Abstract
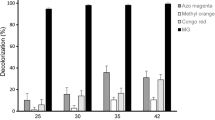
In previous work, decolorization of malachite green (MG) was studied in Aspergillus niger in the presence and absence of calcium chloride stress. Decolorization took place within 24 h, and a signal transduction process that initiated MG decolorization was suggested to be involved. In the present study, further investigation of the relationship between calcium chloride stress and enhanced MG biodegradation was conducted at the sub-cellular level. MG-NADH reductase activity, a key enzyme in MG decolorization, was produced as decolorization commenced, and enzyme activity increased threefold upon exposure to calcium chloride. Inhibitors of cytochrome p450, Ca2+ channel activity as well as activity of the signaling protein phosphoinositide 3-kinase were tested. All three activities were inhibited to different extents resulting in reduced MG decolorization. Spectral analysis of the mitochondrial fraction showed a heme signal at 405 nm and A405/A280 ratio that is characteristic of the porphoryin ring of cytochromes. There were no peaks detected for cytochromes a or b, but a shoulder appearing at 550 nm was observed, which suggested that cytochrome c is involved; the absorbance for cytochrome c doubled after calcium chloride stress supporting this idea. MG decolorization took place via a series of demethylation steps, and cytotoxicity analysis revealed a decrease in the toxicity associated with generation of leucomalachite green.







Similar content being viewed by others
References
Jaszek, M., Grzywnowicz, K., Malarczyk, E., & Leonowicz, A. (2006). Enhanced extracellular laccase activity as a part of the response system of white rot fungi: Trametes versicolor and Abortiporus biennis to paraquat-caused oxidative stress conditions. Pesticide Biochemistry and Physiology, 85, 147–154.
Li, Q., McNeil, B., & Harvey, L. M. (2008). Adaptive response to oxidative stress in the filamentous fungus Aspergillus niger B1-D. Free Radical Biology and Medicine, 44, 394–402.
Gomaa, O. (2012). Ethanol induced response in Phanerochaete chrysosporium and its role in the decolorization of triarylmethane dye. Annals of Microbiology, 62, 1403–1409.
Gomaa, O. M., Selim, N. S., & Linz, J. E. (2012). Biochemical and biophysical response to calcium chloride stress in Aspergillus niger and its role in malachite green degradation. Cell Biochemistry and Biophysics, 65, 413–423. doi:10.1007/s12013-012-9444-0.
Jasinska, A., Rozalska, S., Bernat, P., Paraszkiewicz, K., & Dlugonski, J. (2012). Malachite green decolorization by non-basidiomycete filamentous fungi of Penicillium pinophilum and Myrothecium roridum. International Biodeterioration and Biodegradation, 73, 33–40.
Stammati, A., Nebbia, C., De Angelis, I., Albo, A. G., Carletti, M., Rebecchi, C., et al. (2005). Effects of malachite green (MG) and its major metabolite, leucomalachite green (LMG), in two human cell lines. Toxicology in Vitro, 19, 853–858.
Kim, Y. H., Lee, C., Go, H., Konishi, K., Lee, K., Lau, P. C. K., et al. (2010). Decolorization of malachite green by cytochrome c in mitochondria of the fungus Cunninghamella elegans. Archives of Biochemistry and Biophysics, 494, 159–165.
Ali, H., Ahmad, W., & Haq, T. (2009). Decolorization and degradation of malachite green by Aspergillus flavus and Alternaria solani. African Journal of Biotechnology, 8, 1574–1576.
Kumar, C. G., Mongolla, P., Joseph, J., & Sarma, V. U. M. (2012). Decolorization and biodegradation of triphenylmethane dye, brilliant green, by Aspergillus sp. Isolated from Ladakh, India. Process Biochemistry, 47, 1388–1394.
Ui, M., Okada, T., Hazeki, K., & Hazeki, O. (1995). Wortmannin as a unique probe for an intracellular signaling protein, phosphoinositide 3-kinase. TIBS, 20, 303–307.
Yoshida, Y. (1988). Cytochrome p450 of fungi: primary target for azole antifungal agents. Current Topics in Medical Mycology, 2, 388–418.
Huang, P., Hawthome, W. J., Peng, A., Angeli, G. L., Medbury, H. J., & Fletcher, J. P. (2001). Calcium channel antagonist verapamil inhibits neointimal formation and enhances apoptosis in a vascular graft model. The American Journal of Surgery, 181, 492–498.
Moturi, B., & Singara Charya, M. A. (2010). Triphenyl methane reductase enzyme assay and PCR-based detection of tmr gene in Mucor mucedo. World Applied Science Journal, 10, 462–465.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with phenol-folin reagent. Journal of Biological Chemistry, 193, 265–275.
Hansen, M. B., Nielsen, S. E., & Berg, K. (1989). Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. Journal of Immunological Methods, 119, 203–210.
Jadhav, J. P., & Govindwar, S. P. (2006). Biotransformation of malachite green by Saccharomyces cerevisiae MTCC 463. Yeast, 23, 315–323.
Jang, M. S., Lee, Y. M., Kim, C. H., Lee, J. H., Kang, D. W., Kim, S. J., et al. (2005). Triphenylmethane reductase from Citrobacter sp. strain KCTC18061P: purification, characterization, gene cloning, and overexpression of a functional protein in Escherichia coli. Applied and Environmental Microbiology, 71, 7955–7960.
Silverman-Gavrila, L. B., & Lew, R. R. (2002). An IP3-activated Ca+2 channel regulates fungal tip growth. Journal of Cell Science, 115, 5013–5025.
Xiao, X., Xu, C. C., Wu, Y. M., Cai, P. J., Li, W. W., Du, D. L., et al. (2012). Biodecolorization of Naphthol Green B dye by Shewanella oneidensis MR-1 under anaerobic conditions. Bioresource Technology, 110, 86–90.
Kolekar, Y. M., Nemade, H. N., Markad, V. L., Adav, S. S., Patole, M. S., & Kodam, K. M. (2012). Decolorization and biodegradation of azo dye, reactive blue 59 by aerobic granules. Bioresource Technology, 104, 818–822.
Ott, M., Robertson, J. D., Gogvadze, V., Zhivotovsky, B., & Orrenius, S. (2002). Cytochrome c release from mitochondria proceeds by a two-step process. PNAS, 99, 1259–1263.
Cha, C. J., Doerge, D. R., & Cerniglia, C. E. (2001). Biotransformation of malachite green by the fungus Cunninghamella elegans. Applied and Environmental Microbiology, 67, 4358–4360.
Murugesan, K., Yang, I. H., Kim, Y. M., Jeon, J. R., & Chang, Y. S. (2009). Enhanced transformation of malachite green by laccase of Ganoderma lucidum in the presence of natural phenolic compounds. Applied Microbiology and Biotechnology, 82, 341–350.
Parshetti, G., Kalme, S., Saratale, G., & Govindwar, S. (2006). Biodegradation of malachite green by Kocuria rosea MTCC 1532. Acta Chimica Slovica, 53, 492–498.
Fogh, J., Wright, W. C., & Loveless, J. D. (1977). Absence of HeLa cell contamination in 169 cell lines derived from human tumors. Journal of National Cancer Institute, 58, 209–214.
De Angelis, I., Hoogenboom, L. A. P., Huveneers-Oorsprong, M. B. M., Zucco, F., & Stammati, A. (1994). Established cell lines for safety assessment of food contaminants: Differing furazolidone toxicity to V79, HEp-2 and Caco-2 cells. Food and Chemical Toxicology, 32, 481–488.
Gomaa, O., Abdel El Kareem, H., & Fathey, R. (2012). Assessment of the efficacy of Aspergillus sp. EL-2 in textile waste water treatment. Biodegradation, 23, 243–251.
Acknowledgments
This work was supported by US–Egypt joint Grant, Project ID: 750, Science and Technology Development Fund (STDF), Cairo, Egypt. The authors would also like to thank the Cancer Research Laboratory at the road to Nobel, National Research Center, Cairo, Egypt, for providing their laboratory facilities for the cytotoxicity tests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomaa, O.M., Selim, N.S. & Linz, J.E. A Possible Role of Aspergillus niger Mitochondrial Cytochrome c in Malachite Green Reduction Under Calcium Chloride Stress. Cell Biochem Biophys 67, 1291–1299 (2013). https://doi.org/10.1007/s12013-013-9661-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9661-1