Abstract
Background, aim, and scope
Changes in bioavailability of phosphorus (P) during pedogenesis and ecosystem development have been shown for geogenic calcium phosphate (Ca-P). However, very little is known about long-term changes of biogenic Ca-P in soil.
Materials and methods
Long-term transformation characteristics of biogenic Ca-P were examined using anthropogenic soils along a chronosequence from centennial to millennial time scales.
Results and discussion
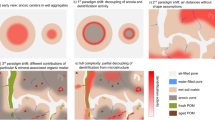
Phosphorus fractionation of Anthrosols resulted in overall consistency with the Walker and Syers model of geogenic Ca-P transformation during pedogenesis. The biogenic Ca-P (e.g., animal and fish bones) disappeared to 3% of total P within the first ca. 2,000 years of soil development. This change concurred with increases in P adsorbed on metal-oxides surfaces, organic P, and occluded P at different pedogenic time. Phosphorus K-edge X-ray absorption near-edge structure (XANES) spectroscopy revealed that the crystalline and therefore thermodynamically most stable biogenic Ca-P was transformed into more soluble forms of Ca-P over time. While crystalline hydroxyapatite (34% of total P) dominated Ca-P species after about 600–1,000 years, β-tricalcium phosphate increased to 16% of total P after 900–1,100 years, after which both Ca-P species disappeared. Iron-associated P was observable concurrently with Ca-P disappearance. Soluble P and organic P determined by XANES maintained relatively constant (58–65%) across the time scale studied.
Conclusions
Disappearance of crystalline biogenic Ca-P on a time scale of a few thousand years appears to be ten times faster than that of geogenic Ca-P.





Similar content being viewed by others
References
Arai Y, Sparks DL (2001) ATR-FTIR spectroscopic investigation on phosphate adsorption mechanisms at the ferrihydrite-water interface. J Colloid Interface Sci 241:317–326
Beauchemin S, Hesterberg D, Chou J, Beauchemin M, Simard RR, Sayers DE (2003) Speciation of phosphorus in phosphorus-enriched agricultural soils using X-ray absorption near-edge structure spectroscopy and chemical fractionation. J Environ Qual 32:1809–1819
Bolan NS, Barrow NJ, Posner AM (1985) Describing the effect of time on sorption of phosphate by iron and aluminum hydroxides. Eur J Soil Sci 36:187–197
Chien SH, Menon RG (1995) Factors affecting the agronomic effectiveness of phosphate rock for direct application. Fert Res 41:227–234
Crews TE, Kitayama K, Fownes JH, Riley RH, Herbert DA, Mueller-Dombois D, Vitousek PM (1995) Changes in soil phosphorus fractions and ecosystem dynamics across a long chronosequence in Hawaii. Ecology 76:1407–1424
Cross AF, Schlesinger WH (1995) A literature review and evaluation of the Hedley fractionation: applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 64:197–214
Gee GW, Or D (2002) Particle-size analysis. In: Dane JH, Topp GC (eds) Methods of soil analysis, Part 4, Physical Methods. Soil Sci Soc Am, Madison, Wisconsin, USA, pp 255–293
Hedin LO, Vitousek PM, Matson PA (2003) Nutrient losses over four million years of tropical forest development. Ecology 84:2231–2255
Holford ICR (1997) Soil phosphorus: its measurement, and its uptake by plants. Aust J Soil Res 35:227–239
Hsu PH (1977) Aluminum oxides and oxyhydroxides. In: Dixon JB, Weed SB (eds) Minerals in soil environments. Soil Sci Soc Am, Madison. Wisconsin, USA, pp 99–144
Hutchison KJ, Hesterberg D, Chou JW (2001) Stability of reduced organic sulfur in humic acid as affected by aeration and pH. Soil Sci Soc Am J 65:704–709
Kern DC, D’Aquino G, Rodrigues TE, Frazão FJL, Sombroek W, Myers TP, Neves EG (2003) Distribution of Amazonian Dark Earths in the Brazilian Amazon. In: Lehmann J (ed) Amazonian Dark Earths: origin, properties and management. Kluwer Academic, Dordrecht, pp 51–75
Lajtha K, Schlesinger WH (1988) The biogeochemistry of phosphorus cycling and phosphorus availability along a desert soil chronosequence. Ecology 69:24–39
Lawrence D, Schlesinger WH (2001) Changes in soil phosphorus during 200 years of shifting cultivation in Indonesia. Ecology 82:2769–2780
Lehmann J, da Silva CM, Zech W (2001) Organic matter stabilization in a Xanthic Ferralsol of the central Amazon as affected by single trees: chemical characterization of density, aggregate, and particle size fractions. Geoderma 99:147–168
Lehmann J, Campos CV, de Macêdo JLV, German L (2004) Sequential P fractionation of relic Anthropogenic Dark Earths of Amazonia. In: Glaser B, Woods WL (eds) Amazonian Dark Earths: explorations in space and time. Springer, Berlin, pp 113–123
Liang B, Lehmann J, Solomon D, Kinyangi J, Grossman J, O’Neill B, Sklemstad JO, Thies J, Luizão FJ, Petersen J, Neves EG (2006) Black carbon increases cation exchange capacity in soils. Soil Sci Soc Am J 70:1719–1730
Lima HN, Schaefer CER, Mello JWV, Gilkes RJ, Ker JC (2002) Pedogenesis and pre-Colombian land use of “Terra Preta Anthrosols” (“Indian black earth”) of Western Amazonia. Geoderma 110:1–17
Lindsay WL, Vlek PLG, Chien SH (1989) Phosphate minerals. In: Dixon JB, Weed SB (eds) Minerals in soil environments, 2nd ed. Soil Sci Soc Am, Madison, Wisconsin, USA, pp 1089–1130
Martin M, Celi L, Barberis E (2002) Extractability and plant availability of phosphate from P-goethite complexes. Commun Soil Sci Plant Anal 33:143–153
McDowell RW, Cade-Menun B, Stewart I (2007) Organic phosphorus speciation and pedogenesis: analysis by solution 31P nuclear magnetic resonance spectroscopy. Eur J Soil Sci 58:1348–1357
McGill WB, Cole CV (1981) Comparative aspects of cycling of organic C, N, S and P through soil organic matter. Geoderma 26:267–286
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Neves EG, Petersen JB, Bartone RN, Silva CAD (2003) Historical and socio-cultural origins of Amazonian Dark Earths. In: Lehmann J (ed) Amazonian Dark Earths: origin, properties and management. Kluwer Academic, Dordrecht, pp 29–50
Parfitt RL, Ross DJ, Coomes DA, Richardson SJ, Smale MC, Dahlgren RA (2005) N and P in New Zealand soil chronosequences and relationships with foliar N and P. Biogeochemistry 75:305–328
Pattan JN, Rao CM, Higgs NC, Colley S, Parthiban G (1995) Distribution of major, trace and rare-earth elements in surface sediments of the Wharton Basin, Indian Ocean. Chem Geol 121:201–215
Peak D, Sims JT, Sparks DL (2002) Solid-state speciation of natural and alum-amended poultry litter using XANES spectroscopy. Environ Sci Technol 36:4253–4261
Rao VP, Naqvi SW, Kumar MD, Cardinal D, Michard A, Borole DV, Jacobs E, Natarajan R (2000) A comparative study of Pleistocene phosphorites from the continental slope off western India. Sedimentology 47:945–960
Ryan J, Zghard MA (1979) Phosphorus transformations with age in a calcareous soil chronosequence. Soil Sci Soc Am J 44:168–169
Sale PWG, Mokwunye AU (1993) Use of phosphate rocks in the tropics. Fertil Res 35:33–45
Sato S, Solomon D, Hyland C, Ketterings QM, Lehmann J (2005) Phosphorus speciation in manure and manure-amended soils using XANES spectroscopy. Environ Sci Technol 39:7485–7491
Schaefer CEG, Lima HN, Gilkes RJ, Mello JWV (2004) Micromorphology and electron microprobe analysis of phosphorus and potassium forms of an Indian Black Earth (IBE) Anthrosol from Western Amazonia. Aust J Soil Res 42:401–409
Schlesinger WH, Bruijnzeel LA, Bush MB, Klein EM, Mace KA, Raikes JA, Whittaker RJ (1998) The biogeochemistry of phosphorus after the first century of soil development on Rakata Island, Kraakatau, Indonesia. Biogeochemistry 40:37–55
Sims JT, Pierzynski GM (2005) Chemistry of phosphorus in soils. In: Tabatabai MA, Sparks DL (eds) Chemical processes in soils. Soil Sci Soc Am, Madison, Wisconsin, USA, pp 151–192
Singleton GA, Lavkulich LM (1987) Phosphorus transformations in a soil chronosequence, Vancouver Island, British Columbia. Can J Soil Sci 67:787–793
Stevens PR, Walker TW (1970) The chronosequence concept and soil formation. Q Rev Biol 45:333–350
Syers JK, Walker TW (1969) Phosphorus transformations in a chronosequence of soils developed on wind-blown sand in New Zealand I. Total and organic phosphorus. J Soil Sci 20:57–64
Tang R, Hass M, Wu W, Gulde S, Nancollas GH (2003a) Constant composition dissolution of mixed phases II. Selective dissolution of calcium phosphates. J Colloid Interface Sci 260:379–384
Tang R, Henneman ZJ, Nancollas GH (2003b) Constant composition kinetics study of carbonated apatite dissolution. J Cryst Growth 239:614–624
Tarawneh K, Moumani K (2006) Petrography, chemistry and genesis of phosphorite concretions in the Eocene Umm Rijam Chert limestone Formation, Ma’an area, South Jordan. J Asian Earth Sci 26:627–635
Thomas RL, Sheard RW, Moyer JR (1967) Comparison of conventional and automated procedures for nitrogen, phosphorus and potassium analysis of plant material using a single digestion. Agron J 59:240–243
Tiessen H, Moir JO (1993) Characterization of available P by sequential extraction. In: Carter MR (ed) Soil sampling and methods of analysis. Can Soc Soil Sci. Lewis, Boca Raton, Florida, USA, pp 75–86
Tiessen H, Stewart JWB (1985) The biogeochemistry of soil phosphorus. In: Caldwell DE, Brierley JA, Brierley CL (eds) Planetary ecology. Van Nostrand Reinhold Company, New York, pp 463–472
Tiessen H, Stewart JW, Cole CV (1984) Pathways of phosphorus transformations in soils of differing pedogenesis. Soil Sci Soc Am J 48:853–858
USDA (1999) Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys, 2nd edn. Natural Resources Conservation Service, USDA, Washington, DC, USA
USEPA (1983) Phosphorus, all forms, Method 365.1. In: Kopp JF, McKee GD (eds) Methods for chemical analysis of water and wastes, EPA-600/4-79-020. USEPA, Cincinnati, pp 365-1.1–365-1.7
USEPA (1995) Microwave assisted acid digestion of siliceous and organically based matrices. In: Test methods for evaluating solid waste, 3rd ed. USEPA, Washington, DC, USA, pp 3052-1-3052-20
Vitousek PM, Farrington H (1997) Nutrient limitation and soil development: experimental test of a biogeochemical theory. Biogeochemistry 37:63–75
Vitousek PM, Sanford RL (1986) Nutrient cycling in moist tropical forest. Annu Rev Ecol Syst 17:137–167
Vose RS, Schmoyer RL, Steurer PM, Peterson TC, Heim R, Karl TR, Eischeid J (1992) The global historical climatology network: long-term monthly temperature, precipitation, sea level pressure, and station pressure data. ORNL/CDIAC-53. Carbon Dioxide Inf. Anal. Cent., Oak Ridge Natl. Lab., Oak Ridge, Tennessee, USA
Walker TW, Syers JK (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–19
Wardle DA, Walker JR, Bardgett RD (2004) Ecosystem properties and forest declines in contrasting long-term chronosequences. Science 305:509–513
Acknowledgements
The authors acknowledge NSF-DEB for funding under project number DEB-0425995. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NSF. We are thankful to Dr. Dean Hesterberg (Department of Soil Science, North Carolina State University) for providing spectral data for several aqueous phosphate species and valuable instruction and assistance for the LCF procedure of the XANES spectra. The authors also thank Dr. W. Caliebe for invaluable assistance with the spectroscopic analyses, performed at the beamline X-19A of the National Synchrotron Light Source, Project No. 4738. The NSLS is supported by the US Department of Energy under the contract No. DE-AC02-76CH00016.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Chengrong Chen
Rights and permissions
About this article
Cite this article
Sato, S., Neves, E.G., Solomon, D. et al. Biogenic calcium phosphate transformation in soils over millennial time scales. J Soils Sediments 9, 194–205 (2009). https://doi.org/10.1007/s11368-009-0082-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-009-0082-0