Abstract
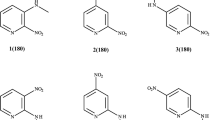
Semiempirical CNDO, AM1, PM3 and ab initio HF/STO-3G, HF/3-21G(d), and HF/6-31(d) methods were employed in the geometry optimization of the phenothiazine and the corresponding radical cation. The results obtained from the PM3 performances were as good as those from the ab initio calculations in the structure optimization of both phenothiazine and phenothiazine radical cation. The PM3 method was used to optimize the structures of a series of N-substituted phenothiazine derivatives and their radical cations. The PM3-optimized results were then analyzed with the ab initio calculation at the 6-311G(d,p) level, which yielded the total energy, frontier molecular orbitals, dipole moments, and charge and spin density distributions of the phenothiazine derivatives and their radical cations.
Similar content being viewed by others
References
P. Mitchell, Austral. New Zealand J. Psych. 27, 370 (1993).
C.M. Gooley, H. Keyzerr, and F. Setchell, Nature 223, 80 (1969).
W.J. Albery, A.W. Foulds, K.J. Hall, A.R. Hillman, R.G. Edgell, and A.F. Orchard, Nature 282, 793 (1979).
I.I. Abu-Abdoun and A. Ledwith, Eur. Polym. J. 33, 1671 (1997).
T. Yamamura, K. Suzuki, T. Yamaguchi, and T. Nishiyama, Bull, Chem. Soc. Jpn. 70, 413 (1997).
N.J. Turro, I.V. Khudyakov, and H. van Willigen, J. Am. Chem. Soc. 117, 12273 (1995).
E. Bosch and J.K. Kochi, J. Chem. Soc. Perkin Tran. 1 1057 (1995).
S. Nath, H. Pal, D.K. Palit, A.V. Sapre, and J.P. Mittal, J. Phys. Chem. A 102, 5822 (1998).
H.-M. Zhang, X.-Q. Ruan, Q.-X. Guo, and Y.-C. Liu, Res. Chem. Intermed. 24, 687 (1998).
H.-M. Zhang, X.-Q. Ruan, X.-Q. Guo and Y.-C. Liu, Chem. Lett. 449 (1998).
D. Clarke, B.C. Gilbert, P. Hanson, C.M. Kirk, J. Chem. Soc. Perkin Trans.2 1103 (1978).
X.-Q. Zheng, X.-Q. Ruan, W. Wang, H.-M. Zhang, Q.-X. Guo, and Y.-C. Liu, Bull. Chem. Soc. Jpn. 72, 253 (1999).
S.C. Chu and D.V. Helm, Acta Cryst. B30, 2489 (1974).
J.J.H. McDowell, Acta Cryst. B32, 5 (1976).
T. Uchida, M. Ito, and K. Kozawa, Bull. Chem. Soc. Jpn. 56, 577 (1983).
M.C. Apreda, F.H. Cano, C. Foces-Foces, F. Lopez-Ruperez, J.C. Conesa, and J. Soria, J. Chem. Soc. Perkin Trans.2 575 (1987).
Y.-C. Liu, Y.-B. Ding, and Z.-L. Liu, Acta Chem. Sin. 48, 1199 (1990).
Y.-C. Liu, Y.-B. Ding, and Z.-L. Liu, JIEGOUHUAXUE 8, 140 (1989).
X.-S. Gao, J.-K. Feng, Q. Jia, Y.-C. Liu, and J.-Z. Sun, Acta Chem. Sin. 54, 1159 (1996).
J.-K. Feng, X.-S. Gao, Q. Jia, Y.-C. Liu, and C.-C. Sun, Chem. J. Chin. Univ. 17, 925 (1996).
V.K. Turchaninov, A.I. Vokin, and N.N. Chipanina, Russ. Chem. Bull. 47, 8 (1998).
GAUSSIAN 94, Revision B.1, M.J. Frisch, G.W. Trucks, H.B. Schlegel, P.M.W. Gill, B.G. Johnson, M.A. Robb, J.R. Cheeseman, T. Keith, G.A. Petersson, J.A. Montgomery, K. Raghavachari, M.A. Al-Laham, V.G. Zakrzewski, J.V. Ortiz, J.B. Foresman, J. Cioslowski, B.B. Stefanov, A. Nanayakkara, M. Challacombe, C.Y. Peng, P.Y. Ayala, W. Chen, M.W. Wong, J.L. Andres, E.S. Replogle, R. Gomperts, R.L. Martin, D.J. Fox, J.S. Binkley, D.J. Defrees, J. Baker, J.P. Stewart, M. Head-Gordon, C. Gonzalez, and J.A. Pople, Gaussian Inc., Pittsburgh PA, 1995.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, XS., Liu, L., Mu, TW. et al. A theoretical study on the structure and properties of phenothiazine derivatives and their radical cations. Res Chem Intermed 26, 375–384 (2000). https://doi.org/10.1163/156856700X00327
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1163/156856700X00327