Abstract
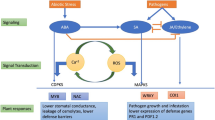
EREBP (ethylene responsive element binding protein) and WRKY (wizz-like transcription factor) are known to play roles in plant tolerance to abiotic stresses, such as low temperatures. Previously, we used cDNA microarrays and Northern blot analysis to determine the mechanisms responsible for those underlying defenses. These analyses led to the identification of CaEREBP-C1, -C2, -C3 and CaWRKY1A genes that encode the ethylene responsive element binding protein and wizz-like transcription factors, respectively, from hot pepper (Capsicum annuum). In that study, we demonstrated that the CaEREBP-C1, -C2, -C3 and CaWRKY1A genes were strongly induced by cold stress. Here, we used Tiplasmid and Agrobecterium-mediated transformation to engineer CaEREBP-C1, -C2, -C3 and CaWRKY1A under control of the CaMV 35S promoter for constitutive expression in transgenic plants. The resultant transgenic plants exhibited significantly increased tolerance to low temperatures. In addition, none of the CaEREBP-C1, -C2, -C3 and CaWRKY1A transgenic plants showed visible phenotypic alteration when compared to wild type plants. Taken together, these results suggest that CaEREBP-C1, -C2, -C3 and CaWRKY1A play important biological roles in conferring plant cold stress tolerance.
Similar content being viewed by others
References
An D, Yang J, Zhang P (2012) Transcriptome profiling of low temperature treated cassava apical shoots showed dynamic responses of tropical plant to cold stress. BMC Genomics 13:64
Bartel DP (2004) MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 116:281–297
Bohnert HJ, Ajoubi P, Borchert C, Bressan RA, Burnap RL, Cushman JC, Cushman MA, Deyholos M, Fischer R, Galbraith DW, Hasegawa PM, Jenks M, Kawasaki S, Koiwa H, Kore-eda S, Lee BH, Michalowski CB, Misawa E, Nomura M, Ozturk N, Postier B, Prade R, Song CP, Tanaka Y, Wang H, Zhu JK (2001) A genomics approach towards salt stress tolerance. Plant Physiol Biochem 39:295–311
Cai M, Qiu D, Yuan T, Ding X, Li H, Duan L, Xu C, Li X, Wang S (2008) Identification of novel pathogen-responsive cis-elements and their binding proteins in the promoter of OsWRKY13, a gene regulating rice disease resistance. Plant Cell Environ 31: 86–96
Chen W, Provart NJ, Glazebrook J, Katagiri F, Chang HS, Eulgem T, Mauch F, Luan S, Zou G, Whitham SA, Budworth PR, Tao Y, Xie Z, Chen X, Lam S, Kreps JA, Harper JF, Si-Ammour A, Mauch-Mani B, Heinlein M, Kobayashi K, Hohn T, Danl JL, Wang X, Zhu T (2002) Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. Plant Cell 14:559–574
Cheong YH, Chang HS, Gupta R, Wang X, Zhu T, Luan S (2002) Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiol 129:661–677
Ciolkowski I, Wanke D, Birkenbihl R, Somssich I (2008) Studies on DNA binding selectivity of WRKY transcription factors lend structural clues into WRKY-domain function. Plant Mol Biol 68:81–92
Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J 33:751–763
Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci 5:199–206
Eulgem T, Somssich IE (2007) Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol 10:366–371
Fujimoto SY, Ohta M, Usui A, Shinshi H, Ohme-Takagi M (2000) Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell 12:393–404
Fujita K, Komatsu K, Tanaka K, Ohshima S, Asami Y, Murata E, Akita M (2006) An in vitro model for studying vascular injury after laser microdissection. Histochem Cell Biol 125:509–514
Giacomelli JI, Weigel D, Chan RL, Manavella PA (2012) Role of recently evolved miRNA regulation of sunflower HaWRKY6 in response to temperature damage. New Phytologist 195:766–773
Gutterson N, Reuber TL (2004) Regulation of disease resistance pathways by AP2/ERF transcription factors. Curr Opin Plant Biol 7:465–471
Hao D, Ohme-Takagi M, Sarai A (1998) Unique mode of GCC box recognition by the DNA-binding domain of ethylene-responsive element-binding factor (REF domain) in plant. J Biol Chem 273: 26857–26861
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2002) Plant cellular and molecular response to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Holster M, De Waele D, Depicker A, Messens E, Van Montagu M, Schell J (1978) Transfection and Transformation of Agrobacterium tumefaciens. Mol Gen Genet 163:181–187
Hwang EW, Kim KA, Park SC, Jeong MJ, Byun MO, Kwon HB (2005) Expression profiles of hot pepper(Capsicum annuum) genes under cold stress conditions. J Biosci 30:657–667
Hwang EW, Park SC, Byun MO, Choi M, Kwon HB (2008) Overexpression of zinc finger protein of Capsicum annuum (PIF1) in tobacco enhances cold tolerance. Genes Genomics 30: 93–99.
Ito Y, Katsura K, Maruyama K, Taji T, Kobayashi M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2006) Functional analysis of rice DREB1/CBF-type transcription factors involved in coldresponsive gene expression in transgenic rice. Plant Cell Physiol 47:141–153
Jaglo KR, Kleff S, Amundsen KL, Zhang X, Haake V, Zhang JZ (2001) Components of the Arabidopsis CRepeat/Dehydrationresponsive element binding factor cold-response pathway are conserved in Brassica napus and other plant species. Plant Physiol 127:910–917
Jian Y, Deyholos MK (2006) Comprehensive transcriptional profiling of NaCl-stressed Arabidopsis roots reveals novel classes of responsive genes. BMC Plant Biol 6:25
Jones-Rhoades MW, Bartel DP, Bartel B (2006) MicroRNAs and their regulatory roles in plants. Annu Rev Plant Biol 57:19–53
Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2012) AP2/ERF family transcription factiors in plant abiotic stress responses. Biochem Biophys Acta 1819:86–96
Kalde M, Barth M, Somssich IE, Lippok B (2003) Members of the Arabidopsis WRKY group III transcription factors are part of different plant defense signaling pathways. Mol Plant Microbe Interact 16:295–305
Khraiwesh B, Zhu JK, Zhu J (2012) Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants. Biochim Biophys Acta 1819:137–148
Kim J, Jung JH, Reyes JL, Kim YS, Kim SY, Chung KS, Kim JA, Lee M, Lee Y, Narry Kim V (2005) MicroRNA-directed cleavage of ATHB15 miRNA regulates vascular development in Arabidopsis inflorescence stems. Plant J 42:84–94
Kim SH, Kim JY, Kim SJ, An KS, An G, Kim SR (2007) Isolation of cold stress-responsive genes in the reproductive organs, and characterization of the OsLti6b gene from rice (Oryza sativa L.). Plant Cell Rep 26:1097–1110
Kizis D, Lumbreras V, Pages M (2001) Role of AP2/EREBP transcription factors in gene regulation during abiotic stress. FEBS Lett 498:187–189
Khraiwesh B, Zhu J K, Zhu J (2011) Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants. BMC Plant Biol 12: 28
Knight H, Knight MR (2001) Abiotic stress signaling pathway: specificity and cross-talk. Trends Plant Sci 6:262–267
Kreps JA, Wu Y, Chang HS, Zhu T, Wang X, Harper (2002) Transcriptome changes for Arabidopsis in response to salt, osmotic and cold stress. Plant Physiol 130:2129–2141
Kwon HB, Park SC, Peng HP, Goodman HM, Dewdney J, Shih MC (1994) Identification of light responsive region of the nuclear gene encoding the B subunit of chloroplast glyceraldehyde 3-phosphate dehydrogenase from Arabidopsis thaliana. Plant Physiol 105:357–367
Lee HE, Shin D, Park SR, Han SE, Jeong MJ, Kwon TR, Lee SK, Park SC, Yi BY, Kwon HB, Byun MO (2007) Ethylene responsive element binding protein 1 (StEREBP1) from Solanum tuberosum increases tolerance to abiotic stress in transgenic potato plants. Biochem Biophys Res Comm 353:863–868
Liu JG, Zhang Z, Qin QL, Peng RH, Xiong AS, Chen JM, Xu F, Zhu H, Yao QH (2007) Isolated and characterization of a cDNA encoding ethylene-responsive element binding protein (EREBP)/AP2-type protein, RCBF2, in Oryza sativa L. Biotechnol Lett 29:165–173
Liu HH, Tian X, Li YJ, Wu C A, Zheng CC (2008) Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA 14:836–843
Llave C, Kasschau KD, Rector M, Carrington JC (2002) Endogenous and silencing-associated small RNAs in plants. Plant Cell 14: 1605–1619
Lu S, Sun YH, Chiang VL (2008) Stress-responsive microRNAs in Populus. Plant J 55:131–151.
Murchison EP, Hannon GJ (2004) miRNAs on the move: miRNA biogenesis and the RNAi machinery. Curr Opin Cell Biol 16: 223–229
Narusaka Y, Narusaka M, Park P, Kubo Y, Hirayama T, Seki M, Shiraishi T, Ishida J, Nakashima M, Enju A, Sakurai T, Satou M, Kobayashi M, Shinozaki K (2004) RCH1, a locus in Arabidopsis that confers resistance to the hemibiotrophic fungal pathogen Colletotrichum Higginsianum. Mol Plant Microbe Interact 17:749–762
Ohme-Takagi M, Suzuki K, Shinshi H (2000) Regulation of ethyleneinduced transcription of defense genes. Plant Cell Physiol 41:1187–1192
Pandrey SP, Somssich IE (2009) The role of WRKY transcription factors in plant immunity. Plant Physiol 150:1648–1655
Park W, Li J, Song R, Messing J, Chen X (2002) CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr Biol 12: 1484–1495
Phillips JR, Dalmay T, Bartels D (2007) The role of small RNAs in abiotic stress. FEBS Lett 581:3592–3597
Qiang L, Nanming Z, Yamaguchi-Shinozaki K, Shinozaki K (2000) Regulatory role of DREB transcription factors in plant drought, salt and cold tolerance. Chinese Sci Bull 45:970–975
Reinhart BJ, Weinstein EG, Rhoades MW, Bartel B, Bartel DP (2002) MicroRNAs in plants. Genes Dev 16:1616–1626
Robatzek S, Somssich IE (2002) Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev 16: 1139–1149
Ross CA, Liu Y, Shen QJ (2007) The WRKY gene family in rice (Oryza sativa). J Integr Plant Biol 49:827–842
Rushton PJ, Reinstadler A, Lipka V, Lippok B, Somssich IE (2002) Synthetic plant promoters containing defined regulatory elements provide novel insight into pathogen- and wound-induced signaling. Plant Cell 14:749–762
Rushton PJ, Somssich IE (1998) Transcriptional control of plant genes responsive to pathogens. Curr Opin. Plant Biol 1:311–315
Sambrook J, Russell DW (2001) In Molecular Cloning; A Laboratory Manual, (3rd ed.). Cold Spring Harbor Laboratory Press, New York, NY, U.S.A.
Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, Manners JM (2000) Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA 97:11655–11660
Seki M, Ishida J, Narusaka M, Fujita M, Nanjo T, Umezawa T, Kamiya A, Nakajima M, Enju A, Sakurai T, Satou M, Akiyama K, Yamaguchi-Shinozaki K, Carninci P, Kawai J, Hayashizaki Y, Shinozaki K (2002) Monitoring the expression pattern of around 7,000 Arabidopsis genes under ABA treatments using a fulllength cDNA microarray. Funct Integr Genomics 2:282–291
Seki M, Narusaka M, Abe H, Kasuga M, Yamaguchi-Shinozaki K, Carninci P, Hayashizaki Y, Shinozaki K (2001) Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell 13:61–72
Shigyo M, Hasebe M, Ito M (2006) Molecular evolution of the AP2 subfamily. Gene 366:256–265
Shinozaki K, Yamaguchi-Shinozaki K (1997) Gene expression and signal transduction in water stress response. Plant Physiol 115: 327–334
Shinozaki K, Yamaguchi-Shinozaki K, Seki M (2003) Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol 6:410–417
Singh K, Foley RC, Onate-Sanchet L (2002) Transcription factors in plant defense and stress respoonses. Curr Opin Plant Biol 5:430–436
Sun C, Palmqvist S, Olsson H, Borén M, Ahlandsberg S, Jansson C (2003) A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter. Plant Cell 15:2076–2092
Sunkar R, Zhu JK (2004) Novel and stress regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16:2001–2019
Thara VK, Tang X, Gu YQ, Martin GB, Zhou JM (1999) Pseudomonas syringae pv. tomato induces the expression of tomato EREBP-like genes Pti4 and Pti5 independent of ethylene, salicylate and jasmonate. Plant J 20:475–483
Thomashow MF (1999) Plant cold acclimation: Freezing tolerance genes and regulatory mechanism. Annu Rev Plant Physiol Plant Mol Biol 50:571–599
van Verk MC, Pappaioannou D, Neeleman L, Bol JF, Linthorst HJM (2008) A novel WRKY transcription factor is required for induction of PR-1A gene expression by salicylic acid and bacterial elicitors. Plant Physiol 146:1983–1995
Wu L, Zhou M, Shen C, Liang J, Lin J (2012) Transgenic tobacco plants over expressing cold regulated protein CbCOR15b from Capsella bursa-pastoris exhibit enhanced cold toleranace. J Plant Physiol 169:1408–1416
Xiong L, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14:S165–S183
Xu P, Narasimhan ML, Samson T, Coca MA, Huh GH, Zhao J, Martin GB, Hasegawa PM, Bressan RA (1998) A nitrilase-like protein interacts with GCC box DNA-binding proteins involved in ethylene and defense responses. Plant Physiol 118:867–874
Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stress. Annu Rev Plant Biol 57:781–803
Zhang J, Xu Y, Huan Q, Chong K (2009) Deep sequencing of Brachypodium small RNAs at the global genome level identifies microRNAs involved in cold stress response. BMC Genomics 10:449
Zhu J K (2001a) Cell signaling under salt, water and cold stresses. Curr Opin Biotechnol 9:214–219
Zhu J K (2001b) Plant salt tolerance. Trends Plant Sci 6:66–71
Zhu T, Provart NJ (2003) Transcriptional responses to low temperature and their regulation in Arabidopsis. Can J Bot Rev Can Bot 81:1168–1174
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, BK., Lee, JH., Shin, SJ. et al. Molecular characterization of cold stress-related transcription factors, CaEREBP-C1, -C2, -C3, and CaWRKY1A from Capsicum annuum L.. J. Plant Biol. 56, 106–114 (2013). https://doi.org/10.1007/s12374-012-0367-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-012-0367-5