Abstract
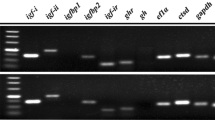
Molecular cloning, characterization, and functional analysis of follicle-stimulating hormone receptor (FSHR) in female turbot (Scophthalmus maximus) were evaluated. Results showed that the full-length FSHR cDNA was 3824 bp long and contained a 2202 bp open reading frame that encoded a mature protein of 733 amino acids (aa) and a signal peptide of 18 aa. Multiple sequence analyses showed that turbot FSHR has high homology with the corresponding genes of other teleosts and significant homology with that of Hippoglossus hippoglossus. Turbot FSHR has the typical structural architecture of glycoprotein hormone receptors consisting of a large N-terminal extracellular domain, seven transmembrane domains and short C-terminal intracellular domain. FSHR mRNA was found to be abundant in the ovaries, but deficient in eyes, intestine, brain, muscle, gills, spleen, stomach, heart and kidney. Furthermore, FSHR mRNA was found to increase gradually from pre-vitellogenesis to migratory nucleus stages, with the highest values observed during the late vitellogenesis stage of the reproductive cycle. However, FSHR mRNA was found to decrease dramatically during the atresia stage. Meanwhile, functional analysis with HEK293T cells continual expressing FSHR demonstrated that FSHR was specifically stimulated by ovine FSH, but not ovine LH. These results indicate that turbot FSHR is mainly involved in the stimulation of vitellogenesis, regulation of oocyte maturation as well as promotion of ovarian development via specific ligand binding. These findings open doors to further investigation of physiological functions of FSHR, which will be valuable for fish reproduction and broodstock management.







Similar content being viewed by others
References
Andersson E, Schulz RW, Male R, Bogerd J, Patiña D, Benedet S, Norberg B, Taranger GL (2013) Pituitary gonadotropin and ovarian gonadotropin receptor transcript levels: seasonal and photoperiod-induced changes in the reproductive physiology of female Atlantic salmon (Salmo salar). Gen Comp Endocrinol 191:247–258
Bogerd J (2007) Ligand-selective determinants in gonadotropin receptors. Mol Cell Endocrinol 260(262):144–152
Bromley PJ, Ravier C, Witthames PR (2000) The influence of feeding regime on sexual maturation, fecundity and atresia in first-time spawning turbot. J Fish Biol 56:264–278
Canipari R (2000) Oocyte-granulosa cell interactions. Hum Reprod Update 6:279–289
Chaffin CL, Vandevoort CA (2013) Follicle growth, ovulation, and luteal formation in primates and rodents: a comparative perspective. Exp Biol Med 238:539–548
Chakraborty P, Roy SK (2015) Expression of FSH receptor in the hamster ovary during perinatal development. Mol Cell Endocrinol 400:41–47
Clelland E, Peng C (2009) Endocrine/paracrine control of zebrafish ovarian development. Mol Cell Endocrinol 312:42–52
Fortune JE (1994) Ovarian follicular growth and development in mammals. Biol Reprod 50:225–232
Hillier SG (2001) Gonadotropic control of ovarian follicular growth and development. Mol Cell Endocrinol 179:39–46
Hirai T, Oba Y, Nagahama Y (2002) Fish gonadotropin receptors: molecular characterization and expression during gametogenesis. Fish Sci Suppl 68:675–678
Hurk R, Zhao J (2005) Formation of mammalian oocytes and their growth, differentiation and maturation within ovarian follicles. Theriogenology 63:1717–1751
Jia YD, Meng Z, Liu XF, Lei JL (2014a) Biochemical composition and quality of turbot (Scophthalmus maximus) eggs throughout the reproductive season. Fish Physiol Biochem 40:1093–1104
Jia YD, Meng Z, Niu HX, Peng H, Lei JL (2014b) Molecular cloning, characterization, and expression analysis of luteinizing hormone receptor gene in turbot (Scophthalmus maximus L). Fish Physiol Biochem 40:1639–1650
Jiang XL, Dias JA, He XL (2014) Structural biology of glycoprotein hormone and their receptors: insights to signaling. Mol Cell Encocrinol 382:424–451
Jones BA (1974) Sexual maturity, fecundity and growth of the turbot Scophthalmus maximus L. J Mar Biol Assoc UK 54:109–125
Kene PS, Dighe RR, Mahale SD (2005) Delineation of regions in the extracellular domain of follicle-stimulating hormone receptor involved in hormone binding and signal transduction. Am J Reprod Immunol 54:38–48
Kobayashi T, Andersen Ø (2008) The gonadotropin receptors FSH-R and LH-R of Atlantic halibut (Hippoglossus hippoglossus), 1: isolation of multiple transcripts encoding full-length and truncated variants of FSH-R. Gen Comp Endocrinol 156:584–594
Kobayashi T, Pakarinen P, Torgersen J, Huhtaniemi I, Andersen Ø (2008) The gonadotropin receptors FSH-R and LH-R of Atlantic halibut (Hippoglossus hippoglossus)-2. Differential follicle expression and asynchronous oogenesis. Gen Comp Endocrinol 156:595–602
Kumar RS, Ijiri S, Trant JM (2001a) Molecular biology of channel catfish gonadotropin receptors: 1. Cloning of a functional luteinizing hormone receptor and preovulatory induction of gene expression. Biol Reprod 64:1010–1018
Kumar RS, Ijiri S, Tran JM (2001b) Molecular biology of the channel catfish gonadotropin receptors: 2. Complementary DNA cloning, functional expression, and seasonal gene expression of the follicle-stimulating hormone receptor. Biol Reprod 65:710–717
Kwok HF, So WK, Wang Y, Ge W (2005) Zebrafish gonadotropins and their receptors: I. Cloning and characterization of zebrafish follicle-stimulating hormone and luteinizing hormone receptors-evidence for their distinct functions in follicle development. Biol Reprod 72:1370–1381
Lan RX, Liu F, He ZB, Chen C, Liu SJ, Shi Y, Liu YL, Yoshimura Y, Zhang M (2014) Immunolocalization of GnRHR I, gonadotropin receptors, PGR, and PGRMC I during follicular development in the rabbit ovary. Theriogenology 81:1139–1147
Levavi-Sivan B, Bogerd J, Mañanós EL, Gómez A, Lareyre JJ (2010) Perspectives on fish gonadotropins and their receptors. Gen Comp Endocrinol 165:412–437
Liang H, Chen L, Zhou Liu, Zhou X (2012) Expression of functional follicle-stimulating hormone receptor and luteinizing hormone/chorionic gonadotrophin receptor in oviduct and uterus in prepubertal gilts. Live Sci 148:74–80
Liu KC, Ge W (2013) Differential regulation of gonadotropin receptors (fshr and lhcgr) by epidermal growth factor (EGF) in the zebrafish ovary. Gen Comp Endocrinol 181:288–294
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2−∆∆CT method. Methods 25:402–408
Lloyd TL, Griswold MD (1995) Sequence analysis of glycoprotein hormone receptors for follicle-stimulating hormone, luteinizing hormone, thyroid-stimulating hormone. Biol Reprod 52(suppl 1):102
Lubzens E, Young G, Bobe J, Cerdà J (2010) Oogenesis in teleosts: how fish eggs are formed. Gen Comp Endocrinol 165:367–389
Luckenbach JA, Dickey JT, Swanson P (2011) Follicle-stimulating hormone regulation of ovarian transcripts for steroidogenesis-related proteins and cell survival, growth and differentiation factors in vitro during early secondary oocyte growth in coho salmon. Gen Comp Endocrinol 171:52–63
Magoffin DA (2005) Ovarian theca cell. Int J Biochem Cell Biol 37:1344–1349
Maugars G, Schmitz M (2006) Molecular cloning and characterization of FSH and LH receptors in Atlantic salmon (Salmo salar L.). Gen Comp Endocrinol 149:108–117
McEvoy LA (1989) Reproduction of turbot Scophthalmus maximus L in captivity. Cuard Aera Cienc Mar Semin Estud Galegos 3:9–28
Menon KMJ, Menon B (2012) Structure, function and regulation of gonadotropin receptors-a perspective. Mol Cell Endocrinol 356:88–97
Mittelholzer C, Andersson E, Taranger GL, Consten D, Hirai T, Senthilkumaran B, Nagahama Y, Norberg B (2009) Molecular characterization and quantification of the gonadotropin receptors FSH-R and LH-R from Atlantic cod (Gadus morhua). Gen Comp Endocrinol 160:47–58
Molés G, Gómez A, Carrillo M, Rocha A, Mylonas CC, Zanuy S (2011) Determination of Fish quantity and bioactivity during sex differentiation and oogenesis in European sea bass. Biol Reprod 85:848–857
Moyle WR, Xing YN, Lin W, Cao DH, Myers RV, Kerrigan JE, Bernard MP (2004) Model of glycoprotein hormone receptor ligand binding and signaling. J Biol Chem 279:44442–44459
Mugnier C, Guennoc M, Lebegue E, Fostier A, Breton B (2000) Induction and synchronisation of spawning in cultivated turbot (Scophth almus maximus L.) broodstock by implantation of a sustained-release GnRH-a pellet. Aquaculture 181:241–255
Nagahama Y, Yamashita M (2008) Regulation of oocyte maturation in fish. Dev Growth Differ 50:S195–S219
Nyuji M, Kitano H, Shimizu A, Lee JM, Kusakabe T, Yamaguchi A, Matsuyama M (2013) Characterization, localization, and stage-dependent gene expression of gonadotropin receptors in chub mackerel (Scomber japonicus) ovarian follicles. Biol Reprod 88(148):1–14
Oba Y, Hirai T, Yoshiura Y, Yoshikuni M, Kawauchi H, Nagahama Y (1999a) Cloning, functional characterization, and expression of a gonadotropin receptor cDNA in the ovary and testis of amago Salmon (Oncorhynchus rhodurus). Biochem Biophys Res Commun 263:584–590
Oba Y, Hirai T, Yoshiura Y, Yoshikuni M, Kawauchi H, Nagahama Y (1999b) The duality of fish gonadotropin receptors: cloning and functional characterization of a second gonadotropin receptor cDNA expressed in the ovary and testis of amago Salmon (Oncorhynchus rhodurus). Biochem Biophys Res Commun 265:366–371
Oba Y, Hira T, Yoshiura Y, Kobayashi T, Nagahama Y (2001) Fish gonadotropin and thyrotropin receptors: the evolution of glycoprotein hormone receptors in vertebrates. Comp Biochem Physiol B 129:441–448
Ohkubo M, Yabu T, Yamashita M, Shimizu A (2013) Molecular cloning of two gonadotropin receptors in mummichog Fundulus heteroclitus and their gene expression during follicular development and maturation. Gen Comp Endocrinol 184:75–86
Ribas L, Pardo BG, Fernández C, Álvarez-Diós JA, Gómez-Tato A, Quiroga MI, Planas JV, Sitjà-Bobadilla A, Martínez P, Piferrer F (2013) A combined strategy involving Sanger and 454 pyrosequencing increases genomic resources to aid in the management of reproduction, disease control and genetic selection in the turbot (Scophthalmus maximus). BMC Genom 14:180
Rocha A, Gómez A, Zanuy S, Cerdá-Reverter JM, Carrillo M (2007) Molecular characterization of two sea bass gonadotropin receptors: cDNA cloning, expression analysis, and functional activity. Mol Cell Endocrinol 272:63–76
Rocha A, Zanuy S, Carrillo M, Gómez A (2009) Seasonal changes in gonadal expression of gonadotropin receptors, steroidogenic acute regulatory protein and steroidogenic enzymes in the European sea bass. Gen Comp Endocrinol 162:265–275
Sambroni E, Le Gac F, Breton B, Lareyre JJ (2007) Functional specificity of the rainbow trout (Oncorhynchus mykiss) gonadotropin receptors as assayed in a mammalian cell line. J Endocrinol 195:213–228
Suquet M, Billard R, Cosson J, Normant Y, Fauvel C (1995) Artificial insemination in turbot (Scophthalmus maximus): determination of the optimal sperm to egg ratio and time of gamete contact. Aquaculture 133:83–90
Swanson P, Dickey JT, Campbell B (2003) Biochemistry and physiology of fish gonadotropins. Fish Physiol Biochem 28:53–59
Ulloa-Aguirre A, Zariñán T, Pasapera A, Casas-González P, Dias J (2007) Multiple facets of follicle-stimulating hormone receptor function. Endocrine 32:251–263
Vassart G, Pardo L, Costagliola S (2004) A molecular dissection of the glycoprotein hormone receptors. Trends Biochem Sci 29:119–126
Vischer HF, Bogerd J (2003) Cloning and functional characterization of a gonadal luteinizing hormone receptor complementary DNA from the African Catfish (Clarias gariepinus). Biol Reprod 68:262–271
Wallace RA, Selman K (1981) Cellular and dynamic aspects of oocyte growth in teleosts. Am Zool 21:325–343
Ziecik AJ, Derecka-Reszka K, Rzucidło SJ (1992) Extragonadal gonadotropin receptors, their distribution and function. Physiol Pharmacol 43:33–49
Acknowledgments
This study was supported by China Agriculture Research System (CRAS-50), National Natural Science Foundation of China (31302205, 31402315 and 31402284), Natural Science Foundation of Shandong Province (ZR2012CQ024 and BS2013SW004) and the China Postdoctoral Science Foundation (2012M511559 and 2013T60690). We thank Chunren Gao and Xinfu Liu (Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences) for help in the experiment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yudong Jia and Ai Sun have equal contribution to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jia, Y., Sun, A., Meng, Z. et al. Molecular characterization and quantification of the follicle-stimulating hormone receptor in turbot (Scophthalmus maximus). Fish Physiol Biochem 42, 179–191 (2016). https://doi.org/10.1007/s10695-015-0128-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0128-8