trilobite larvae of T. gigas also suggested that the most favourable temperature condition was at 30 °C (Suniza et al. 2011). They also suggested that higher temperature (40 and 50 °C) was unfavourable to trilobite larvae. It is known fact that as the temperature rises, the capacity of the water to dissolve oxygen also decreases simultaneously (Taylor 1981). Faster growth and better moulting have been reported for trilobite larvae of C. rotundicauda reared in the laboratory at 28 °C (Zadeh et al. 2009). The development rate of trilobite larvae of L. polyphemus was observed to be rapid at temperature 30 °C (Laughlin 1983), but the development rate had a negative effect when the temperature was above and below this temperature (Laughlin 1983; Botton et al. 1992). Taken together with the results of the present study, Suniza et al. (2011) and other studies revealed that the trilobite larvae prefer temperature range similar to that of their natural habitat. The average annual temperature at spawning ground of horseshoe crabs in Kranji, Singapore, is reported to be about 28–30 °C (Cartwright-Taylor et al. 2012) and in Pahang coast Malaysia, it was reported as 24.08 ± 2.91 °C at Balok and 22.97 ± 3.26 °C at Pekan (John et al. 2011). Other studies also indicated that temperature has an important role in the native habitats of horseshoe crabs (Lee and Morton 2009; Faurby et al. 2010; Botton et al. 2010). Though not statistically proven, overall trend from our study suggests that the temperature between 20 and 30 °C could be the best condition for rearing trilobite larvae, since there was more or less consistent oxygen consumption rate than temperatures lower and higher than these two temperatures.
Tidally influenced salinity cycles are very common in the mangrove eco-system and estuaries; thus it is important to understand the effect that salinity cycle has on the physiology of marine animals inhabiting in such environment. Any deviation of the salinity from its ambient level can disrupt the osmotic balance in many aquatic organisms and for readjusting the osmotic concentration they may have to exchange a considerable quantity of energy (Huni and Aravindan 1985). The trilobite larvae of horseshoe crab showed a considerable degree of respiratory independence since they were able to maintain their oxygen consumption rates approximately constant over a wide range of salinity tested in the present study. This shows that the larval stages of horseshoe crabs have an efficient osmo-regulation mechanism, enabling it to function in a wide range of salinities. Similar to our study, the larvae of T. gigas (Suniza et al. 2011) also had no significant effect on the oxygen consumption and they also suggested that the animal can thrive well in a wide range of salinity changes. This wide range of salinity tolerance could be an adaptation of horseshoe crabs for an easy distribution in estuarine systems. Such adaptations are common in other crustaceans especially penaeid shrimp (Rowe 2002; Stickle et al. 2007). Many holo-euryhaline or extremely euryhaline invertebrates like Eriocheir sinensis, Milne-Edwards, 1854, Artemia salina, Linnaeus, 1758 and their nauplii have been reported to have the ability to maintain a constant metabolic rate in media of varying salinity (Moreira et al. 1982). According to Kinne (1971), an efficient respiration of many invertebrates occurs efficiently at salinities to which they are genetically adjusted, or to which they have been acclimated over prolonged periods of time. However, the response of crustaceans to changes in salinity and temperature is more complex with one variable acting as a modulating factor on the effects of other (Vernberg 1983; Moreira and Nelson 1990). Unlike the results of oxygen consumption rate in trilobite larvae of horseshoe crabs, most other studies on various crustaceans have shown that the changes in salinity have a critical effect on their oxygen consumption (Chen and Sen-Huan 1993; Chen and Lin 1995; Rosas et al. 1999). In general, at low salinity concentrations, crustaceans that osmoregulate increase their metabolic rate, while osmoconformers reduce their metabolic rate under these conditions (Mantel and Farmer 1983). Moreover, high locomotor activity (Gross 1957) and higher solubility of oxygen coupled with oxygen-dependent respiration (Lange et al. 1972) also can influence the increased metabolism at low salinities.
A clear understanding on the inter-relation between environmental salinity and oxygen consumption is of considerable importance to larval biology, which could deliver specific information regarding the rearing conditions in which better growth can be achieved with minimum energy expenditure. In our study, though the statistical test did not indicate an effect of salinity on the oxygen consumption pattern of trilobite larvae of C. rotundicauda, the trend may indicate that a more or less uniform oxygen consumption pattern at 20 ppt until 12 h revealing the most preferred salinity among all the tested salinities. Salinity has been found to have an important influence on growth rate, survival and moulting in horseshoe crab larvae. Our study on growth and survival rate of trilobite larvae of C. rotundicauda also showed that the best salinity parameter was at 20 ppt (Srijaya 2011). According to Zaleha et al. (2011), the optimal salinity ranges for the laboratory development of T. gigas larvae were found to range between 25 and 35 ppt. Similarly, the embryos and posthach larval stages of L. polyphemus were found to develop and moult in the shortest time when exposed to salinities in the range of about 20 and 30 ppt (Shuster CN Jr 1982). Although L. polyphemus can survive at salinity as low as 10 ppt and as high as 40 ppt, growth rates were slowed significantly at these salinity extremes (Jegla and Costlow 1982). In another study by Smith et al. (2011), salinity tolerance of horseshoe crab larvae of L. polyphemus was observed to range from 5 to 35 ppt. Smith et al. (2011) observed that larval survival was less at low salinities, but the juvenile and adult horseshoe crabs could be properly maintained for long term at water salinity of 27 ppt. Laughlin (1983) also reported a wide salinity tolerance in trilobite larvae of L. polyphemus, and he suggested that it may be an adaptation allowing them to exploit the large range of habitats encountered with wide deviations in temperature and salinity, occurring randomly in degree and duration from year to year.
Unfavourable environmental parameters usually produce changes in pH level that have been reported to cause considerable stress affecting the growth and other normal metabolic activities of the animals (Chizinski et al. 2008). Moreover, changes in pH level can exert profound effects on ionoregulation and acid–base balance in decapod crustaceans (Ellis and Morris 1995). The normal variation of pH in oceanic water of 35 ppt salinity is 7.8–8.2 (Knutzen 1981). However, in estuarine habitats the pH level can drop due to mixing of freshwater and values of between 6.9 and 7.8 are common (John et al. 2011). In addition, intensive photosynthesis (Bowmer and Muirhead 1987); respiration (Knutzen 1981); organic decomposition (Albrecht et al. 1997) and industrial effluents (Boyd 1982) can alter the pH level. Most of the mangrove soil is fully buffered, having a pH in the range of 6–7, but some have a low pH, due to reduction of sulphate to sulphide (a common constituent of mangrove soils). In some cases, increased rates of sulphate reduction occurs leading to anaerobiosis and increased sulphide in the mangrove sediments, thus giving a much lower value of pH than normal (Kathiresan and Bingham 2001). Considering such variation of pH level in mangrove areas, a wide range of pH from 5 to 9 was taken for the present experimentation. In our study, trilobite larvae of C. rotundicauda were influenced by changes in the pH level considerably on the oxygen demand. The maximum oxygen consumptions were calculated mostly in the alkaline pH 8 and 9 during the early 4 h of the experiment. However, the acidic pH (5 and 6) tested had showed somewhat consistent oxygen consumption pattern during the 12-h experiment period. This result was similar to that reported by Suniza et al. (2011), where they showed that a wide fluctuation in oxygen consumption was observed under different pH ranges tested with normal oxygen consumption falling at pH 6. This suggests that the trilobite larvae tend to prefer slightly acidic conditions for their regular physiological activities. This could be because of their habitat preference, as these larvae remain for a portion of their life cycle near inshore waters and estuaries. An increase in oxygen consumption was observed at pH 5, 6 and 7 after a duration of 12 h in our study. This pattern was also observed in T. gigas trilobite larvae, where a sudden increase in the consumption rate at pH 6, 7 and 8 was reported. Overall, the results from both these studies suggests that the variable pH is rather critically important for trilobite larvae as any variation of the optimal parameter can make larvae under stress, even within a short time interval. In other crustaceans also changes in pH level have shown to affect their feeding behaviour, growth and even the oxygen consumption, which directly affected their metabolic rates (Allan and Maguire 1992; Zanotto and Wheatly 1993; Chen and Lin 1995; Chen and Chen 2003).
Light is the primary entraining factor in regulating the diurnal rhythms of the aquatic animals, but light as an environmental parameter has been given little importance as a stressor affecting animal function rate (Prosser 1957). Although more consistent oxygen consumption pattern was recorded under dark condition, in general, no significant pattern of oxygen consumption rate was observed. However, swimming activity of trilobite larvae of horseshoe crabs have been strongly correlated with endogenous rhythms and this is one of the common behavioural and physiological processes of many organisms inhabiting intertidal and estuarine areas (Ehlinger and Tankersley 2006). Trilobite larvae exhibit strong nocturnal activity and they swim much more actively in darkness than during daylight, suggesting a strong endogenous rhythm (Botton and Loveland 2003). Moreover, the larvae are positively phototactic to dim light sources such as moonlight (Rudloe 1979, 1985). It has also been reported that, the largest spawning activities in horseshoe crabs occur primarily at night, which also fits well with their nocturnal behaviour and biological activities (Barlow 1981). Previous study suggests that the juvenile horseshoe crabs are also found to be nocturnally active under natural cyclic lighting and maintained rhythmic activity in constant darkness (Borst and Barlow 2002). Thus we assume that the comfortable oxygen consumption pattern observed in our study could be due to their endogenous larval behaviour of horseshoe crab, whose activity increases under dark condition. Similar to horseshoe crab larvae, many other subtidal decapods also show typically diurnal rhythm in their behaviour patterns and they exhibited increased oxygen consumption at night (Du Preez 1983; Dall 1986; Crear and Forteath 2000).
Conclusion
Knowledge of the oxygen consumption rate of a species is of great interest in aquaculture since it gives the best accurate measurement of metabolic expenditure. This could provide crucial information regarding factors affecting larval survival and development. The apparent wide tolerance of trilobite larvae of horseshoe crabs under a wide range of environmental condition, as evidenced by its natural range and preferred environment, suggests that it is possible to culture early stages of horseshoe crabs under captive condition. Based on the findings of this experiment, trilobite larvae of C. rotundicauda should be reared at 20–30o C temperature, 20 ppt salinity, slightly acidic pH of 6–7 and appropriate dark and light cycles, to achieve optimal physiological homeostasis without any stress under culture conditions. Moreover, a better strategic plan could be developed considering the optimal parameters tested in this experiment, for protecting this species by managing their habitat following appropriate measures.
References
Albrecht SL, Baune HLM, Rasmussen PE, Douglas JCL (1997) Light fraction soil organic matter in long-term agro ecosystems. Columbia Basin Agricultural Research Annual Report 977:38–42
Aldrich JC (1975) Individual variability in oxygen consumption rates of fed and starved Cancer pagurus and Maia squinado. Comp Biochem Physiol A 51:175–183
Ali MH, Salman SD, Al-Adhub A (2000) Oxygen consumption of the freshwater crab Elamenopsis kempi (Chopra and Das, 1930) from the Garmat-Ali river, Iraq. Sci Mar 64(3):311–317
Allan GL, Maguire GB (1992) Effects of pH and salinity on survival, growth and osmoregulation in Penaeus monodonFabricius. Aquaculture 107:33–47
Anderson LI, Shuster CN (2003) Throughout geologic time: where have they lived?. In: The American Horseshoe Crab. Harvard Press, Cambridge
Barlow RB (1981) Vision in Limulus mating behaviour. Biol Bull 161:339–340
Borst D, Barlow R (2002) Circadian rhythms in locomotor activity of juvenile horseshoe crabs. Biol Bull 203:227–228
Botton ML (2000) Toxicity of cadmium and mercury to horseshoe crab (Limulus polyphemus) embryos and larvae. Bull Environ Contam Toxicol 64:137–143
Botton ML, Loveland RE (2003) Abundance and dispersal potential of horseshoe crab (Limulus polyphemus) larvae in the Delaware estuary. Estuaries 26(6):1472–1479
Botton ML, Loveland RE, Jacobsen TR (1992) Overwintering by trilobite larvae of the horseshoe crab Limulus polyphemus on a sandy beach of Delaware Bay (New Jersey, USA). Mar Ecol Prog Ser 88:289–292
Botton ML, Johnson K, Helleby L (1998a) Effects of copper and zinc on embryos and larvae of the horseshoe crab, Limulus polyphemus. Arch Environ Contam Toxicol 35:25–32
Botton ML, Hodge M, Gonzalez TI (1998b) High tolerance to tributyltin in embryos and larvae of the horseshoe crab, Limulus polyphemus. Estuaries 21:340–346
Botton ML, Pogorzelska M, Smoral L, Shehata A, Hamilton MG (2006) Thermal biology of horseshoe crab embryos and larvae: a role for heat shock proteins. J Exp Mar Biol Ecol 336:65–73
Botton ML, Tankersley RA, Loveland RE (2010) Developmental ecology of the American horseshoe crab Limulus polyphemus. Curr Zool 56:550–562
Bowmer KH, Muirhead WA (1987) Inhibition of algal photosynthesis to control pH and reduce ammonia volatilization from rice floodwater. Nutr Cycl Agroecosyst 13(1):13–29
Boyd CE (1982) Water quality management for warm water fish culture. Developments in aquaculture and fisheries science. Elsevier, Amsterdam
Brockman HJ, Smith MD (2009) Reproductive competition and sexual selection in horseshoe crabs. In: Biology and Conservation of Horseshoe Crabs. Springer Science, New York
Brockmann HJ (2003) Male competition and satellite behavior. In: The American horseshoe crab. Harvard University Press, Cambridge
Carmichael RH, Brush E (2012) Three decades of horseshoe crab rearing: a review of conditions for captive growth and survival. Rev Aquacul 4:32–43
Cartwright-Taylor L, Hsu CC (2012) Follow-up study on population structure and breeding pattern of the mangrove horseshoe crab Carcinoscorpius rotundicauda in Singapore. Aquat Biol 14:217–222
Cartwright-Taylor L, Lee J, Hsu CC (2009) Population structure and breeding pattern of the mangrove horseshoe crab Carcinoscorpius rotundicauda in Singapore. Aquat Biol 8:61–69
Cartwright-Taylor L, Ng HN, Goh TY (2012) Tracked mangrove horseshoe crab Carcinoscorpius rotundicauda remain resident in a tropical estuary. Aquat Biol 17:235–245
Carvalho PSM, Phan VN (1997) Oxygen consumption and ammonia excretion of Xiphopenaeus kroyeri, Heller (Penaeidae) in relation to mass temperature and experimental procedures. Shrimp oxygen uptake and ammonia excretion. J Exp Mar Biol Ecol 209:143–156
Chatterji A, Parulerkar AH (1992) Fecundity of the Indian Horseshoe crab Carcinoscorpius rotundicauda (Latreille). Tropical Ecol 33(1):97–102
Chatterji A, Kotnala S, Mathew R (2004) Effect of salinity on larval growth of horseshoe crab, Tachypleus gigas (Müller). Curr Sci 87(2):248–249
Chen S, Chen J (2003) Effects of pH on survival, growth, molting and feeding of giant freshwater prawn Macrobrachium rosenbergii. Aquaculture 218:613–623
Chen J, Lin C (1995) Responses of oxygen consumption, ammonia-N excretion and Urea-N excretion on Penaeus chinensis exposed to ambient NH3 at different salinity and pH levels. Aquaculture 55:297–306
Chen J, Sen-Huan L (1993) Effects of temperature and salinity on oxygen consumption and ammonia-N excretion of juvenile Penaeus japonicus Bate. J Exp Mar Biol Ecol 165:161–170
Chiu HMC, Morton B (2004) The behaviour of juvenile horse-shoe crabs, Tachypleus tridentatus (Xiphosura), on a nursery beach at Shui Hau Wan, Hong Kong. Hydrobiologia 523:29–35
Chizinski CJ, Sharm B, Pope KL, Patinos R (2008) A bioenergetic model for zebrafish, Danio rerio (Hamilton). J Fish Biol 73:35–43
Cockcroft AC, Wooldridge T (1985) The effect of mass, temperature and molting on the respiration of Macropetasma Africanus balss (Decapoda: Penaeoidea). Comp Biochem Physiol A 81:143–148
Crear BJ, Forteath GNR (2000) The effect of extrinsic and intrinsic factors on oxygen consumption by the southern rock lobster, Jasus edwardsii. J Exp Mar Biol Ecol 252:129–147
Dall W (1986) Estimation of routine metabolic rate in a penaeid prawn, Penaeus esculentus, Haswell. J Exp Mar Biol Ecol 96:7–74
Daoud D, Chabot D, Audet C, Lambert Y (2007) Temperature induced variation in oxygen consumption of juvenile and adult stages of the northern shrimp, Pandalus borealis. J Exp Mar Biol Ecol 347:30–40
Davidson GWH, Ng PKL, Ho HC (2008) The Singapore Red Data Book: threatened plants and animals of Singapore. The Nature Society (Singapore), Singapore
Du Preez HH (1983) The effects of temperature, season and activity on the respiration of the three spot swimming crab, Ovalipes punctatus. Comp Biochem Physiol A 75:353–362
Ehlinger GS, Tankersley RA (2004) Survival and development of horseshoe crab (Limulus polyphemus) embryos and larvae in hypersaline conditions. Biol Bull 206:87–94
Ehlinger GS, Tankersley RA (2006) Endogenous rhythms and entrainment cues of larval activity in the horseshoe crab Limulus polyphemus. J Exp Mar Biol Ecol 337:205–214
Ehlinger GS, Tankersley RA (2009) Ecology of horseshoe crabs in microtidal lagoons. In: Biology and conservation of horseshoe crabs. Springer, New York, pp 149–162
Ehlinger GS, Tankersley RA, Bush MB (2003) Spatial and temporal patterns of spawning and larval hatching by the horseshoe crab, Limulus polyphemus, in a microtidal coastal lagoon. Estuaries 26:631–640
Ellis BA, Morris S (1995) Effects of extreme pH on the physiology of the Australian ‘Yabby’ Cherax destructor: acute and chronic changes in haemolymph oxygen levels, oxygen consumption and metabolite levels. J Exp Biol 198:409–418
Farley RD (2010) Book gill development in embryos and first and second instars of the horseshoe crab, Limulus polyphemus L. (Chelicerata, Xiphosura). Arthropod Struct Dev 39:369–381
Faurby S, King TL, Obst M, Hallerman EM, Pertoldi C, Funch P (2010) Population dynamics of American horseshoe crabs-historic climatic events and recent anthropogenic pressures. Mol Ecol 19:3088–3100
Fry FEJ (1971) The effect of environmental factors on the physiology of fish. In: Environmental relations and behavior. Academic, New York
Gaudy R, Sloane L (1981) Effect of salinity on oxygen consumption in post larvae of the penaeid shrimps, Penaeus monodon and P. stylirostris without and with acclimation. Mar Biol 65:297–301
Greene MP, Hamilton MG, Botton ML (2011) Physiological responses of horseshoe crab (Limulus polyphemus) embryos to osmotic stress and a possible role for stress proteins (HSPs). Mar Biol 158:1691–1698
Gross WJ (1957) An analysis of response to osmotic stress in selected decapod crustacea. Biol Bull Mar Biol Lab (Woods Hole) 112:43–62
Harper C, Wolf JC (2009) Morphologic effects of the stress response in fish. ILAR J 50(4):387–396
Hu M, Wang Y, Chen Y, Cheung SG, Shin PKS, Li Q (2009) Summer distribution and abundance of juvenile Chinese horseshoe crabs Tachypleus tridentatus along and intertidal zone in southern China. Aquat Biol 7:107–112
Huni AAD, Aravindan CM (1985) The effect of salinity on the oxygen consumption of two intertidal crustaceans. Comp Biochem Physiol A 81(4):869–871
Itow T, Mishra JK, Ahmed ATA (2004) Horseshoe crabs (King crabs) in the Bay of Bengal, South Asia. Bull Fac Educ Shizuoka Univ Natl Sci Ser 54:13–30
IUCN (International Union for Conservation of Nature) (2010) Red List of threatened species. Available at: www.iucnredlist.org
Jackson NL, Smith DR, Nordstrom KF (2005) Comparison of sediment grain size characteristics on nourished and un-nourished esturine beaches and impacts on horseshoe crab habitat, Deleware Bay, New Jersey. Zeitschrift fur Geomorphologie 141:31–45
Jegla TC, Costlow JD Jr (1982) Temperature and salinity effects on developmental and early posthatch stages of Limulus. In: Physiology and biology of horseshoe crabs: studies on normal and environmentally stressed animals. Liss, New York
John BA, Kamaruzzaman BY, Jalal KCA, Zaleha K (2011) Hydrology of the horseshoe crab nesting grounds at Pahang coast, Malaysia. Oriental J Chem 27(4):1475–1483
Kathiresan K, Bingham BL (2001) Biology of mangroves and mangrove ecosystems. Advan Mar Biol 40:81–251
Ketpadung R, Tangkrockolan N (2006) Changes in oxygen consumption and heart rate of the blue swimming crab, Portunus pelagicus (Linnaeus, 1766) following exposure to sublethal concentrations of copper. J Env Biol 27:7–12
Kinne O (1971) Salinity: invertebrates. In: Marine Ecology, Wiley Interscience
Knutzen J (1981) Effects of decreased pH on marine organisms. Mar Poll Bull 12:25–29
Lange R, Staaland H, Mostad A (1972) The effect of salinity and temperature on solubility of oxygen and respiratory rate in oxygen dependent marine invertebrates. J Exp Mar Biol Ecol 9:217–229
Laughlin R (1983) The effect of temperature and salinity on larval growth of the horseshoe crab Limulus polyphemus. Biol Bull 164:93–103
Le Moullac G, Haffner P (2000) Environmental factors affecting immune responses in Crustacea. Aquaculture 191:121–131
Lee CN, Morton B (2009) Emergence behaviour of Tachypleus tridentatus under simulated tidal conditions in the laboratory and at two different sediment temperatures. In: Biology and Conservation of Horseshoe Crabs. Springer, New York
Mantel LH, Farmer LL (1983) Osmotic and ionic regulation. In: The Biology of Crustacea. Academic Press, London
Mishra JK, Mishra A (2011) Anthropogenic effect on the horseshoe crab breeding ground and impact on their population along the North-East Coast of India. International workshop on scientific conservation of Asian horseshoe crabs, Hong Kong
Moreira GS, Nelson S (1990) The effects of temperature on the respiratory metabolism of Calcinus Laevimanus (Randall, 1839) (Crustacea, Anomura), in different salinities. J Thermal Biol 15(1):37–39
Moreira GS, McNamara JC, Moreira PS (1982) The effect of salinity on the metabolic rates of palaemonid shrimp larvae. Aquaculture 29:95–100
Mukke VK, Chinte DN (2012) Effect of sub lethal concentration of mercury and copper on oxygen consumption of fresh water crab, Barytelphusa guerini. Recent Research in Science and Technology 4(5):15–17
Palumbi SR, Johnson BA (1982) A note on the influence of life-history stage on metabolic adaptation: the responses of Limulus eggs and larvae to hypoxia. In: Physiology and biology of horseshoe crabs: studies on normal and environmentally stressed animals. Liss, New York
Prosser CL (1957) Physiological variation in animals. Biol Rev 30:229–262
Romero MC, Vanella F, Tapella F, Lovrich GA (2006) Assimilation and oxygen uptake associated with two different feeding habits of Munida gregaria (M. subrugosa) (Crustacea, Decapoda). J Exp Mar Biol Ecol 333:40–48
Rosas C, Martinez E, Gaxiola G, Brito R, Sanchez A, Luis A, Soto LA (1999) The effect of dissolved oxygen and salinity on oxygen consumption, ammonia excretion and osmotic pressure of Penaeus setiferus (Linnaeus) juveniles. J Exp Mar Biol Ecol 234:41–57
Rowe CL (2002) Differences in maintenance energy expenditure by two estuarine shrimp (Palaemonetes pugio and P. vulgaris) that may permit partitioning of habitats by salinity. Comp Biochem Physiol A 132:341–351
Rudloe A (1979) Locomoter and light responses of larvae of the horseshoe crabs, Limulus Polyphemus (L.). Biol Bull 157:494–505
Rudloe A (1985) Variations in the expression of lunar and tidal behavioural rhythms in the horseshoe crab Limulus polyphemus. Bull Mar Sci 36(2):388–395
Salvato B, Cuomo V, Di Muro P, Beltramini M (2001) Effects of environmental parameters on the oxygen consumption of four marine invertebrates: a comparative factorial study. Mar Biol 138:659–668
Sekiguchi K (1988) Biology of horseshoe crabs. Science House, Tokyo
Sekiguchi K, Seshimo H, Sugita H (1988a) Post-embryonic development of the horseshoe crab. Biol Bull 174:337–345
Sekiguchi K, Yamamichi Y, Seshimo H, Sugita H (1988b) Normal development. In: Biology of Horseshoe Crabs, Science House Company, Tokyo
Shin P, Li H, Cheung SG (2009) Horseshoe crabs in Hong Kong: current population status and human exploitation. In: Biology and Conservation of Horseshoe Crabs. Springer Science + Business Media, New York
Shuster CN Jr (1982) Physiology and biology of horseshoe crabs: studies on normal and environmentally stressed animals. In: A pictorial review of the natural history and ecology of the horseshoe crab Limulus polyphemus with reference to other Limulidae. Alan R. Liss, Inc, New York
Shuster CN Jr (1990) The American horseshoe crab, Limulus polyphemus. In: Clinical applications of the Limulus amoebocyte lysate test. CRC Press, Boca Raton
Shuster C, Sekiguchi K (2003) Growing up takes about ten years and eighteen stages. In: The American Horseshoe Crab. Harvard University Press, Harvard
Smith SA, Scimeca JM, Mainous ME (2011) Culture and maintenance of selected invertebrates in the laboratory and classroom. ILAR J 52:153–164
Srijaya TC (2011) Embrology and physiology of Malaysian horseshoe crab, Carcinoscorpius rotundicauada (Latreille). Ph.D. Thesis, University Malaysia Terenggannu, Institute of Tropical Aquaculture
Srijaya TC, Pradeep PJ, Mithun S, Hassan A, Chatterji A (2010a) A new record on the morphometric variations in the populations of Horseshoe crab (Carcinoscorpius rotundicauda) obtained from two different ecological habitats of Peninsular Malaysia. Our Nature 8:204–211
Srijaya TC, Pradeep PJ, Mithun S, Hassan A, Shaharom F, Chatterji A (2010b) A new record on variability in different body measurements of the horseshoe crab Carcinoscorpius rotundicauda (Latreille) collected from Setiu and Gelang Patah habitats in Peninsular Malaysia. J Bombay Nat Hist Soc 107(2):130–134
Stern S, Borut A, Cohen D (1984) The effect of salinity and ion composition on oxygen consumption and nitrogen excretion of Macrobrachium rosenbergi (De Man). Comp Biochem Physiol A 19(2):271–274
Stickle WB, Wyler HJ, Dietz TH (2007) Effects of salinity on the juvenile crab physiology and agonistic interactions between two species of blue crabs, Callinectes sapidus and C. similis from coastal Louisiana. J Exp Mar Biol Ecol 352:361–370
Sugita H (1988) Environmental adaptations of embryos. In: Biology of horseshoe crabs. Science House, Tokyo
Suniza AMS, Zaleha K, Chatterji A (2011) Effects of different environmental parameters on the respiratory metabolism of the larvae of Malaysian horseshoe crab, Tachypleus gigas (Muller). Pertanika J Sci Technol 19(1):1–9
Taylor EW (1981) Some effects of temperature on respiration in decapodan crustaceans. J Therm Biol 6:239–248
Towle DW, Henry RP (2003) Coping with environmental changes: physiological challenges. In: The American horseshoe crab. Harvard Press, Cambridge
Valverde JC, Hernández MD, Aguado-Giménez F, García BG (2009) Oxygen consumption in spider crab (Maja brachydactyla): effect of weight, temperature, sex, feeding and daily light–dark cycle. Aquaculture 298:131–138
Vernberg FJ (1983) Respiratory adaptations. In: The biology of crustacea: environmental adaptations. Academic Press, New York
Villarreal H, Hernandez-Llamas A, Hewitt R (2003) Effect of salinity on growth, survival and oxygen consumption of juvenile brown shrimp, Farfantepenaeus californiensis (Holmes). Aquatic Res 34:187–193
Walls EA, Berkson J, Smith SA (2002) The horseshoe crab, Limulus polyphemus: 200 million years of existence, 100 years of study. Rev Fish Sci 10(1):39–73
Widener JW, Barlow RB (1999) Decline of a horseshoe crab population on Cape Cod. Biolo Bull 197:300–302
Zadeh SSh, Christianus A, Saad CR, Hajeb P, Kamarudin MS (2009) Comparisons in prosomal width and body weight among early instar stages of Malaysian horseshoe crabs, Carcinoscorpius rotundicauda and Tachypleus gigas, in the laboratory. In: Biology and conservation of horseshoe crabs. Springer, New York
Zaleha K, Hazwani I, Hamidah HS, Kamaruzzaman BY, Jalal KCA (2011) Effect of salinity on the egg hatching and early larvae of horseshoe crab Tachpleus gigas (Muller, 1785) in laboratory culture. J Appl Sci 11(14):2620–2626
Zanotto FP, Wheatly M (1993) The effect of ambient pH on electrolyte regulation during the post moult period in freshwater crayfish Procambrus clarkia. J Exp Biol 178:1–19
Zhou H, Morton B (2004) The diets of juvenile horseshoe crabs, Tachypleus tridentatus and Carcinoscorpius rotundicauda (Xiphosura), from nursery beaches proposed for conservation in Hong Kong. J Nat Hist 38:1915–1925
Acknowledgments
This work was part of the Ph.D. study of STC at University Malaysia Terengganu. The authors are grateful to the University for providing SKS fellowships to PJP, STC and principal research fellowship to AC. They thank Dr. Alessio Papini from University of Florence for assisting them in performing the statistical analysis.
Conflict of interest
All authors declare that they have no competing interests.
Author contribution
STC and AC planned and designed the experiment. STC and PJP performed the experiments and analyses. STC interpreted the results. PJP, STC and AJ, edited and drafted the manuscript. STC, PJP, AH, AC and FS contributed to the sampling, discussion of the study and reviewing. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Srijaya, T.C., Pradeep, P.J., Hassan, A. et al. Oxygen consumption in trilobite larvae of the mangrove horseshoe crab (Carcinoscorpius rotundicauda; Latreille, 1802): effect of temperature, salinity, pH, and light–dark cycle. Int Aquat Res 6, 60 (2014). https://doi.org/10.1007/s40071-014-0060-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40071-014-0060-z