Abstract
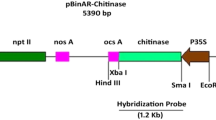
A Rice chitinase-3 under enhance version of CaMV 35S was introduced into peanut (Arachis hypogaea L.) through Agrobacterium mediation. Agrobacterium tumefaciens strain LB4404 was used harboring the binary vector (pB1333-EN4-RCG3) containing the chitinase (chit) and hygromycin resistance (hpt) gene as selectable marker. Putative transgenic shoots were regenerated and grown on MS medium supplemented with 5 mg/l BAP, 1 mg/l kinetin, and 30 mg/l hygromycin. Elongated shoots were examined for the presence of the integrated rice chitinase gene along with hygromycin gene as selectable. The integration pattern of transgene in the nuclear genome of the putative transformed plants (T0) was confirmed through Southern hybridization analysis of the genomic DNA. Survival rate of the in vitro regenerated plantlets was over 60% while healthy putatively transgenic (T0) plants with over 42% transformation frequency were produced through Agrobacterium mediated gene transfer of the rice chitinase gene and all the plants flowered and set seed normally. T1 plants were tested for resistance against Cercospora arachidicola by infection with the microspores. Transgenic strains exhibited a higher resistance than the control (non-transgenic plants). chitinase gene expression in highly resistant transgenic strains was compared to that of a susceptible control. A good correlation was observed between chitinase activity and fungal pathogen resistance.







Similar content being viewed by others
Abbreviations
- BAP:
-
Benzyl amino purine
- SIM:
-
Shoot induction medium
- RIM:
-
Root induction medium
- LB:
-
Luria broth
References
Broglie, K., Chet, I., Holliday, M., Cressman, R., Biddle, P., Knowlton, S., et al. (1991). Transgenic plants with enhanced resistance to the fungal pathogen Rizoctonia solani. Science, 254, 1194–1197.
Grison, R., Grezes-Besset, B., Schneider, M., Lucante, N., Olsen, L., Leguay, J. J., et al. (1996). Field tolerance to fungal pathogens of Brassica napus constitutively expressing a chimeric chitinase gene. Nature Biotechnology, 14, 643–646.
Taylor, M. G., & Vasil, I. K. (1991). Histology of and physical factors affecting transient GUS expression in pearl millet (Pennisetum glaucum (L.) R. Br.) embryos following microprojectile bombard-ment. Plant Cell Reports, 10, 120–125.
Devi, P., Zhong, H., & Sticklen, M. B. (2000). In vitro morphogenesis of pearl millet [Pennisetum glaucum (L.) R. Br.]: Efficient production of multiple shoots and inflorescences from shoot-apices. Plant Cell Reports, 19, 546–550.
Naeem, U. D., Mahmood, A., Shah, G. S., Saeed, I., & Hassan, M. F. (2009). High yielding groundnut (Arachis hypogaea L.) variety “GOLDEN”. Pakistan Journal of Botany, 41(5), 2217–2222.
Murashige, T., & Skoog, K. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum, 15, 473–497.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: A laboratory manual. New York: Cold Spring Harbor Laboratory Press.
Murray, M. G., & Thompson, W. F. (1980). Rapid isolation of high molecular weight plant DNA. Nucleic Acids Research, 19, 4321–4326.
Satyavathi, V. V., Prasad, V., Khandelwal, A., Shaila, M. S., & Sita, G. L. (2003). Expression of haemagglutinin protein of Rinderpest virus in transgenic pigeonpea [Cajanus cajan (L.) Millsp.] plant. Plant Cell Reports, 21, 651–658.
Eapen, S., & George, L. (1994). Agrobacterium tumefaciens-mediated gene transfer in peanut (Arachis hypogaea L.). Plant Cell Reports, 13, 582–586.
Cheng, M., Jarret, R. L., Li, Z., Xing, A., & Demski, J. W. (1996). Production of fertile transgenic peanut (Arachis hypogaea L.) plants using Agrobacterium tumefaciens. Plant Cell Reports, 15, 653–657.
Rohini, V. K., & Sankara, R. K. (2001). Transformation of peanut (Arachis hypogaea L.) with tobacco chitinase gene: Variable response of transformants to leaf spot disease. Plant Science, 160, 883–892.
Kone, M., Kouakou, T. H., Kone, D., Kouadio, Y. J., Zouzou, M., & Ochatt, S. J. (2009). Factors affecting regeneration of bambara peanut [Vigna subterranea (L.) Verdc.] from mature embryo axes. In Vitro Cellular and Developmental Biology—Plant, 45, 769–775.
Venkatachalam, P., Geetha, N., Khandelwal, A., Shaila, M. S., & LakshmiSita, G. (2000). Agrobacterium-mediated genetic transformation and regeneration of transgenic plants from cotyledon explants of peanut (Arachis hypogaea L.) via somatic embryogenesis. Current Science, 78, 1130–1136.
Sharma, K. K., & Anjaiah, V. (2000). An efficient method for the production of transgenic plants of peanut (Arachis hypogaea L.) through Agrobacterium tumefaciens-mediated genetic transformation. Plant Science, 159(1), 7–19.
Yang, H. Y., Nairn, J., & Ozias-Akins, P. (2003). Transformation of peanut using a modified bacterial mercuric ion reductase gene driven by an actin promoter from Arabidopsis thaliana. Journal of Plant Physiology, 160, 945–952.
Anuradha, T. S., Jami, S. K., Datla, R. S., & Kirti, P. B. (2006). Genetic transformation of peanut (Arachis hypogaea L.) using cotyledonary node as explant and a promoterless gus:nptII fusion gene based vector. Journal of Biosciences, 31, 235–246.
Mikula, A., Tykarska, T., Kuras, M., & Rybczynski, J. J. (2005). Somatic embryogenesis of Gentiana Cruciata (L.): Histological and ultrastructural changes in seedling hypocotyl explant. In Vitro Cellular and Developmental Biology—Plant, 41, 686–694.
Tiwari, S., & Rakesh, T. (2008). Factors promoting efficient in vitro regeneration from de-embryonated cotyledon explants of Arachis hypogaea L. Plant Cell, Tissue and Organ Culture, 92, 15–24.
Lei, S., Guiying, T., Pingli, X., Zhanji, L., & Yuping, B. (2009). High efficiency in vitro plant regeneration from epicotyls explants of Chinese peanut cultivars. In Vitro Cellular and Developmental Biology—Plant, 45, 525–531.
Matand, K., & Prakash, C. S. (2007). Evaluation of peanut genotypes for in vitro plant regeneration using thidiazuron. Journal of Biotechnology, 130(2), 202–207.
Grant, J. J., Gelvin, S. B., Meilan, R., & Morris, R. O. (1996). Transfer RNA is the source of extracellular isopentyladenine in a Ti-plasmidless strain of Agrobacterium tumefaciens. Plant Physiology, 110, 431–438.
Leeuwen, L. W., Ruttink, T., Borst-Vrenssen, A. W., Van, D. P., Van-Der, L. H., & Krol, A. R. (2001). Characterization of position induced spatial and temporal regulation of transgene promoter activity in plants. Journal of Experimental Botany, 52, 949–959.
Acknowledgments
The authors are highly thankful to acknowledge the cooperation of Dr. Ghulam Muhammad Ali, Chief Scientific Officer, National Institute for Genomics and Advanced Biotechnology (NIGAB), NARC, Islamabad, Pakistan and Director Barani Agricultural Research Institute (BARI) Chakwal, Pakistan for the providing the vector pB1333-EN4-RCG3 carrying rice chitinase gene expression cassette and the seeds of peanut varieties, respectively. The authors also acknowledge the support of Dr. Aish Muhammad for his guidance and support during the studies conducted at Plant Tissue Culture Lab, NARC, Islamabad, Pakistan. The authors are also very thankful to Dr. Daniel Tan, Senior Lecturer, University of Sydney, Australia for language improvement of this manuscript and Dr. Nausherwan Nobel Nawab for his valuable guidance in writing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iqbal, M.M., Nazir, F., Ali, S. et al. Over Expression of Rice chitinase Gene in Transgenic Peanut (Arachis hypogaea L.) Improves Resistance Against Leaf Spot. Mol Biotechnol 50, 129–136 (2012). https://doi.org/10.1007/s12033-011-9426-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-011-9426-2