Abstract
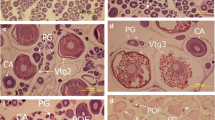
Studies on reproduction of the dragonfishes, Bathydraconidae, are scarce, and within this family, the reproductive biology of Parachaenichthys charcoti was poorly understood. Herein we present a histologic analysis of P. charcoti ovaries together with data on reproductive effort using fish collected with trammel nets in austral summer at Potter Cove, South Shetland Islands (SSI), and compare this information with that reported for the South Georgia congener Parachaenichthys georgianus. In gravid females of P. charcoti, GSI of 16–31%, mature oocytes of 1.8–3.9 mm and total fecundity (TF) of 9025–18,937 oocytes/individual (X ± SD = 12,617 ± 4019, n = 7) were recorded. The histology of the ovaries confirmed the common characteristics of the Notothenioidei observed macroscopically, i.e., two distinct batches of oocytes, one in the previtellogenic stage (primary growing or cortical alveoli stages) and the other in vitellogenesis and likely to be released in the current season. A longer incubation period of P. charcoti compared with P. georgianus is associated to the colder waters at the SSI. Based on our sampling and reproductive effort data, together with the reported nesting behavior for P. charcoti, it is assumed that this species spawns in nearshore, sheltered waters in summer, presumably from late December to February. Spawning periods of both congeners differ from those reported for other notothenioids in the same Seasonal Pack-ice Zone, suggesting divergence in some aspects of the life strategies in the genus Parachaenichthys. Likewise, although there are no substantial differences between P. charcoti and other notothenioids regarding gonadal development, the genus Parachaenichthys shows distinct features in its reproductive strategies (e.g., higher TF) compared with other bathydraconid species.








Similar content being viewed by others
References
Acuña E, Mujica A, Apablaza H (1992) Fish captured as by-catch during the 1991 Chilean Antarctic krill fishery. Document WG-KRILL- 92/32. CCAMLR, Hobart, Australia
Atkinson A, Whitehouse MJ, Priddle J, Cripps GC, Ward P, Brandon MA (2001) South Georgia, Antarctica: a productive, cold water, pelagic ecosystem. Mar Ecol Prog Ser 216:279–308
Barrera-Oro E (2002) The role of fish in the Antarctic marine food web: differences between inshore and offshore waters in the southern Scotia Arc and west Antarctic Peninsula. Antarct Sci 14:293–309
Barrera-Oro ER, Lagger C (2010) Egg-guarding behaviour in the Antarctic bathydraconid dragonfish Parachaenichthys charcoti. Polar Biol 33:1585–1587
Bellisio NB (1967) Peces Antárticos del sector Argentino (Parte 4), Parachaenichthys charcoti, P. georgianus y Harpagifer bispinis antarcticus de Bahía Luna. Servicio de Hidrografía Naval 904:1–57
Brown-Peterson NJ, Wyanski DM, Saborido-Rey F, Macewicz BJ, Lowerre-Barbieri SK (2011) A standardized terminology for describing reproductive development in fishes. Mar Coast Fish 3:52–70
Burchett MS (1983) Abundance of the nearshore fish population at South Georgia (Antarctica) sampled by trammel net. Br Antarct Surv Bull 61:39–43
Casaux R, Mazzotta A, Barrera-Oro ER (1990) Seasonal aspects of the biology and diet of nearshore nototheniid fish at Potter Cove, South Shetland Islands, Antarctica. Polar Biol 11:63–72
Casaux R, Barrera-Oro E, Baroni A, Ramon A (2003) Ecology of inshore notothenioid fish from the Danco Coast, Antarctic Peninsula. Polar Biol 26:157–165
Daniels RA (1982) Feeding ecology of some fishes of the Antarctic Peninsula. Fish Bull US 80:575–588
Duhamel G, Kock KH, Balguerias E, Hureau JC (1993) Reproduction in fish of the Weddell Sea. Polar Biol 13:193–200
Eastman JT, Eakin RR (2000) An updated species list for notothenioid fish (Perciformes; Notothenioidei), with comments on Antarctic species. Arch Fish Mar Res 48:11–20
Eastman JT, Sidell BD (2002) Measurements of buoyancy for some Antarctic notothenioid fishes from the South Shetland Islands. Polar Biol 25:753–760
Eastman JT, Witmer LM, Ridgely RC, Kuhn KL (2014) Divergence in skeletal mass and bone morphology in Antarctic notothenioid fishes. J Morphol 275:841–861
Efremenko VN (1983) Atlas of fish larvae of the Southern Ocean. Cybium 7:1–74
Ekau W (1991) Reproduction in high Antarctic fishes (Notothenioidei). Meeresforschung 33:159–167
Evans CW, Cziko P, Cheng CHC, DeVries AL (2005) Spawning behaviour and early development in the naked dragonfish Gymnodraco acuticeps. Antarct Sci 17(3):319–327
Gon O (1990) Bathydraconidae. In: Gon O, Heemstra PC (eds) Fishes of the Southern ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 364–380
Kamler E (1992) Early life history of fish: an energetic approach. Chapman & Hall, London, p 267
Kellermann A (1989) The larval fish community in the zone of seasonal ice cover and its seasonal and interannual variability. Arch FischWiss 39:81–109
Kellermann A (1990) Catalogue of early life stages of Antarctic notothenioid fish. Ber Polarforsch 67:45–136
Kock K-H, Kellermann A (1991) Reproduction in Antarctic notothenioid fish. Antarct Sci 3:125–150
Kompowski A (1992) Food and feeding behaviour of Parachaenichthys georgianus (Fischer, 1885) and Parachaenichthys charcoti (Vaillant, 1906) (Pisces, Bathydraconidae). Acta Ichthyol Piscat 22:15–29
Kompowski A, Rojas C (1994) On some biological characters of Parachaenichthys georgianus (Fischer, 1885) (Pisces, Notothenioidei, Bathydraconidae) from the shelf waters of South Georgia (Antarctica). Acta Ichthyol Piscat 24:61–75
Kuhn K, Near T, Detrich W, Eastman J (2011) Biology of the Antarctic dragonfish Vomeridens infuscipinnis (Notothenioidei: Bathydraconidae). Antarct Sci 23:18–26
La Mesa M, Caputo V, Eastman JT (2007) Gametogenesis in the dragon fishes Akarotaxis nudiceps and Bathydraco marri (Pisces, Notothenioidei: Bathydraconidae) from the Ross Sea. Antarct Sci 19:64–70
La Mesa M, Catalano B, Kock KH, Jones CD (2012) Age and growth of the Antarctic dragon fish Parachaenichthys charcoti (Pisces, Bathydraconidae) from the southern Scotia Arc. Polar Biol 35:1545–1553
La Mesa M, Riginella E, Jones CD (2017) Early life history traits and geographical distribution of Parachaenichthys charcoti. Antarct Sci 29:41–416. https://doi.org/10.1017/S0954102017000189
Matallanas J, Olaso I (2007) Fishes of the Bellingshausen Sea and Peter I Island. Polar Biol 30:333–341
Motta CM, Capriglione T, Frezza V, Simoniello P, Tammaro S, Filosa S (2005) Oogenesis at subzero temperatures: a comparative study of the oocyte morphology in nine species of Notothenioids. Tissue Cell 37:233–240
Murua H, Kraus G, Saborido-Rey F, Witthames PR, Thorsen A, Junquera S (2003) Procedures to estimate fecundity of marine fish species in relation to their reproductive strategy. J Northwest Atl Fish Sci 33:33–54
Near TJ et al (2012) Ancient climate change, antifreeze, and the evolutionary diversification of Antarctic fishes. Proc Nat Acad Sci USA 109:3434–3439
North AW (1991) Review of the early life history of Antarctic notothenioid fish. In: Di Prisco G, Maresca B, Tota B (eds) Biology of Antarctic fish. Springer, Berlin, pp 70–86
North AW (2001) Early life history strategies of notothenioids at South Georgia. J Fish Biol 58:496–505
North AW, White MG (1987) Reproductive strategies of Antarctic fish. In: Kullander SO, Fernholm B (eds) Proceedings of the V congress of the European ichthyological society 1985, Stockholm, pp 381–390
Nybelin O (1951) Subantarctic and Antarctic Fishes. Scientific results of the ‘‘Brategg’’ expedition 1947–1948, vol 2. Publication Christensen Hvalfanstmuseum, pp 1–32
Permitin YY (1973) Fecundity and reproductive biology of icefish (fam. Chaenichthyidae), fish of the family Muraenolepidae and dragonfish (Bathydraconidae) of the Scotia Sea (Antarctic). J Ichthyol 13:204–215
Slosarczyk W, Rembiszewski JM (1982) The occurrence of juvenile Notothenioidei (Pisces) within krill concentrations in the región of the Bransfield Strait and the Southern Drake Passage. Pol Polar Res 3:299–312
Targett TE (1981) Trophic ecology and structure of coastal Antarctic fish community. Mar Ecol Progr Ser 4:243–263
Van Der Molen S, Matallanas J (2003) Oocyte development and maturity classification of Gerlachea australis from the Weddell Sea, Antarctica. Polar Biol 26:653–658
Wallace RA, Selman K (1981) Cellular and dynamic aspects of oocyte growth in teleost. Am Zool 21:325–343
Acknowledgements
We are grateful to C. Bellisio, O. Gonzalez, J. Piscicelli and R. Varela for their help in field activities and laboratory tasks and to M. Estrada and H. Brachetta for preparation of the histologic sections. We thank three anonymous referees for their constructive comments. Funding was provided by Fondo para la Investigación Científica y Tecnológica (grant no. PICTO0100-2010) and Instituto Antártico Argentino.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Novillo, M., Moreira, E., Macchi, G. et al. Reproductive biology in the Antarctic bathydraconid dragonfish Parachaenichthys charcoti. Polar Biol 41, 2239–2248 (2018). https://doi.org/10.1007/s00300-018-2359-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-018-2359-5