Abstract
A system of partial differential equations is derived as a model for the dynamics of a honey bee colony with a continuous age distribution, and the system is then extended to include the effects of a simplified infectious disease. In the disease-free case, we analytically derive the equilibrium age distribution within the colony and propose a novel approach for determining the global asymptotic stability of a reduced model. Furthermore, we present a method for determining the basic reproduction number \(R_0\) of the infection; the method can be applied to other age-structured disease models with interacting susceptible classes. The results of asymptotic stability indicate that a honey bee colony suffering losses will recover naturally so long as the cause of the losses is removed before the colony collapses. Our expression for \(R_0\) has potential uses in the tracking and control of an infectious disease within a bee colony.



Similar content being viewed by others
References
Ball B, Allen M (1988) The prevalence of pathogens in honey bee (Apis mellifera) colonies infested with the parasitic mite Varroa jacobsoni. Ann Appl Biol 113:237–244
Becher MA, Osborne JL, Thorbek P, Kennedy PJ, Grimm V (2013) Review: towards a systems approach for understanding honeybee decline: a stocktaking and synthesis of existing models. J Appl Ecol 50:868–880
Becher MA, Grimm V, Thorbek P, Horn J, Kennedy PJ, Osborne JL (2014) Beehave: a systems model of honeybee colony dynamics and foraging to explore multifactorial causes of colony failure. J Appl Ecol 51:470–482
Betti MI, Wahl LM, Zamir M (2014) Effects of infection on honey bee population dynamics: a model. PLoS ONE 9:e110237
Betti MI, Wahl LM, Zamir M (2016) Age structure is critical to the population dynamics and survival of honey bee colonies, Open Science. Accepted
Botias C, Martin-Hernandez R, Barrios L, Meana A, Higes M (2013) Nosema spp. infection and its negative effects on honey bees (Apis mellifera iberiensis) at the colony level. Vet Res 44:1–15
Boyce W, DiPrima R (2008) Differential equations and boundary value problems. Wiley, New York
Brauer F, Castillo-Chavez C, Castillo-Chavez C (2001) Mathematical models in population biology and epidemiology, vol 1. Springer, Berlin
Calderone NW (2012) Insect pollinated crops, insect pollinators and US agriculture: trend analysis of aggregate data for the period 1992–2009. PLoS ONE 7:e37235
Cannon RH (2003) Dynamics of physical systems. Courier Corporation, North Chelmsford
Castillo-Chavez C, Feng Z (1998) Global stability of an age-structure model for TB and its applications to optimal vaccination strategies. Math Biosci 151:135–154
Corless RM, Fillion N (2013) A graduate introduction to numerical methods. AMC 10:12
Diekmann O, Heesterbeek J, Metz JA (1990) On the definition and the computation of the basic reproduction ratio \({R}_0\) in models for infectious diseases in heterogeneous populations. J Math Biol 28:365–382
Domoshnitsky A (2004) Exponential stability of convolution integro-differential equations. Funct Differ Equ 5:297–307
Domoshnitsky A, Goltser Y (2001) One approach to study of stability of integro-differential equations. Nonlinear Anal Theory Methods Appl 47:3885–3896
Dukas R (2008) Mortality rates of honey bees in the wild. Insectes Soc 55:252–255
Eberl HJ, Frederick MR, Kevan PG (2010) Importance of brood maintenance terms in simple models of the honeybee—Varroa destructor—acute bee paralysis virus complex, Electron J Differ Equ [electronic only], 2010, pp 85–98
Fahrbach S, Robinson G (1996) Juvenile hormone, behavioral maturation and brain structure in the honey bee. Dev Neurosci 18:102–114
Goltser Y, Litsyn E (2005) Volterra integro-differential equations and infinite systems of ordinary differential equations. Math Comput Model 42:221–233
Grossmann C, Roos H-G, Stynes M (2007) Numerical treatment of partial differential equations. Springer, Berlin
Hale JK, Waltman P (1989) Persistence in infinite-dimensional systems. SIAM J Math Anal 20:388–395
Heffernan J, Smith R, Wahl L (2005) Perspectives on the basic reproductive ratio. J R Soc Interface 2:281–293
Ho M-W, Cummins J (2007) Mystery of disappearing honeybees. Sci Soc 34:35–36
Huang Z-Y, Robinson GE (1996) Regulation of honey bee division of labor by colony age demography. Behav Ecol Sociobiol 39:147–158
Hyman JM, Li J (2000) An intuitive formulation for the reproductive number for the spread of diseases in heterogeneous populations. Math Biosci 167:65–86
Inaba H (1990) Threshold and stability results for an age-structured epidemic model. J Math Biol 28:411–434
Jaycox ER, Skowronek W, Guynn G (1974) Behavioral changes in worker honey bees (apis mellifera) induced by injections of a juvenile hormone mimic. Ann Entomol Soc Am 67:529–534
Jones JC, Helliwell P, Beekman M, Maleszka R, Oldroyd B (2005) The effects of rearing temperature on developmental stability and learning and memory in the honey bee, Apis mellifera. J Comp Physiol A 191:1121–1129
Khoury DS, Myerscough MR, Barron AB (2011) A quantitative model of honey bee colony population dynamics. PLoS ONE 6:e18491
Khoury DS, Barron AB, Myerscough MR (2013) Modelling food and population dynamics in honey bee colonies. PLoS ONE 8:e59084
Leoncini I, Le Conte Y, Costagliola G, Plettner E, Toth AL, Wang M (2004) Regulation of behavioral maturation by a primer pheromone produced by adult worker honey bees. Proc Natl Acad Sci USA 101:17559–17564
Li J, Brauer F (2008) Continuous-time age-structured models in population dynamics and epidemiology. In: Brauer F, van den Driessche P, Wu J (eds) Mathematical epidemiology. Springer, Berlin, pp 205–227
Magal P, McCluskey C, Webb G (2010) Lyapunov functional and global asymptotic stability for an infection-age model. Appl Anal 89:1109–1140
Martin S (2000) Hygienic behaviour: an alternative view. Bee Improv 7:6–7
Martin SJ (2001) The role of varroa and viral pathogens in the collapse of honeybee colonies: a modelling approach. J Appl Ecol 38:1082–1093
McKendrick A, Pai MK (1911) The rate of multiplication of microorganisms: a mathematical study. In: Proceedings of the Royal Society of Edinburgh, vol. 31, pp 649–655
Neumann P, Carreck NL (2010) Honey bee colony losses. J Apic Res 49:1–6
Odoux J-F, Aupinel P, Gateff S, Requier F, Henry M, Bretagnolle V (2014) Ecobee: a tool for long-term honey bee colony monitoring at the landscape scale in west european intensive agroecosystems. J Apic Res 53:57–66
Olver PJ (2014) Introduction to partial differential equations. Springer, Berlin
Parker S (2003) McGraw-Hill dictionary of scientific and technical terms. McGraw-Hill, New York
Perry CJ, Søvik E, Myerscough MR, Barron AB (2015) Rapid behavioral maturation accelerates failure of stressed honey bee colonies. Proc Natl Acad Sci 112:3427–3432
Petric A, Guzman-Novoa E, Eberl HJ (2016) A mathematical model for the interplay of nosema infection and forager losses in honey bee colonies. J Biol Dyn. doi:10.1080/17513758.2016.1237682
Ratnieks FL, Keller L (1998) Queen control of egg fertilization in the honey bee. Behav Ecol Sociobiol 44:57–61
Ratti V, Kevan PG, Eberl HJ (2013) A mathematical model for population dynamics in honeybee colonies infested with Varroa destructor and the Acute Bee Paralysis Virus. Can Appl Math Q 21:63–93
Ratti V, Kevan PG, Eberl HJ (2015) A mathematical model of the honeybee-Varroa destructor-Acute Bee Paralysis Virus complex with seasonal effects. Bull Math Biol 77(8):1493–1520
Robinson GE, Page RE, Strambi C, Strambi A (1992) Colony integration in honey bees: mechanisms of behavioral reversion. Ethology 90:336–348
Russell S, Barron AB, Harris D (2013) Dynamic modelling of honey bee (Apis mellifera) colony growth and failure. Ecol Model 265:158–169
Sakagami S, Fukuda H (1968) Life tables for worker honeybees. Res Popul Ecol 10:127–139
Schmickl T, Crailsheim K (2007) Hopomo: A model of honeybee intracolonial population dynamics and resource management. Ecol Model 204:219–245
Seeley TD (2009) The wisdom of the hive: the social physiology of honey bee colonies. Harvard University Press, Cambridge
Seeley TD (2010) Honeybee democracy. Princeton University Press, Princeton
Smith GD (1985) Numerical solution of partial differential equations: finite difference methods. Oxford University Press, Oxford
Smith ML (2012) The honey bee parasite Nosema ceranae: Transmissible via food exchange? PLoS ONE 7:e43319
Southwick EE, Southwick L Jr (1992) Estimating the economic value of honey bees (Hymenoptera: Apidae) as agricultural pollinators in the United States. J Econ Entomol 85:621–633
Stevanovic J, Simeunovic P, Gajic B, Lakic N, Radovic D, Fries I, Stanimirovic Z (2013) Characteristics of Nosema ceranae infection in Serbian honey bee colonies. Apidologie 44:522–536
Stewart J (2011) Multivariable calculus. Cengage Learning, Boston
Sumpter DJT, Martin SJ (2004) The dynamics of virus epidemics in Varroa-infested honey bee colonies. J Anim Ecol 73:51–63
Tereshko V, Loengarov A (2005) Collective decision making in honey-bee foraging dynamics. Comput Inf Syst 9:1
Thieme HR (2009) Spectral bound and reproduction number for infinite-dimensional population structure and time heterogeneity. SIAM J Appl Math 70:188–211
Van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180:29–48
van der Steen JJ, Cornelissen B, Donders J, Blacquière T, van Dooremalen C (2012) How honey bees of successive age classes are distributed over a one storey, ten frames hive. J Apic Res 51:174–178
Vance JT, Williams JB, Elekonich MM, Roberts SP (2009) The effects of age and behavioral development on honey bee (apis mellifera) flight performance. J Exp Biol 212:2604–2611
vanEngelsdorp D, Evans J D, Saegerman C, Mullin C, Haubruge E, Nguyen B K, Frazier M, Frazier J, Cox-Foster D, Chen Y, Underwood R, Tarpy D R, Pettis J S (2009) Colony collapse disorder: a descriptive study. PLoS ONE 4:e6481
Wang W, Zhao X-Q (2012) Basic reproduction numbers for reaction–diffusion epidemic models. SIAM J Appl Dyn yst 11:1652–1673
Watanabe ME (2008) Colony collapse disorder: many suspects, no smoking gun. Bioscience 58:384–388
Winston M (1987) The biology of the honey bee. Harvard University Press, Cambridge
Acknowledgements
Funding was provided by Natural Sciences and Engineering Research Council of Canada (Grant No.s R3128A02002 and RGPIN/8103-2011).
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
1.1 Test: Uniform Age Distribution
We test the validity of this bifurcation parameter by reducing Eqs. (70) and (71) to a system in which all parameters are constant with respect to age. In doing so, we find from Eq. (89) that
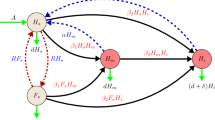
This can be verified by using the next-generation matrix on the infected classes of the following reduced model
which are a reduced form of Eqs. (5) and (7). The ratio of the disease-free equilibrium values of \(F_S,H_S\) will always be such that
This ratio is found by setting \(H_I=F_I=\dfrac{\mathrm {d}{H_S}}{\mathrm {d}{t}}=\dfrac{\mathrm {d}{F_S}}{\mathrm {d}{t}}=0\) in Eqs. (91), (92), (93) and (94).
From these reduced equations, we find the matrices,
which yield the next-generation matrix
Each term in this matrix has a biological interpretation which is the expected number of infections in each class (H or F) caused by a single infected individual in each class. For example, the term
gives the expected number of susceptible hive bees that an infected hive bee will infect while it is still a hive bee. The term
represents the probability that an infected hive bee will be recruited to foraging duties during its life time, multiplied by the expected number of susceptible hive bees that would then become infected. The expected number of susceptible hive bees infected by a single forager is given by
The interpretations for the second row of matrix (98) are similar, but give the expected numbers of susceptible foragers that will become infected.
The basic reproduction number for this uniform age distribution model is then determined by the largest eigenvalue of the matrix \(FV^{-1}\). Since we have the relation (95), matrix (98) is rank 1. Therefore, one of its eigenvalues is zero and the other is given by its trace. We can see that the trace of matrix (98) gives the same expression for the basic reproduction number as (90).
The three terms that appear in (89) are analogous to the three terms that appear in Eq. (90). This suggests that (89) correctly determines not only the threshold for disease persistence, but also correctly estimates the number of secondary infections subsequent to one primary infection (Heffernan et al. 2005).
Rights and permissions
About this article
Cite this article
Betti, M.I., Wahl, L.M. & Zamir, M. Reproduction Number and Asymptotic Stability for the Dynamics of a Honey Bee Colony with Continuous Age Structure. Bull Math Biol 79, 1586–1611 (2017). https://doi.org/10.1007/s11538-017-0300-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-017-0300-7