Abstract
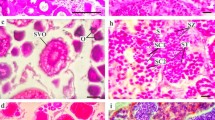
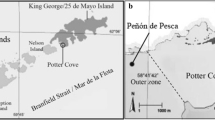
Several fish species of the suborder Notothenioidei (Perciformes) predominate in the Antarctic Convergence Zone; nevertheless, reproductive studies are scarce due to difficulties on regular sampling. This study takes the research area of reproductive biology of notothenioids to a new level by providing, for the first time, data on sex hormone and vitellogenin detection in the blood of females of Notothenia rossii and N. coriiceps and correlates this data with morphological maturity indices as well as ovarian histology. Fish were captured during the Antarctic summer at Potter Cove, 25 de Mayo (King George) Island, and blood and ovary were collected. Histological analysis revealed that females of both fish possess group synchronous ovarian development with two distinct clutches of oocytes: a more advanced batch of vitellogenic oocytes ready for spawning and a second batch of previtellogenic oocytes for the next spawning event. Since liver vitellogenin synthesis is stimulated by estradiol produced by the ovaries, gonadal development, estradiol levels, and vitellogenin showed that both species were at a more advanced stage of maturation in March than in January. On the other hand and irrespectively of the month, gonadosomatic index and plasma estradiol levels of N. coriiceps were higher than those of N. rossii. Furthermore, females of N. coriiceps showed an advanced stage of vitellogenesis or were ready for spawn, contrary to results of previous studies. Our results indicate the successful use of gonadal morphology, estradiol, and vitellogenin detection for the estimation of sexual maturity stage of female adults.





Similar content being viewed by others
References
Aida K, Ngan PV, Hibiya T (1973) Physiological studies on gonadal maturation of fishes. 1. Sexual difference in composition of plasma-protein of ayu in relation to gonadal maturation. B Jpn Soc Sci Fish 39:1091–1106
Antarctic Treaty Consultative Meeting (ATCM) (2007) Final report presented to ATCM XXX. India, 30 April –11 May 2007. http://www.asoc.org/storage/documents/Meetings/ATCM/XXX/asoc%20report%20on%20xxx%20atcm%20-%20new%20delhi%202007.pdf
Babin PJ, Cerdà J, Lubzens E (2007) The fish oocyte: from basic studies to biotechnological applications. Springer, The Netherlands
Barrera-Oro E (2002) The role of fish in the Antarctic marine food web: differences between inshore and offshore waters in the southern Scotia Arc and west Antarctic Peninsula. Antarct Sci 14:293–309
Barrera-Oro ER, Casaux RJ (1996) Validation of age determination in Notothenia coriiceps, by means of a tag-recapture experiment at Potter Cove, South Shetland Islands. Arch Fish Mar Res 43:205–216
Barrera-Oro ER, Casaux R (2008) General ecology of coastal fish from the South Shetland Island and west Antarctic Peninsula areas. Ber Polar Meeresforsch 571:95–110
Barrera-Oro ER, Marschoff ER (2007) Information on the status of fjord Notothenia rossii Gobionotothen gibberifrons and Notothenia coriiceps in the lower South Shetland Islands derived from the 2000–2006 monitoring program at Potter Cove. CCAMLR Sci 14:83–87
Barrera-Oro ER, Marschoff ER, Casaux RJ (2000) Trends in relative abundance of fjord Notothenia rossii, Gobionotothen gibberifrons and Notothenia coriiceps at Potter Cove, South Shetland Islands, after commercial fishing in the area. CCAMLR Sci 7:43–52
Berg AH, Westerlund L, Olsson PE (2004) Regulation of Arctic char (Salvelinus alpinus) egg shell proteins and vitellogenin during reproduction and in response to 17β-estradiol and cortisol. Gen Comp Endocrinol 135:276–285
Brickle P, Laptikhovsky V, Arkhipkin A, Portela J (2006) Reproductive biology of Patagonotothen ramsayi (Regan, 1913) (Pisces: Nototheniidae) around the Falkland Islands. Polar Biol 29:570–580
Bromwich DH, Nicolas JP, Monaghan AJ, Lazzara MA, Keller LM, Weidner GA, Wilson AB (2013) Central West Antarctica among the most rapidly warming regions on Earth. Nature Geosci 6:139–145
Brooks S, Tyler CR, Sumpter JP (1997) Egg quality in fish: what makes a good egg? Rev Fish Biol Fisher 7:387–416
Burchett MS (1983) The life-cycle of Notothenia rossii from south Georgia. Brit Antarct Surv Bull 61:71–73
Canapa A, Barucca M, Gorbi S, Benedetti M, Zucchi S, Biscotti MA, Regoli F (2007) Vitellogenin gene expression in males of the Antarctic fish Trematomus bernacchii from Terra Nova Bay (Ross Sea): a role for environmental cadmium? Chemosphere 66:1270–1277
Casaux RJ, Barrera-Oro ER (2002) Effect of a shore-based sampling programme on Notothenia coriiceps populations. Antarct Sci 14:221–224
Casaux RJ, Mazzotta AS, Barrera-Oro ER (1990) Seasonal aspects of the biology and diet of nearshore nototheniid fish at Potter Cove South Shetland Islands Antarctica. Polar Biol 11(1):63–72
Chakrabarti P, Chatterjee N (2014) Seasonal changes in the architecture of hepatocytes in relation to ovarian activities during growth, maturation, spawning and post-spawning phases in Mystus vittatus (Bloch, 1790). J Entomol Zool Stud 2:212–217
Christiansen JS, Fevolden SE, Karamushko OV, Karamushko LI (1998) Maternal output in polar fish reproduction. In: di Prisco G, Pisano E, Clarke A (eds) Fishes of Antarctica. Springer, Berlin, pp 41–52
Clarke A, Johnston IA (1996) Evolution and adaptive radiation of Antarctic fishes. Trends Ecol Evol 11:212–218
Clarke A, Murphy EJ, Meredith MP, King JC, Peck LS, Barnes DK, Smith RC (2007) Climate change and the marine ecosystem of the western Antarctic Peninsula. Philos Trans R Soc B 362:149–166
Dahle R, Taranger GL, Karlsen Ø, Kjesbu OS, Norberg B (2003) Gonadal development and associated changes in liver size and sexual steroids during the reproductive cycle of captive male and female Atlantic cod Gadus morhua. Comp Biochem Physiol A 136:641–653
De Vlaming V, Wiley HS, Delahunty G, Wallace RA (1980) Goldfish (Carassius auratus) vitellogenin: induction, isolation, properties and relationship to yolk proteins. Comp Biochem Physiol B Biochem Mol Biol 67:613–623
Dearborn JH (1965) Reproduction in the nototheniid fish Trematomus bernacchii. Boulenger at McMurdo Sound, Antarctica. Copeia 1965:302–308
Denslow ND, Chow MC, Kroll KJ, Green L (1999) Vitellogenin as a biomarker of exposure for estrogen or estrogen mimics. Ecotoxicology 8:385–398
DeWitt HH (1971) Coastal and deep-water benthic fishes of the Antarctic. In: Bushnell VC (ed) Antarctic map folio series, folio 15. American Geographical Society, New York, pp 1–10
DeWitt HH, Heemstra PC, Gon O (1990) Nototheniidae. In: Gon O, Heemstra PC (eds) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 279–331
Duhamel G (1982) Biology and population dynamics of Notothenia rossii rossii from the Kerguelen Islands (Indian Sector of Southern Ocean). Polar Biol 1:141–151
Duhamel G, Kock KH, Balguerias E, Hureau JC (1993) Reproduction in fish of the Weddell Sea. Polar Biol 13:193–200
Eastman JT (2005) The nature of the diversity of Antarctic fishes. Polar Biol 28:93–107
Eastman JT, Grande L (1989) Evolution of the Antarctic fish fauna with emphasis on the recent notothenioids. In: Crame JA (ed) Origins and evolution of the Antarctic biota, vol 47. Geological Society of London, Special Publication, London, pp 241–252
Everson I (1970) Reproduction in Notothenia neglecta Nybelin. Brit Antarct Surv B 23:81–92
Everson I (1977) The living resources of the Southern Ocean. FAO GLO/SO/77/1, Rome
Everson I (1984) Fish biology. In: Laws RM (ed) Antarctic ecology, vol 2. Academic Press, London, pp 491–532
Finn RN (2007) The maturational disassembly and differential proteolysis of paralogous vitellogenins in a marine pelagophil teleost: a conserved mechanism of oocyte hydration. Biol Reprod 76:936–948
Fostier A, Jalabert B, Billard R, Breton B, Zohar Y (1983) The Gonadal Steroids. In: Hoar WS, Randall DJ, Donaldson EM (eds) Fish physiology, vol IX., part AAcademic Press, London, pp 277–372
Freytag G (1979) Age determination in Notothenia rossii marmorata. In: International council for the exploration of the sea council conference and meeting documents, G. vol 11, pp 1–7
Garrison DL, Buck KR (1989) The biota of Antarctic pack ice in the Weddell Sea and Antarctic Peninsula regions. Polar Biol 10:211–219
Genovese G, Da Cuña R, Towle DW, Maggese MC, Lo Nostro FL (2011) Early expression of zona pellucida proteins under octylphenol exposure in Cichlasoma dimerus (Perciformes, Cichlidae). Aquat Toxicol 101:175–185
Harmin SA, Crim LW, Wiegand MD (1995) Plasma sex steroid profiles and the seasonal reproductive cycle in male and female winter flounder Pleuronectes americanus. Mar Biol 121:601–610
Heppell SA, Denslow ND, Folmar LC, Sullivan CV (1995) Universal assay of vitellogenin as a biomarker for environmental estrogens. Environ Health Persp 103:9–15
Holden MJ, Raitt DFS (1974) Manual of fisheries science. Part II. Methods of resource investigation and their application. FAO, Rome
Hunter JR, Macewicz BJ (1985) Measurement of spawning frequency in multiple spawning fishes. In: Lasker R (ed) An egg production method for estimating spawning biomass of pelagic fish: application to the northern anchovy (Engraulis mordax). National Marine Fisheries Services. Technical Report 36, California, pp 79–94
Hureau JC (1970) Biologie comparée de quelques Poissons antarctiques (Notothenidae). Bulletin de l’Institut océanographique de Monaco 68:1–244
Jobling S, Nolan M, Tyler CR, Brighty G, Sumpter JP (1998) Widespread sexual disruption in wild fish. Environ Sci Technol 32:2498–2506
Karlsen Ø, Holm JC, Kjesbu OS (1995) Effects of periodic starvation on reproductive investment in first-time spawning Atlantic cod (Gadus morhua). Aquaculture 133:159–170
Kjesbu OS, Witthames PR, Solemdal P, Walker MG (1990) Ovulatory rhythm and a method to determine the stage of spawning in Atlantic cod (Gadus morhua). Can J Fish Aquat Sci 47:1185–1193
Knox GA (2006) Biology of the southern ocean. CRC Press Taylor and Francis Group, Boca Raton
Kobayashi M, Aida K, Hanyu I (1988) Hormone changes during the ovulatory cycle in goldfish. Gen Comp Endocrinol 69:301–307
Kock KH (1992) Antarctic fish and fisheries. Cambridge University Press, Cambridge
Kock KH, Kellermann A (1991) Reproduction in Antarctic notothenioid fish. Antarct Sci 3:125–150
Kumar P, Arasu ART, Kailasam M, Sukumarran K, Subburj R, Tyagraj G, Natarajan M (2015) Gonadal development and steroid hormone profile of wild caught grey mullet (Mugil cephalus). Biol Rhythm Res 46:601–610
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Le Menn F (2002) Fish oocyte yolk accumulation mediated by specific receptors. A comparative approach in oviparous vertebrates. Fish Sci 68:1198–1201
Lee WK, Yang SW (2002) Relationship between ovarian development and serum levels of gonadal steroid hormones, and induction of oocyte maturation and ovulation in the cultured female Korean spotted sea bass Lateolabrax maculatus (Jeom-nong-eo). Aquaculture 207:169–183
Lowry OM, Rosenbrough NJ, Farr L, Randall RJ (1951) Protein measurement with the phenol reagent. J Biol Chem 193:265–275
Macchi GJ, Barrera-Oro E (1995) Histological study on the ovarian development of mackerel icefish (Champsocephalus gunnari) from the South Georgia Islands. CCAMLR Sci 2:35–49
Marrari M (2008) Characterization of the western Antarctic Peninsula ecosystem: environmental controls on the zooplankton community. Proquest Web. http://search.proquest.com/docview/21103836?accountid=28953. Accessed 17 June 2015
Marschoff ER, Barrera-Oro ER, Alescio NS, Ainley DG (2012) Slow recovery of previously depleted demersal fish at the South Shetland Islands 1983–2010. Fish Res 125:206–213
Matsubara T, Nagae M, Ohkubo N, Andoh T, Sawaguchi S, Hiramatsu N, Sullivan CV, Hara A (2003) Multiple vitellogenins and their unique roles in marine teleosts. Fish Physiol Biochem 28:295–299
Meredith MP, King JC (2005) Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys Res Lett 32:604–608
Meucci V, Arukwe A (2005) Detection of vitellogenin and zona radiata protein expressions in surface mucus of immature juvenile Atlantic salmon (Salmo salar) exposed to waterborne nonylphenol. Aquat Toxicol 73:1–10
Mommsen TP, Walsh PJ (1988) Vitellogenesis and oocyte assembly. In: Hoar WS, Randall DJ (eds) Fish physiology, vol XI. Academic Press, New York, pp 347–406
Moon HW, Wan Hussin WMR, Kim HC, Ahn IY (2015) The impacts of climate change on Antarctic nearshore mega-epifaunal benthic assemblages in a glacial fjord on King George Island: responses and implications. Ecol Indic 57:280–292
Nilsen BM, Berg K, Arukwe A, Goksøyr A (1998) Monoclonal and polyclonal antibodies against fish vitellogenin for use in pollution monitoring. Mar Environ Res 46:153–157
Pankhurst NW, Munday PL (2011) Effects of climate change on fish reproduction and early life history stages. Mar Freshwater Res 62:1015–1026
Patiño R, Sullivan CV (2002) Ovarian follicle growth, maturation, and ovulation in teleost fish. Fish Physiol Biochem 26:57–70
Prut’ko VG (2012) Materials on some aspects of reproductive biology of Whitson’s grenadier Macrourus whitsoni (Macrouridae) in the Ross Sea. J Appl Ichthyol 52:77–84
Rae GA, Calvo J (1995) Fecundity and reproductive habits in Patagonotothen tessellata (Richardson 1845) from the Beagle Channel Argentina. Antarct Sci 7:235–240
Raga G, Pichler HA, Zaleski T, da Silva FBV, Machado C, Rodrigues E, Gonçalves Kawall H, Sant’Anna Rios F, Donatti L (2014) Ecological and physiological aspects of the antarctic fishes Notothenia rossii and Notothenia coriiceps in Admiralty Bay, Antarctic Peninsula. Environ Biol Fish 98:775–788
Romano M, Rosanova P, Anteo C, Limatola E (2004) Vertebrate yolk proteins: a review. Mol Reprod Dev 69:109–116
Rønnestad I, Thorsen A, Finn RN (1999) Fish larval nutrition: a review of recent advances in the roles of amino acids. Aquaculture 177:201–216
Russo A, Angelini F, Carotenuto R, Guarino FM, Falugi C, Campanella C (2000) Spermatogenesis in some Antarctic teleosts from the Ross Sea: histological organisation of the testis and localisation of bFGF. Polar Biol 23:279–287
Sapota MR (1999) Gonad development and embryogenesis of Notothenia coriiceps from South Shetlands–Antarctica. Polar Biol 22:164–168
Scott AP, MacKenzie DS, Stacey NE (1984) Endocrine changes during natural spawning in the white sucker Catostomus commersoni: II steroid hormones. Gen Comp Endocrinol 56:349–359
Siciński J, Jażdżewski K, De Broyer C, Presler P, Ligowski R, Nonato EF, Campos LS (2011) Admiralty Bay benthos diversity—a census of a complex polar ecosystem. Deep Sea Res Pt II 58:30–48
Skora KE, Neyelov AV (1992) Fish of Admiralty Bay (King George Island South Shetland Islands Antarctica). Polar Biol 12:469–476
Sundararaj BI, Nath P (1981) Steroid-induced synthesis of vitellogenin in the catfish, Heteropneustes fossilis (Bloch). Gen Comp Endocrinol 43:201–210
Tiedtke JE, Kock KH (1989) Structure and composition of the demersal fish fauna around Elephant Island. Arch Fisch Wiss 39:143–169
Turner J, Maksym T, Phillips T, Marshall GJ, Meredith MP (2013) The impact of changes in sea ice advance on the large winter warming on the western Antarctic Peninsula. Int J Climatol 33:852–861
Tveiten H, Mayer I, Johnsen HK, Jobling M (1998) Sex steroids growth and condition of Arctic charr broodstock during an annual cycle. J Fish Biol 53:714–727
Tyler CR, Van der Eerden B, Jobling S, Panter G, Sumpter JP (1996) Measurement of vitellogenin, a biomarker for exposure to oestrogenic chemicals, in a wide variety of cyprinid fish. J Comp Physiol B 166:418–426
Vaughan DG (2008) West Antarctic Ice Sheet collapse–the fall and rise of a paradigm. Clim Change 91:65–79
Wahli W (1988) Evolution and expression of vitellogenin genes. Trends Genet 4:227–232
Wallace RA, Selman K (1981) Cellular and dynamic aspects of oocyte growth in teleosts. Am Zool 21:325–343
Webb MA, Feist GW, Foster EP, Schreck CB, Fitzpatrick MS (2002) Potential classification of sex and stage of gonadal maturity of wild white sturgeon using blood plasma indicators. Trans Am Fish Soc 131:132–142
White MG, North AW (1987) Postlarval Notothenioidei and midwater fish collected during the SIBEX cruise by British Antarctic Survey, 1985. In: Kullander SO, Fernholm B (eds) Proceedings, fifth congress of european ichthyologists, vol 1985. Swedish Museum of Natural History, Stockholm, pp 405–411
Williams VN, Reading BJ, Hiramatsu N, Amano H, Glassbrook N, Hara A, Sullivan CV (2014) Multiple vitellogenins and product yolk proteins in striped bass, Morone saxatilis: molecular characterization and processing during oocyte growth and maturation. Fish Physiol Biochem 40:395–415
Yaron Z, Cocos M, Salzer H (1980) Effects of temperature and photoperiod on ovarian recrudescence in the cyprinid fish Mirogrex terraesanctae. J Fish Biol 16:371–382
Acknowledgments
We are grateful to Gabriel Rosa, Fernando Meijide, Hernán Sacristan, and Carlos Bellisio for sampling collection during scientific campaigns. The financial support was provided by Agencia Nacional de Promoción Científica y Tecnológica (PICTO 0091, Ansaldo, Genovese, Lo Nostro). Grants from Universidad de Buenos Aires (UBACyT 056, Lo Nostro) and CONICET (PIP 1021, Lo Nostro) partially contributed to this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferreira, M.F., Varela, M.L., Lo Nostro, F. et al. Reproductive aspects of Notothenia rossii and N. coriiceps (Perciformes, Nototheniidae) at Potter Cove, 25 de Mayo (King George) Island during austral summer. Polar Biol 40, 1–11 (2017). https://doi.org/10.1007/s00300-016-1918-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-016-1918-x