Abstract
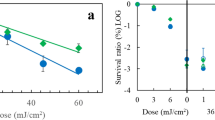
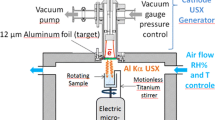
Organisms are often exposed to different types of ionizing radiation that, directly or not, will promote damage to DNA molecules and/or other cellular structures. Because of that, organisms developed a wide range of response mechanisms to deal with these threats. Endonuclease III is one of the enzymes responsible to detect and repair oxidized pyrimidine base lesions. However, the effect of radiation on the structure/function of these enzymes is not clear yet. Here, we demonstrate the effect of UV-C radiation on E. coli endonuclease III through several techniques, namely UV–visible, fluorescence and Mössbauer spectroscopies, as well as SDS-PAGE and electrophoretic mobility shift assay. We demonstrate that irradiation with a UV-C source has dramatic consequences on the absorption, fluorescence, structure and functionality of the protein, affecting its [4Fe–4S] cluster and its DNA-binding ability, which results in its inactivation. An UV-C radiation-induced conversion of the [4Fe–4S]2+ into a [2Fe–2S]2+ was observed for the first time and proven by Mössbauer and UV–visible analysis. This work also shows that the DNA-binding capability of endonuclease III is highly dependent of the nuclearity of the endogenous iron–sulfur cluster. Thus, from our point of view, in a cellular context, these results strengthen the argument that cellular sensitivity to radiation can also be due to loss of radiation-induced damage repair ability.





Similar content being viewed by others
References
Agarwalla S, Stroud RM, Gaffney BJ (2004) Redox reactions of the iron–sulfur cluster in a ribosomal RNA methyltransferase, RumA—Optical and EPR studies. J Biol Chem 279(33):34123–34129. doi:10.1074/jbc.M405702200
Asahara H, Wistort PM, Bank JF, Bakerian RH, Cunningham RP (1989) Purification and characterization of Escherichia coli endonuclease III from the cloned nth gene. Biochemistry 28(10):4444–4449. doi:10.1021/bi00436a048
Boal AK, Yavin E, Barton JK (2007) DNA repair glycosylases with a [4Fe–4S] cluster: a redox cofactor for DNA-mediated charge transport? J Inorg Biochem 101(11–12):1913–1921. doi:10.1016/j.jinorgbio.2007.05.001
Borsarelli CD, Falomir-Lockhart LJ, Ostatna V, Fauerbach JA, Hsiao HH, Urlaub H, Palecek E, Jares-Erijman EA, Jovin TM (2012) Biophysical properties and cellular toxicity of covalent crosslinked oligomers of alpha-synuclein formed by photoinduced side-chain tyrosyl radicals. Free Radic Biol Med 53(4):1004–1015. doi:10.1016/j.freeradbiomed.2012.06.035
Bottger LH, Miller EP, Andresen C, Matzanke BF, Kupper FC, Carrano CJ (2012) Atypical iron storage in marine brown algae: a multidisciplinary study of iron transport and storage in Ectocarpus siliculosus. J Exp Bot 63(16):5763–5772. doi:10.1093/Jxb/Ers225
Bruge F, Tiano L, Cacciamani T, Principi F, Littarru GP (2003) Effect of UV-C mediated oxidative stress in leukemia cell lines and its relation to ubiquinone content. BioFactors 18(1–4):51–63. doi:10.1002/biof.5520180207
Castruita M, Saito M, Schottel PC, Elmegreen LA, Myneni S, Stiefel EI, Morel FMM (2006) Overexpression and characterization of an iron storage and DNA-binding Dps protein from Trichodesmium erythraeum. Appl Environ Microb 72(4):2918–2924. doi:10.1128/Aem.72.4.2918-2924.2006
Chakraborty M, Bhattacharya D, Mukhopadhyay C, Chakrabarti A (2010) Structure and conformational studies on dityrosine formation in the DNA binding domain of RFX5. Biophys Chem 149(3):92–101. doi:10.1016/j.bpc.2010.04.005
Correia M, Neves-Petersen MT, Jeppesen PB, Gregersen S, Petersen SB (2012) UV-Light Exposure of Insulin: Pharmaceutical Implications upon Covalent Insulin Dityrosine Dimerization and Disulphide Bond Photolysis. Plos One 7(12). doi:10.1371/journal.pone.0050733
Crack JC, Gaskell AA, Green J, Cheesmant MR, Le Brun NE, Thomson AJ (2008a) Influence of the environment on the [4Fe–4S](2+) to [2Fe–2S](2+) cluster switch in the transcriptional regulator FNR. J Am Chem Soc 130(5):1749–1758. doi:10.1021/Ja077455
Crack JC, Le Brun NE, Thomson AJ, Green J, Jervis AJ (2008b) Reactions of nitric oxide and oxygen with the regulator of fumarate and nitrate reduction, a global transcriptional regulator, during anaerobic growth of Escherichia coli. Methods Enzymol 437:191–209. doi:10.1016/S0076-6879(07)37011-0
Cunningham RP, Asahara H, Bank JF, Scholes CP, Salerno JC, Surerus K, Munck E, McCracken J, Peisach J, Emptage MH (1989) Endonuclease III is an iron–sulfur protein. Biochemistry 28(10):4450–4455. doi:10.1021/bi00436a049
Daly MJ (2012) Death by protein damage in irradiated cells. DNA Repair 11(1):12–21. doi:10.1016/j.dnarep.2011.10.024
Endres RG, Cox DL, Singh RRP (2004) Colloquium: the quest for high-conductance DNA. Rev Mod Phys 76(1):195–214. doi:10.1103/RevModPhys.76.195
Fink HW, Schonenberger C (1999) Electrical conduction through DNA molecules. Nature 398(6726):407–410. doi:10.1038/18855
Flint DH, Emptage MH, Finnegan MG, Fu W, Johnson MK (1993) The role and properties of the iron–sulfur cluster in Escherichia coli dihydroxy-acid dehydratase. J Biol Chem 268(20):14732–14742
Girard PM, Francesconi S, Pozzebon M, Graindorge D, Rochette P, Drouin R, Sage E (2011) UVA-induced damage to DNA and proteins: direct versus indirect photochemical processes. J Phys Conf Ser 261. doi:10.1088/1742-6596/261/1/012002
Gomes PJ, Ribeiro PA, Shaw D, Mason NJ, Raposo M (2009) UV degradation of deoxyribonucleic acid. Polym Degrad Stab 94(12):2134–2141. doi:10.1016/j.polymdegradstab.2009.09.013
Gomes PJ, Coelho M, Dionisio M, Ribeiro PA, Raposo M (2012) Probing radiation damage by alternated current conductivity as a method to characterize electron hopping conduction in DNA molecules. Appl Phys Lett 101(12). doi:10.1063/1.4754287
Hoeijmakers JH (2001) Genome maintenance mechanisms for preventing cancer. Nature 411(6835):366–374. doi:10.1038/35077232
Huynh BH, Moura JJG, Moura I, Kent TA, Legall J (1980) Evidence for a 3-Iron Center in a Ferredoxin from Desulfovibrio-gigas. J Biol Chem 255(8):3242–3244
Jervis AJ, Crack JC, White G, Artymiuk PJ, Cheesman MR, Thomson AJ, Le Brun NE, Green J (2009) The O-2 sensitivity of the transcription factor FNR is controlled by Ser24 modulating the kinetics of [4Fe–4S] to [2Fe–2S] conversion. P Natl Acad Sci USA 106(12):4659–4664. doi:10.1073/pnas.0804943106
Krebs C, Henshaw TF, Cheek J, Huynh BH, Broderick JB (2000) Conversion of 3Fe–4S to 4Fe–4S clusters in native pyruvate formate-lyase activating enzyme: mossbauer characterization and implications for mechanism. J Am Chem Soc 122(50):12497–12506. doi:10.1021/Ja003335p
Kungl AJ, Visser AJWG, Kauffmann HF, Breitenbach M (1994) Time-resolved fluorescence studies of dityrosine in the outer layer of intact yeast ascospores. Biophys J 67(1):309–317. doi:10.1016/S0006-3495(94)80482-5
Lau AT, Wang Y, Chiu JF (2008) Reactive oxygen species: current knowledge and applications in cancer research and therapeutic. J Cell Biochem 104(2):657–667. doi:10.1002/jcb.21655
Lehrer SS, Fasman GD (1967) Ultraviolet irradiation effects in Poly-l-Tyrosine and model compounds. Identification of bityrosine as a photoproduct. Biochemistry 6(3):757–767. doi:10.1021/Bi00855a017
Lim PS, Cheng YM, Yang SM (2007) Impairments of the biological properties of serum albumin in patients on haemodialysis. Nephrology 12(1):18–24. doi:10.1111/j.1440-1797.2006.00745.x
Long YT, Li CZ, Kraatz HB, Lee JS (2003) AC impedance spectroscopy of native DNA and M-DNA. Biophys J 84(5):3218–3225. doi:10.1016/S0006-3495(03)70046-0
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193(1):265–275
Lukianova OA, David SS (2005) A role for iron–sulfur clusters in DNA repair. Curr Opin Chem Biol 9(2):145–151. doi:10.1016/j.cbpa.2005.02.006
Malencik DA, Anderson SR (2003) Dityrosine as a product of oxidative stress and fluorescent probe. Amino Acids 25(3–4):233–247. doi:10.1007/s00726-003-0014-z
Mena S, Ortega A, Estrela JM (2009) Oxidative stress in environmental-induced carcinogenesis. Mutat Res 674(1–2):36–44. doi:10.1016/j.mrgentox.2008.09.017
Moura I, Xavier AV, Cammack R, Bruschi M, Legall J (1978) Comparative spectroscopic study of 2 non-heme iron proteins lacking labile sulfide from Desulphovibrio-gigas. Biochim Biophys Acta 533(1):156–162. doi:10.1016/0005-2795(78)90559-7
Muh U, Buckel W, Bill E (1997) Mossbauer study of 4-hydroxybutyryl-CoA dehydratase—Probing the role of an iron–sulfur cluster in an overall non-redox reaction. Eur J Biochem 248(2):380–384. doi:10.1111/j.1432-1033.1997.t01-1-00380.x
Pereira AS, Tavares P, Folgosa F, Almeida RM, Moura I, Moura JJG (2007) Superoxide reductases. Eur J Inorg Chem 18:2569–2581. doi:10.1002/ejic.200700008
Pfeifer GP, You YH, Besaratinia A (2005) Mutations induced by ultraviolet light. Mutat Res 571(1–2):19–31. doi:10.1016/j.mrfmmm.2004.06.057
Porello SL, Cannon MJ, David SS (1998) A substrate recognition role for the [4Fe–4S]2+ cluster of the DNA repair glycosylase MutY. Biochemistry 37(18):6465–6475. doi:10.1021/bi972433t
Ramirez-Guadiana FH, Barraza-Salas M, Ramirez-Ramirez N, Ortiz-Cortes M, Setlow P, Pedraza-Reyes M (2012) Alternative excision repair of ultraviolet B- and C-induced DNA damage in dormant and developing spores of Bacillus subtilis. J Bacteriol 194(22):6096–6104. doi:10.1128/JB.01340-12
Romano CA, Sontz PA, Barton JK (2011) Mutants of the base excision repair glycosylase, endonuclease III: DNA charge transport as a first step in lesion detection. Biochemistry 50(27):6133–6145. doi:10.1021/Bi2003179
Santos AL, Oliveira V, Baptista I, Henriques I, Gomes NCM, Almeida A, Correia A, Cunha A (2013) Wavelength dependence of biological damage induced by UV radiation on bacteria. Arch Microbiol 195(1):63–74. doi:10.1007/s00203-012-0847-5
Scharer OD (2003) Chemistry and biology of DNA repair. Angew Chem Int Ed Engl 42(26):2946–2974. doi:10.1002/anie.200200523
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to imageJ: 25 years of image analysis. Nat Methods 9(7):671–675. doi:10.1038/Nmeth.2089
Silva ACT, Asad LMBO, Felzenszwalb I, Asad NR (2012) The role of Fpg protein in UVC-induced DNA lesions. Redox Rep 17(3):95–100. doi:10.1179/1351000212y.0000000006
Slade D, Radman M (2011) Oxidative stress resistance in Deinococcus radiodurans. Microbiol Mol Biol R 75(1):133. doi:10.1128/Mmbr.00015-10
Sowa M, Arthurs BJ, Estes BJ, Morgan WF (2006) Effects of ionizing radiation on cellular structures, induced instability and carcinogenesis. EXS 96:293–301. doi:10.1007/3-7643-7378-4_12
Staples CR, Ameyibor E, Fu WG, GardetSalvi L, StrittEtter AL, Schurmann P, Knaff DB, Johnson MK (1996) The function and properties of the iron–sulfur center in spinach ferredoxin:thioredoxin reductase: a new biological role for iron–sulfur clusters. Biochemistry 35(35):11425–11434. doi:10.1021/Bi961007p
Swinger KK, Rice PA (2004) IHF and HU: flexible architects of bent DNA. Curr Opin Struc Biol 14(1):28–35. doi:10.1016/j.sbi.2003.12.003
Tetlow JA, Wilson AL (1964) Absorptiometric determination of iron in boiler feed-water. 3. Method for determining total iron content. Analyst 89(1060):442–452. doi:10.1039/An9648900442
Timoteo CG, Guilherme M, Penas D, Folgosa F, Tavares P, Pereira AS (2012) Desulfovibrio vulgaris bacterioferritin uses H2O2 as a co-substrate for iron oxidation and reveals DPS-like DNA protection and binding activities. Biochem J 446:125–133. doi:10.1042/Bj20111439
Wang SSS, Wen WS (2010) Examining the influence of ultraviolet C irradiation on recombinant human gamma D-crystallin. Mol Vis 16(298–00):2777–2790
Wei HC, Cai QY, Rahn R, Zhang XS (1997) Singlet oxygen involvement in ultraviolet (254 nm) radiation-induced formation of 8-hydroxy-deoxyguanosine in DNA. Free Radic Bio Med 23(1):148–154. doi:10.1016/S0891-5849(96)00526-6
Yoo SJ, Meyer J, Achim C, Peterson J, Hendrich MP, Munck E (2000) Mossbauer, EPR, and MCD studies of the C9S and C42S variants of Clostridium pasteurianum rubredoxin and MCD studies of the wild-type protein. J Biol Inorg Chem 5(4):475–487. doi:10.1007/s007750050008
Zhang XS, Rosenstein BS, Wang Y, Lebwohl M, Wei HC (1997) Identification of possible reactive oxygen species involved in ultraviolet radiation-induced oxidative DNA damage. Free Radic Bio Med 23(7):980–985. doi:10.1016/S0891-5849(97)00126-3
Acknowledgments
This research was supported by Fundação para a Ciência e Tecnologia, Ministério da Educação e Ciência, grant PTDC/SAU-SAP/111482/2009 (P.T.), PTDC/QUI/64248/2006 (to A.S.P), grant SFRH/BPD/48430/2008, a Post-Doctoral grant to F.F. Requimte was funded in part by grant PEst-C/EQB/LA0006/2011 from FCT/MEC.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Folgosa, F., Camacho, I., Penas, D. et al. UV radiation effects on a DNA repair enzyme: conversion of a [4Fe–4S]2+ cluster into a [2Fe–2S]2+ . Radiat Environ Biophys 54, 111–121 (2015). https://doi.org/10.1007/s00411-014-0569-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-014-0569-y