ABSTRACT
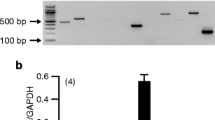
Sodium-dependent multivitamin transporter (SMVT) is a vital transmembrane protein responsible for translocating biotin and other essential cofactors such as pantothenate and lipoate. Unlike primary cultures of corneal and retinal pigment epithelial (RPE) cells, immortalized cells can be subcultured many times, yet maintain their physiological properties. Hence, the purpose of this study was to delineate the functional and molecular aspects of biotin uptake via SMVT on immortalized human corneal epithelial (HCEC) and RPE (D407) cells. Functional aspects of [3H] biotin uptake were studied in the presence of different concentrations of unlabeled biotin, pH, temperature, metabolic inhibitors, ions, substrates, structural analogs and biotinylated prodrug (Biotin-Acyclovir (B-ACV)). Molecular identity of SMVT was examined with reverse transcription–polymerase chain reaction. Biotin uptake was found to be saturable in HCEC and D407 cells with K m of 296.2 ± 25.9 and 863.8 ± 66.9 μM and V max of 77.2 ± 2.2 and 308.3 ± 10.7 pmol/mg protein/min, respectively. Uptake was found to be pH, temperature, energy, and sodium-dependent. Inhibition of biotin uptake was observed in the presence of structural analogs and specific substrates. Further, uptake was lowered in the presence of B-ACV indicating the translocation of biotinylated prodrug by SMVT. A distinct band at 774 bp confirmed the molecular existence of SMVT in both the cells. This study shows for the first time the functional and molecular presence of SMVT in HCEC and D407 cells. Therefore, these cell lines may be utilized as in vitro models to study the cellular translocation of biotin-conjugated prodrugs.









Similar content being viewed by others
REFERENCES
Ohkura Y, Akanuma S, Tachikawa M, Hosoya K. Blood-to-retina transport of biotin via Na+-dependent multivitamin transporter (SMVT) at the inner blood-retinal barrier. Exp Eye Res. 2010;91(3):387–92.
Janoria KG, Boddu SH, Wang Z, Paturi DK, Samanta S, Pal D, et al. Vitreal pharmacokinetics of biotinylated ganciclovir: role of sodium-dependent multivitamin transporter expressed on retina. J Ocul Pharmacol Ther. 2009;25(1):39–49.
Janoria KG, Hariharan S, Paturi D, Pal D, Mitra AK. Biotin uptake by rabbit corneal epithelial cells: role of sodium-dependent multivitamin transporter (SMVT). Curr Eye Res. 2006;31(10):797–809.
Bonjour JP. Biotin. In: Machlin LJ, editor. Handbook of vitamins nutritional biochemical and clinical aspects. New York: Marcel Dekker; 1984. p. 403–35.
Sweetman L, Nyhan WL. Inheritable biotin-treatable disorders and associated phenomena. Annu Rev Nutr. 1986;6:317–43.
Mall GK, Chew YC, Zempleni J. Biotin requirements are lower in human Jurkat lymphoid cells but homeostatic mechanisms are similar to those of HepG2 liver cells. J Nutr. 2010;140(6):1086–92.
Dakshinamurti K, Cheah-Tan C. Liver glucokinase of the biotin deficient rat. Can J Biochem. 1968;46(1):75–80.
Zempleni J, Mock DM. Uptake and metabolism of biotin by human peripheral blood mononuclear cells. Am J Physiol. 1998;275(2 Pt 1):C382–8.
Said HM. Cellular uptake of biotin: mechanisms and regulation. J Nutr. 1999;129(2S Suppl):490S–3S.
Said HM, Ortiz A, McCloud E, Dyer D, Moyer MP, Rubin S. Biotin uptake by human colonic epithelial NCM460 cells: a carrier-mediated process shared with pantothenic acid. Am J Physiol. 1998;275(5 Pt 1):C1365–71.
Said HM, Ma TY, Kamanna VS. Uptake of biotin by human hepatoma cell line, Hep G2: a carrier-mediated process similar to that of normal liver. J Cell Physiol. 1994;161(3):483–9.
Ma TY, Dyer DL, Said HM. Human intestinal cell line Caco-2: a useful model for studying cellular and molecular regulation of biotin uptake. Biochim Biophys Acta. 1994;1189(1):81–8.
Said HM, Redha R, Nylander W. A carrier-mediated, Na+ gradient-dependent transport for biotin in human intestinal brush-border membrane vesicles. Am J Physiol. 1987;253(5 Pt 1):G631–6.
Balamurugan K, Ortiz A, Said HM. Biotin uptake by human intestinal and liver epithelial cells: role of the SMVT system. Am J Physiol Gastrointest Liver Physiol. 2003;285(1):G73–7.
Prasad PD, Wang H, Huang W, Fei YJ, Leibach FH, Devoe LD, et al. Molecular and functional characterization of the intestinal Na+-dependent multivitamin transporter. Arch Biochem Biophys. 1999;366(1):95–106.
Wang H, Huang W, Fei YJ, Xia H, Yang-Feng TL, Leibach FH, et al. Human placental Na+-dependent multivitamin transporter. Cloning, functional expression, gene structure, and chromosomal localization. J Biol Chem. 1999;274(21):14875–83.
Prasad PD, Wang H, Kekuda R, Fujita T, Fei YJ, Devoe LD, et al. Cloning and functional expression of a cDNA encoding a mammalian sodium-dependent vitamin transporter mediating the uptake of pantothenate, biotin, and lipoate. J Biol Chem. 1998;273(13):7501–6.
Scholz M, Schrunder S, Gartner S, Keipert S, Hartmann C, Pleyer U. Ocular drug permeation following experimental excimer laser treatment on the isolated pig eye. J Ocul Pharmacol Ther. 2002;18(2):177–83.
Prausnitz MR, Noonan JS. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci. 1998;87(12):1479–88.
Lee VH, Robinson JR. Topical ocular drug delivery: recent developments and future challenges. J Ocul Pharmacol. 1986;2(1):67–108. Winter.
Maurice DM, Mishima S. Ocular Pharmakokinetics. In: Sears ML, editor. Pharmacology of the eye. Berlin: Springer; 1984. p. 19–116.
Reichl S, Bednarz J, Muller-Goymann CC. Human corneal equivalent as cell culture model for in vitro drug permeation studies. Br J Ophthalmol. 2004;88(4):560–5.
Reichl S. Cell culture models of the human cornea - a comparative evaluation of their usefulness to determine ocular drug absorption in-vitro. J Pharm Pharmacol. 2008;60(3):299–307.
Hornof M, Toropainen E, Urtti A. Cell culture models of the ocular barriers. Eur J Pharm Biopharm. 2005;60(2):207–25.
Barar J, Asadi M, Mortazavi-Tabatabaei SA, Omidi Y. Ocular drug delivery; impact of in vitro cell culture models. J Ophthalmic Vis Res. 2009;4(4):238–52.
Davis AA, Bernstein PS, Bok D, Turner J, Nachtigal M, Hunt RC. A human retinal pigment epithelial cell line that retains epithelial characteristics after prolonged culture. Invest Ophthalmol Vis Sci. 1995;36(5):955–64.
Hunt RC, Davis AA. Altered expression of keratin and vimentin in human retinal pigment epithelial cells in vivo and in vitro. J Cell Physiol. 1990;145(2):187–99.
Notara M, Daniels JT. Characterisation and functional features of a spontaneously immortalised human corneal epithelial cell line with progenitor-like characteristics. Brain Res Bull. 2010;81(2–3):279–86.
Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36(3):614–21.
Vellonen KS, Mannermaa E, Turner H, Hakli M, Wolosin JM, Tervo T, et al. Effluxing ABC transporters in human corneal epithelium. J Pharm Sci. 2010;99(2):1087–98.
Karla PK, Quinn TL, Herndon BL, Thomas P, Pal D, Mitra A. Expression of multidrug resistance associated protein 5 (MRP5) on cornea and its role in drug efflux. J Ocul Pharmacol Ther. 2009;25(2):121–32.
Karla PK, Earla R, Boddu SH, Johnston TP, Pal D, Mitra A. Molecular expression and functional evidence of a drug efflux pump (BCRP) in human corneal epithelial cells. Curr Eye Res. 2009;34(1):1–9.
Becker U, Ehrhardt C, Daum N, Baldes C, Schaefer UF, Ruprecht KW, et al. Expression of ABC-transporters in human corneal tissue and the transformed cell line, HCE-T. J Ocul Pharmacol Ther. 2007;23(2):172–81.
Karla PK, Pal D, Quinn T, Mitra AK. Molecular evidence and functional expression of a novel drug efflux pump (ABCC2) in human corneal epithelium and rabbit cornea and its role in ocular drug efflux. Int J Pharm. 2007;336(1):12–21.
Ranta VP, Laavola M, Toropainen E, Vellonen KS, Talvitie A, Urtti A. Ocular pharmacokinetic modeling using corneal absorption and desorption rates from in vitro permeation experiments with cultured corneal epithelial cells. Pharm Res. 2003;20(9):1409–16.
Toropainen E, Ranta VP, Vellonen KS, Palmgren J, Talvitie A, Laavola M, et al. Paracellular and passive transcellular permeability in immortalized human corneal epithelial cell culture model. Eur J Pharm Sci. 2003;20(1):99–106.
Toropainen E, Ranta VP, Talvitie A, Suhonen P, Urtti A. Culture model of human corneal epithelium for prediction of ocular drug absorption. Invest Ophthalmol Vis Sci. 2001;42(12):2942–8.
Saarinen-Savolainen P, Jarvinen T, Araki-Sasaki K, Watanabe H, Urtti A. Evaluation of cytotoxicity of various ophthalmic drugs, eye drop excipients and cyclodextrins in an immortalized human corneal epithelial cell line. Pharm Res. 1998;15(8):1275–80.
Wan WJ, Cui DM, Yang X, Hu JM, Li CX, Hu SL, et al. Expression of adenosine receptors in human retinal pigment epithelium cells in vitro. Chin Med J (Engl). 2011;124(8):1139–44.
Mannermaa E, Vellonen KS, Ryhanen T, Kokkonen K, Ranta VP, Kaarniranta K, et al. Efflux protein expression in human retinal pigment epithelium cell lines. Pharm Res. 2009;26(7):1785–91.
Constable PA, Lawrenson JG, Dolman DE, Arden GB, Abbott NJ. P-Glycoprotein expression in human retinal pigment epithelium cell lines. Exp Eye Res. 2006;83(1):24–30.
Maenpaa H, Gegelashvili G, Tahti H. Expression of glutamate transporter subtypes in cultured retinal pigment epithelial and retinoblastoma cells. Curr Eye Res. 2004;28(3):159–65.
Kennedy BG, Mangini NJ. P-glycoprotein expression in human retinal pigment epithelium. Mol Vis. 2002;8:422–30.
Talluri RS, Katragadda S, Pal D, Mitra AK. Mechanism of l-ascorbic acid uptake by rabbit corneal epithelial cells: evidence for the involvement of sodium-dependent vitamin C transporter 2. Curr Eye Res. 2006;31(6):481–9.
Vadlapudi AD, Vadlapatla RK, Kwatra D, Earla R, Samanta SK, Pal D, et al. Targeted lipid based drug conjugates: a novel strategy for drug delivery. Int J Pharm. 2012;434(1–2):315–24.
Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45.
Vadlapudi AD, Vadlapatla RK, Mitra AK. Sodium dependent multivitamin transporter (SMVT): a potential target for drug delivery. Curr Drug Targets. 2012;13(7):994–1003.
Vellonen KS, Hakli M, Merezhinskaya N, Tervo T, Honkakoski P, Urtti A. Monocarboxylate transport in human corneal epithelium and cell lines. Eur J Pharm Sci. 2010;39(4):241–7.
Maenpaa H, Mannerstrom M, Toimela T, Salminen L, Saransaari P, Tahti H. Glutamate uptake is inhibited by tamoxifen and toremifene in cultured retinal pigment epithelial cells. Pharmacol Toxicol. 2002;91(3):116–22.
Toimela TA, Tahti H. Effects of mercuric chloride exposure on the glutamate uptake by cultured retinal pigment epithelial cells. Toxicol In Vitro. 2001;15(1):7–12.
Luo S, Kansara VS, Zhu X, Mandava NK, Pal D, Mitra AK. Functional characterization of sodium-dependent multivitamin transporter in MDCK-MDR1 cells and its utilization as a target for drug delivery. Mol Pharm. 2006;3(3):329–39.
Said HM, Redha R, Nylander W. Biotin transport in the human intestine: site of maximum transport and effect of pH. Gastroenterology. 1988;95(5):1312–7.
Prasad PD, Ganapathy V. Structure and function of mammalian sodium-dependent multivitamin transporter. Curr Opin Clin Nutr Metab Care. 2000;3(4):263–6.
Jwala J, Vadlapatla RK, Vadlapudi AD, Boddu SH, Pal D, Mitra AK. Differential expression of folate receptor-alpha, sodium-dependent multivitamin transporter, and amino acid transporter (B ((0, +))) in human retinoblastoma (Y-79) and retinal pigment epithelial (ARPE-19) cell lines. J Ocul Pharmacol Ther Off J Assoc Ocul Pharmacol Ther. 2012;28(3):237–44.
ACKNOWLEDGMENTS
This study has been supported by NIH grants R01EY09171-16 and R01EY010659-14. The authors would like to acknowledge Dr. Vadivel Ganapathy and Dr. Pamela Martin from the Department of Biochemistry and Molecular Biology at Georgia Health Sciences University for their generous help in providing human corneal RNA used in our studies. Also, the authors would like to thank Matthew Scrivner at the UMKC Writing Center for his assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 82 kb)
Rights and permissions
About this article
Cite this article
Vadlapudi, A.D., Vadlapatla, R.K., Pal, D. et al. Functional and Molecular Aspects of Biotin Uptake via SMVT in Human Corneal Epithelial (HCEC) and Retinal Pigment Epithelial (D407) Cells. AAPS J 14, 832–842 (2012). https://doi.org/10.1208/s12248-012-9399-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-012-9399-5