Abstract
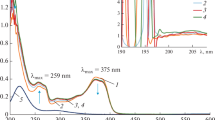
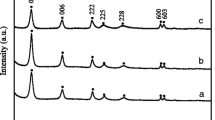
Gold nanoparticles (AuNPs) were synthesized in a microemulsion water/Triton X-100/1-pentanol/cyclohexane using various reducing agents. Basically, three microemulsion syntheses of AuNPs were studied: (i) one using a strong chemical reducing agent (NaBH4), (ii) another using γ-irradiation under moderately strong reducing/oxidizing conditions, and (iii) yet another under highly oxidizing conditions (with the addition of NaOH aqueous solution). All the three were performed at room temperature. When a strong chemical reducing agent NaBH4 was used in the microemulsion, gold crystallites 11.7 nm in size were obtained, as determined on the basis of X-ray powder diffraction line broadening. The γ-irradiation of nitrogen-saturated microemulsion at the acidic pH produced AuNPs about 12 nm in size, which under the isolation by centrifugation aggregated into large preconcentrated AuNPs about 150 nm in size. These AuNPs possess thixotropic properties. The microemulsion stirred at room temperature and at the pH < 7 under oxidizing conditions did not produce gold nanoparticles. Under the identical experimental conditions and at the pH > 7 (stronger oxidizing conditions), well-dispersed AuNPs 12 nm in size were formed. The microemulsion synthesis of AuNPs in the alkaline range but not at an acidic pH was explained by the oxidation of alcohol groups (–OH) into carbonyl groups (>C=O) due to the catalytic action of hydroxyl ions and gold. In parallel with the catalytic oxidation of alcohol groups in microemulsion, the Au(III) were reduced with the subsequent formation of gold nanoparticles. The synthesis of AuNPs in 1-pentanol by adding the aqueous NaOH solution at room temperature without using microemulsions confirmed the role of the base-catalyzed oxidation of alcohols in the formation of AuNPs. Based on the findings in this study, we propose the base-catalyzed alcohol oxidation at room temperature as a new, simple, and versatile synthesis route for obtaining gold nanoparticles. The results of this study suggest that the classical approach of using a reducing agent for the synthesis of AuNPs is not a determining factor, since a diametrically opposite approach to the synthesis of AuNPs can be used, namely, stimulating the oxidation of the functional organic groups in close proximity to gold ions.









Similar content being viewed by others
References
Pal A (1998) Photoinitiated gold sol generation in aqueous Triton X-100 and its analytical application for spectrophotometric determination of gold. Talanta 46:583–587. doi:10.1016/S0039-9140(97)00320-2
Kwon MJ, Lee J, Wark AW, Lee HJ (2012) Nanoparticle-enhanced surface plasmon resonance detection of proteins at attomolar concentrations: comparing different nanoparticle shapes and sizes. Anal Chem 84:1702–1707. doi:10.1021/ac202957h
Jiao PF, Zhou HY, Chen LX, Yan B (2011) Cancer-targeting multifunctionalized gold nanoparticles in imaging and therapy. Curr Med Chem 18:2086–2102. doi:10.2174/092986711795656199
Dykman L, Khlebtsov N (2012) Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem Soc Rev 41:2256–2282. doi:10.1039/C1CS15166E
Ide MS, Davis RJ (2014) The important role of hydroxyl on oxidation catalysis by gold nanoparticles. Acc Chem Res 47:825–833. doi:10.1021/ar4001907
Kwon Y, Lai SCS, Rodriguez P, Koper MTM (2011) Electrocatalytic oxidation of alcohols on gold in alkaline media: base or gold catalysis? J Am Chem Soc 133:6914–6917. doi:10.1021/ja200976j
Vinod CP, Wilson K, Lee AF (2011) Recent advances in the heterogeneously catalysed aerobic selective oxidation of alcohols. J Chem Technol Biotechnol 86:161–171. doi:10.1002/jctb.2504
Yuan Y, Yan N, Dyson PJ (2011) pH-sensitive gold nanoparticle catalysts for the aerobic oxidation of alcohols. Inorg Chem 50:11069–11074. doi:10.1021/ic201608j
Zope BN, Hibbitts DD, Neurock M, Davis RJ (2010) Reactivity of the gold/water interface during selective oxidation catalysis. Science 330:74–78. doi:10.1126/science.1195055
Nguyen DT, Kim D-J, Kim K-S (2011) Controlled synthesis and biomolecular probe application of gold nanoparticles. Micron 42:207–227. doi:10.1016/j.micron.2010.09.008
Chang C-C, Chen C-P, Lee C-H et al (2014) Colorimetric detection of human chorionic gonadotropin using catalytic gold nanoparticles and a peptide aptamer. Chem Commun (Camb) 50:14443–14446. doi:10.1039/c4cc06366j
Tiwari PM, Vig K, Dennis VA, Singh SR (2011) Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 1:31–63. doi:10.3390/nano1010031
Hainfeld JF, Slatkin DN, Smilowitz HM (2004) The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol 49:N309–N315
Reuveni T, Motiei M, Romman Z et al (2011) Targeted gold nanoparticles enable molecular CT imaging of cancer: an in vivo study. Int J Nanomedicine 6:2859–2864. doi:10.2147/IJN.S25446
Hainfeld JF, Slatkin DN, Focella TM, Smilowitz HM (2006) Gold nanoparticles: a new X-ray contrast agent. Br J Radiol 79:248–253. doi:10.1259/bjr/13169882
Jain S, Coulter JA, Hounsell AR et al (2011) Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int J Radiat Oncol Biol Phys 79:531–539. doi:10.1016/j.ijrobp.2010.08.044
Butterworth KT, McMahon SJ, Currell FJ, Prise KM (2012) Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale 4:4830–4838. doi:10.1039/c2nr31227a
Zhao P, Li N, Astruc D (2013) State of the art in gold nanoparticle synthesis. Coord Chem Rev 257:638–665. doi:10.1016/j.ccr.2012.09.002
Turkevich J, Stevenson PC, Hillier J (1951) A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc 11:55–75. doi:10.1039/DF9511100055
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature 241:20–22. doi:10.1038/10.1038/physci241020a0
Hanžić N, Jurkin T, Maksimović A, Gotić M (2015) The synthesis of gold nanoparticles by a citrate-radiolytical method. Radiat Phys Chem 106:77–82. doi:10.1016/j.radphyschem.2014.07.006
Zieliska-Jurek A, Reszczyska J, Grabowska E, Zalesk A (2012) Nanoparticles Preparation Using Microemulsion Systems. Microemulsions - An Introduction to Properties and Applications
Gotić M, Jurkin T, Musić S (2007) Factors that may influence the micro-emulsion synthesis of nanosize magnetite particles. Colloid Polym Sci 285:793–800. doi:10.1007/s00396-006-1624-2
Gotić M, Jurkin T, Musić S (2009) From iron(III) precursor to magnetite and vice versa. Mater Res Bull 44:2014–2021. doi:10.1016/j.materresbull.2009.06.002
Uskoković V, Drofenik M (2005) Synthesis of materials within reverse micelles. Surf Rev Lett 12:239–277. doi:10.1142/S0218625X05007001
Moulik SP, Paul BK (1998) Structure, dynamics and transport properties of microemulsions. Adv Colloid Interf Sci 78:99–195. doi:10.1016/S0001-8686(98)00063-3
Yang X-H, Wu Q-S, Li L et al (2005) Controlled synthesis of the semiconductor CdS quasi-nanospheres, nanoshuttles, nanowires and nanotubes by the reverse micelle systems with different surfactants. Colloids Surf A Physicochem Eng Asp 264:172–178. doi:10.1016/j.colsurfa.2005.05.059
Spirin MG, Brichkin SB, Razumov VF (2005) Synthesis and stabilization of gold nanoparticles in reverse micelles of Aerosol OT and Triton X-100. Colloid J 67:485–490. doi:10.1007/s10595-005-0122-4
Spirin MG, Brichkin SB, Razumov VF (2008) Studies on absorption spectra of uniform gold nanoparticles prepared in Triton X-100 reverse micelles. J Photochem Photobiol A Chem 196:174–179. doi:10.1016/j.jphotochem.2007.12.014
Vô KDN, Kowandy C, Dupont L, Coqueret X (2015) Evidence of chitosan-mediated reduction of Au(III) to Au(0) nanoparticles under electron beam by using OH˙ and e−aq scavengers. Chem Commun 51:4017–4020. doi:10.1039/C4CC09346A
Polte J, Ahner TT, Delissen F et al (2010) Mechanism of gold nanoparticle formation in the classical citrate synthesis method derived from coupled in situ XANES and SAXS evaluation. J Am Chem Soc 132:1296–1301. doi:10.1021/ja906506j
Haiss W, Thanh NTK, Aveyard J, Fernig DG (2007) Determination of size and concentration of gold nanoparticles from UV−Vis spectra. Anal Chem 79:4215–4221. doi:10.1021/ac0702084
Khlebtsov NG (2008) Determination of size and concentration of gold nanoparticles from extinction spectra. Anal Chem 80:6620–6625. doi:10.1021/ac800834n
Perkowski J, Mayer J (1994) Oxygen effect in the radiolysis of Triton X-100 aqueous solution. J Radioanal Nucl Chem Lett 188:211–217. doi:10.1007/BF02164594
Gachard E, Remita H, Khatouri J et al (1998) Radiation-induced and chemical formation of gold clusters. New J Chem 22:1257–1265. doi:10.1039/A804445G
Von Sonntag, C., Formation of Reactive Free Radicals in an Aqueous Environment, in: Free-Radical-Induced DNA Damage and Its Repair, Springer, Berlin, Heidelberg, 2006, pp. 7–46. http://link.springer.com/chapter/10.1007/3-540-30592-0_2
Abidi W, Remita H (2010) Gold based nanoparticles generated by radiolytic and photolytic methods. Recent Patents Eng 4:170–188. doi:10.2174/187221210794578556
Dey GR, El Omar AK, Jacob JA et al (2011) Mechanism of trivalent gold reduction and reactivity of transient divalent and monovalent gold ions studied by gamma and pulse radiolysis. J Phys Chem A 115:383–391. doi:10.1021/jp1096597
Urucu OA, Gündüz ZY, Yetimoğlu EK (2013) Cloud point preconcentration of gold (III) and determination by flame atomic absorption spectrometry. Indian J Chem Technol 20:106–110. http://nopr.niscair.res.in/handle/123456789/16542
El-Naggar WS, Lasheen TA, Nouh E-SA, Ghonaim AK (2010) Cloud point extraction and preconcentration of gold in geological matrices prior to flame atomic absorption determination. Cent Eur J Chem 8:34–40. doi:10.2478/s11532-009-0093-0
Samaddar P, Sen K (2014) Cloud point extraction: a sustainable method of elemental preconcentration and speciation. J Ind Eng Chem 20:1209–1219. doi:10.1016/j.jiec.2013.10.033
Melnyk A, Namieśnik J, Wolska L (2015) Theory and recent applications of coacervate-based extraction techniques. TrAC Trends Anal Chem 71:282–292. doi:10.1016/j.trac.2015.03.013
Schalek E, Szegvari A (1923) Die langsame Koagulation konzentrierter Eisenoxydsole zu reversiblen Gallerten. Kolloid-Zeitschrift 33:326–334. doi:10.1007/BF01427565
Barnes HA (1997) Thixotropy—a review. J Non-Newtonian Fluid Mech 70:1–33. doi:10.1016/S0377-0257(97)00004-9
Schon E-M, Bachl J, Diaz Diaz D (2014) Thixotropic and injectable nature of supramolecular aqueous gels derived from N, N’-dibenzoyl-L-cystine and the effects of esterification. Nanosci Nanotechnol Asia 4:31–37. doi:10.2174/2210681204666140905222744
Acknowledgments
The financial support of the Centre of Excellence for Advanced Materials and Sensors, “Ruđer Bošković” Institute, Croatia, is gratefully acknowledged. Goran Dražić acknowledges the financial support of the Slovenian Research Agency (ARRS) through Program No. P2-0393 and Project J2-6754. We thank Mr. Igor Sajko for the technical assistance on γ-irradiation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Scheme S1
Experimental procedure for the preparation of samples MAu-1 through MAu-7 and sample Au-8. (DOC 125 kb)
Table S1
UV-Vis, TEM and SEM characterization of samples. The gold nanoparticle size was determined from the UV-Vis spectra using a procedure presented by Haiss et al. [S1]. The method is based on the relative A SPR/A 450 ratio. For samples MAu-1 and MAu-3 the nanoparticle size was determined using a procedure presented by Khlebtsov [S2]. The mean particle size was calculated from TEM images using the normal function. (DOCX 15.5 kb)
Fig. S1
UV-Vis spectra of samples MAu-1 and MAu-3. Sample MAu-1 was diluted with “pure” microemulsion at a ratio of 1 : 2 (33 % dilution), whereas sample MAu-3 was recorded as synthesized. (DOCX 209 kb)
Fig. S2
SEM images of samples MAu-2 (A) and MAu-3 (B) isolated by centrifugation and recorded on a carbon support. Below the images are corresponding particle size distributions. For sample MAu-2 two size distributions are given, for small (14.9 nm) and large (153.2 nm) nanoparticles. The mean particle size was calculated using the normal function, where \( \overline{D} \) and σ stand for the mean particle diameter and standard deviation, respectively. (DOCX 5074 kb)
Fig. S3
SEM images and corresponding EDS analyses of samples MAu-2 (A) and MAu-3 (B) upon centrifugation. (DOCX 374 kb)
Fig. S4
SEM images and corresponding EDS analyses of sample MAu-3 before (A) and after centrifugation (B). The relative concentration of gold rises and the size of nanoparticles increases upon centrifugation. (DOCX 655 kb)
Fig. S5
SEM images and corresponding EDS analyses of sample MAu-2 before (A) and after the addition of acetone (B). Both samples were analyzed as microemulsion (there was no centrifugation). The size of nanoparticles did not change before or after the addition of acetone. The relative concentration of gold was higher on addition of acetone, which suggests that the samples contained an amount of Au(I) ions that were reduced upon adding acetone. Generally, acetone is added to the microemulsion prior to centrifugation in order to impair its stability and facilitate the isolation of gold nanoparticles in the form of the precipitate (powder). (DOCX 258 kb)
Rights and permissions
About this article
Cite this article
Jurkin, T., Guliš, M., Dražić, G. et al. Synthesis of gold nanoparticles under highly oxidizing conditions. Gold Bull 49, 21–33 (2016). https://doi.org/10.1007/s13404-016-0179-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13404-016-0179-3