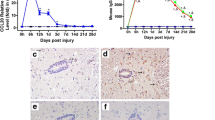
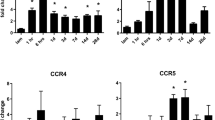
Abstract
In spite of many promising experimental studies, an effective treatment dramatically eliminating the secondary damage after spinal cord injury (SCI) is still missing. Since clinical data on the therapeutical effect after methylprednisolone treatment are not conclusive, new therapeutical modalities targeting specific components of secondary spinal cord damage needs to be developed. It is known that immune cells are recruited to injury sites by chemokines, which are small, structurally similar proteins released locally at the site of inflammation. Hence, this review was aimed to summarize possible roles of chemokines in the inflammation following SCI as well as to identify possible new therapeutical targets which can potentially be effective in ameliorating individual components of this inflammatory response. Data concerning inflammation reduction together with techniques improving axonal growth, cell replacement and remyelinization, may be crucial to move a small step forward in an attempt to make paraplegic and quadriplegic patients to walk.
Similar content being viewed by others
References
Ahn YH, Lee G, Kang SK (2006) Molecular insights of the injured lesions of rat spinal cords: inflammation, apoptosis, and cell survival. Biochem Biophys Res Commun 348:560–570. doi:10.1016/j.bbrc.2006.07.105
Amar AP, Levy ML (1999) Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery 44:1027–1039. doi:10.1097/00006123-199905000-00052
Arakaki R, Tamamura H, Premanathan M, Kanbara K, Ramanan S, Mochizuki K, Baba M, Fujii N, Nakashima H (1999) T134, a small-molecule CXCR4 inhibitor, has no cross-drug resistance with AMD3100, a CXCR4 antagonist with a different structure. J Virol 73:1719–1723
Baba M, Nishimura O, Kanzaki N, Okamoto M, Sawada H, Iizawa Y, Shiraishi M, Aramaki Y, Okonogi K, Ogawa Y, Meguro K, Fujino M (1999) A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci USA 96:5698–5703. doi:10.1073/pnas.96.10.5698
Barnes PJ (2006) How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br J Pharmacol 148:245–254. doi:10.1038/sj.bjp.0706736
Basso DM, Beattie MS, Bresnahan JC (1996) Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 139:244–256. doi:10.1006/exnr.1996.0098
Behrmann DL, Bresnahan JC, Beattie MS, Shah BR (1992) Spinal cord injury produced by consistent mechanical displacement of the cord in rats: behavioral and histologic analysis. J Neurotrauma 9:197–217
Benzel EC, Lancon JA, Bairnsfather S, Kesterson L (1990) Effect of dosage and timing of administration of naloxone on outcome in the rat ventral compression model of spinal cord injury. Neurosurgery 27:597–601. doi:10.1097/00006123-199010000-00016
Blight AR, Cohen TI, Saito K, Heyes MP (1995) Quinolinic acid accumulation and functional deficits following experimental spinal cord injury. Brain 118:735–752. doi:10.1093/brain/118.3.735
Bracken MB, Collins WF, Freeman DF, Shepard MJ, Wagner FW, Silten RM, Hellenbrand KG, Ransohoff J, Hunt WE, Perot PL Jr (1984) Efficacy of methylprednisolone in acute spinal cord injury. JAMA 251:45–52. doi:10.1001/jama.251.1.45
Bracken MB, Shepard MJ, Hellenbrand KG, Collins WF, Leo LS, Freeman DF, Wagner FC, Flamm ES, Eisenberg HM, Goodman JH (1985) Methylprednisolone and neurological function 1 year after spinal cord injury. Results of the National Acute Spinal Cord Injury Study. J Neurosurg 63:704–713
Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers L, Maroon J (1990) A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med 322:1405–1411
Bracken MB, Shepard MJ, Collins WF Jr, Holford TR, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers L, Maroon JC, Marshall LF (1992) Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J Neurosurg 76:23–31
Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings M, Herr DL, Hitchon PW, Marshall LF, Nockels RP, Pascale V, Perot PL Jr, Piepmeier J, Sonntag VK, Wagner F, Wilberger JE, Winn HR, Young W (1997) Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 277:1597–1604. doi:10.1001/jama.277.20.1597
Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings MG, Herr DL, Hitchon PW, Marshall LF, Nockels RP, Pascale V, Perot PL Jr, Piepmeier J, Sonntag VK, Wagner F, Wilberger JE, Winn HR, Young W (1998) Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg 89:699–706
Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR (2005) Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med 202:145–156. doi:10.1084/jem.20041918
Brodmerkel CM, Huber R, Covington M, Diamond S, Hall L, Collins R, Leffet L, Gallagher K, Feldman P, Collier P, Stow M, Gu X, Baribaud F, Shin N, Thomas B, Burn T, Hollis G, Yeleswaram S, Solomon K, Friedman S, Wang A, Xue CB, Newton RC, Scherle P, Vaddi K (2005) Discovery and pharmacological characterization of a novel rodent-active CCR2 antagonist, INCB3344. J Immunol 175:5370–5378
Byrnes KR, Waynant RW, Ilev IK, Wu X, Barna L, Smith K, Heckert R, Gerst H, Anders JJ (2005) Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg Med 36:171–185. doi:10.1002/lsm.20143
Campbell SJ, Hughes PM, Iredale JP, Wilcockson DC, Waters S, Docagne F, Perry VH, Anthony DC (2003) CINC-1 is an acute-phase protein induced by focal brain injury causing leukocyte mobilization and liver injury. FASEB J 17:1168–1170
Campbell SJ, Perry VH, Pitossi FJ, Butchart AG, Chertoff M, Waters S, Dempster R, Anthony DC (2005) Central nervous system injury triggers hepatic CC and CXC chemokine expression that is associated with leukocyte mobilization and recruitment to both the central nervous system and the liver. Am J Pathol 166:1487–1497
Chan CC (2008) Inflammation: beneficial or detrimental after spinal cord injury? Recent Pat CNS Drug Discov 3:189–199. doi:10.2174/157488908786242434
Čížková D, Rosocha J, Vanický I, Jergová S, Čízek M (2006) Transplants of human mesenchymal stem cells improve functional recovery after spinal cord injury in the rat. Cell Mol Neurobiol 26:1167–1180
Damon I, Murphy PM, Moss B (1998) Broad spectrum chemokine antagonistic activity of a human poxvirus chemokine homolog. Proc Natl Acad Sci USA 95:6403–6407. doi:10.1073/pnas.95.11.6403
De Lucca GV, Kim UT, Vargo BJ, Duncia JV, Santella JB 3rd, Gardner DS, Zheng C, Liauw A, Wang Z, Emmett G, Wacker DA, Welch PK, Covington M, Stowell NC, Wadman EA, Das AM, Davies P, Yeleswaram S, Graden DM, Solomon KA, Newton RC, Trainor GL, Decicco CP, Ko SS (2005) Discovery of CC chemokine receptor-3 (CCR3) antagonists with picomolar potency. J Med Chem 48:2194–2211. doi:10.1021/jm049530m
DeVivo MJ (1997) Causes and costs of spinal cord injury in the US. Spinal Cord 35:809–813. doi:10.1038/sj.sc.3100501
Dusart I, Schwab ME (1994) Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J NeuroSci 6:712–724. doi:10.1111/j.1460-9568.1994.tb00983.x
Dwyer MP, Yu Y, Chao J, Aki C, Chao J, Biju P, Girijavallabhan V, Rindgen D, Bond R, Mayer-Ezel R, Jakway J, Hipkin RW, Fossetta J, Gonsiorek W, Bian H, Fan X, Terminelli C, Fine J, Lundell D, Merritt JR, Rokosz LL, Kaiser B, Li G, Wang W, Stauffer T, Ozgur L, Baldwin J, Taveras AG (2006) Discovery of 2-hydroxy-N, N-dimethyl-3-{2-[[(R)-1-(5-methylfuran-2-yl)propyl]amino]-3, 4-dioxocyclobut-1-enylamino}benzamide (SCH 527123): a potent, orally bioavailable CXCR2/CXCR1 receptor antagonist. J Med Chem 49:7603–7606. doi:10.1021/jm0609622
Egberink HF, De Clercq E, Van Vliet AL, Balzarini J, Bridger GJ, Henson G, Horzinek MC, Schols D (1999) Bicyclams, selective antagonists of the human chemokine receptor CXCR4, potently inhibit feline immunodeficiency virus replication. J Virol 73:6346–6352
Eidelberg E, Staten E, Watkins CJ, Smith JS (1976) Treatment of experimental spinal cord injury in ferrets. Surg Neurol 6:243–246
Fawcett JW, Asher RA (1999) The glial scar and central nervous system repair. Brain Res Bull 49:377–391. doi:10.1016/S0361-9230(99)00072-6
Fehlings MG (2001) Editorial: Recommendations regarding the use of methylprednisolone in acute spinal cord injury: making sense out of the controversy. Spine 26(24S):S56–S57. doi:10.1097/00007632-200112151-00012
Fouad K, Dietz V, Schwab ME (2001) Improving axonal growth and functional recovery after experimental spinal cord injury by neutralizing myelin associated inhibitors. Brain Res Brain Res Rev 36:204–212. doi:10.1016/S0165-0173(01)00096-0
Gale K, Kerasidis H, Wrathall JR (1985) Spinal cord contusion in the rat: behavioral analysis of functional neurologic impairment. Exp Neurol 88:123–126. doi:10.1016/0014-4886(85)90118-9
Gladue RP, Tylaska LA, Brissette WH, Lira PD, Kath JC, Poss CS, Brown MF, Paradis TJ, Conklyn MJ, Ogborne KT, McGlynn MA, Lillie BM, DiRico AP, Mairs EN, McElroy EB, Martin WH, Stock IA, Shepard RM, Showell HJ, Neote K (2003) CP-481, 715, a potent and selective CCR1 antagonist with potential therapeutic implications for inflammatory diseases. J Biol Chem 278:40473–40480. doi:10.1074/jbc.M306875200
Glaser J, Gonzalez R, Perreau VM, Cotman CW, Keirstead HS (2004) Neutralization of the chemokine CXCL10 enhances tissue sparing and angiogenesis following spinal cord injury. J Neurosci Res 77:701–708. doi:10.1002/jnr.20204
Glaser J, Gonzalez R, Sadr E, Keirstead HS (2006) Neutralization of the chemokine CXCL10 reduces apoptosis and increases axon sprouting after spinal cord injury. J Neurosci Res 84:724–734. doi:10.1002/jnr.20982
Gonzalez R, Glaser J, Liu MT, Lane TE, Keirstead HS (2003) Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp Neurol 184:456–463. doi:10.1016/S0014-4886(03)00257-7
Gonzalez R, Hickey MJ, Espinosa JM, Nistor G, Lane TE, Keirstead HS (2007) Therapeutic neutralization of CXCL10 decreases secondary degeneration and functional deficit after spinal cord injury in mice. Regen Med 2:771–783. doi:10.2217/17460751.2.5.771
Gruner JA (1992) A monitored contusion model of spinal cord injury in the rat. J Neurotrauma 9:123–128
Hausmann ON (2003) Post-traumatic inflammation following spinal cord injury. Spinal Cord 41:369–378. doi:10.1038/sj.sc.3101483
Hedlund E, Hefferan MP, Marsala M, Isacson O (2007) Cell therapy and stem cells in animal models of motor neuron disorders. Eur J NeuroSci 26:1721–1737. doi:10.1111/j.1460-9568.2007.05780.x
Hefferan MP, Fuchigami T, Marsala M (2006) Development of baclofen tolerance in a rat model of chronic spasticity and rigidity. Neurosci Lett 403:195–200. doi:10.1016/j.neulet.2006.04.048
Heise CE, Pahuja A, Hudson SC, Mistry MS, Putnam AL, Gross MM, Gottlieb PA, Wade WS, Kiankarimi M, Schwarz D, Crowe P, Zlotnik A, Alleva DG (2005) Pharmacological characterization of CXC chemokine receptor 3 ligands and a small molecule antagonist. J Pharmacol Exp Ther 313:1263–1271. doi:10.1124/jpet.105.083683
Hiruma S, Otsuka K, Satou T, Hashimoto S (1999) Simple and reproducible model of rat spinal cord injury induced by a controlled cortical impact device. Neurol Res 21:313–323
Holtz A, Nystrom B, Gerdin B (1990) Relation between spinal cord blood flow and functional recovery after blocking weight-induced spinal cord injury in rats. Neurosurgery 26:952–957. doi:10.1097/00006123-199006000-00005
Horuk R, Clayberger C, Krensky AM, Wang Z, Grone HJ, Weber C, Weber KS, Nelson PJ, May K, Rosser M, Dunning L, Liang M, Buckman B, Ghannam A, Ng HP, Islam I, Bauman JG, Wei GP, Monahan S, Xu W, Snider RM, Morrissey MM, Hesselgesser J, Perez HD (2001) A non-peptide functional antagonist of the CCR1 chemokine receptor is effective in rat heart transplant rejection. J Biol Chem 276:4199–4204. doi:10.1074/jbc.M007457200
Hugenholtz H (2003) Methylprednisolone for acute spinal cord injury: not a standard of care. CMAJ 168:1145–1146
Hurlbert RJ (2000) Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg 93:1–7
Imai M, Watanabe M, Suyama K, Osada T, Sakai D, Kawada H, Matsumae M, Mochida J (2008) Delayed accumulation of activated macrophages and inhibition of remyelination after spinal cord injury in an adult rodent model. J Neurosurg Spine 8:58–66. doi:10.3171/SPI-08/01/058
Jayasuriya H, Herath KB, Ondeyka JG, Polishook JD, Bills GF, Dombrowski AW, Springer MS, Siciliano S, Malkowitz L, Sanchez M, Guan Z, Tiwari S, Stevenson DW, Borris RP, Singh SB (2004) Isolation and structure of antagonists of chemokine receptor (CCR5). J Nat Prod 67:1036–1038. doi:10.1021/np049974l
Ji C, Zhang J, Dioszegi M, Chiu S, Rao E, Derosier A, Cammack N, Brandt M, Sankuratri S (2007) CCR5 small-molecule antagonists and monoclonal antibodies exert potent synergistic antiviral effects by cobinding to the receptor. Mol Pharmacol 72:18–28. doi:10.1124/mol.107.035055
Jones TB, Hart RP, Popovich PG (2005) Molecular control of physiological and pathological T-cell recruitment after mouse spinal cord injury. J Neurosci 25:6576–6583. doi:10.1523/JNEUROSCI.0305-05.2005
Kakulas BA (1987) The clinical neuropathology of spinal cord injury: a guide to the future. Paraplegia 25:212–216
Kauffman GS, Watson PS, Nugent WA (2006) Strategy for the enantioselective synthesis of trans-2, 4-disubstituted piperidines: application to the CCR3 antagonist IS811. J Org Chem 71:8975–8977. doi:10.1021/jo0616963
Kavanagh RJ, Kam PC (2001) Lazaroids: efficacy and mechanism of action of the 21-aminosteroids in neuroprotection. Br J Anaesth 86:110–119. doi:10.1093/bja/86.1.110
Kawa S, Kimura S, Hakomori S, Igarashi Y (1997) Inhibition of chemotactic motility and trans-endothelial migration of human neutrophils by sphingosine 1-phosphate. FEBS Lett 420:196–200. doi:10.1016/S0014-5793(97)01516-0
Keilhoff G, Stang F, Goihl A, Wolf G, Fansa H (2006) Transdifferentiated mesenchymal stem cells as alternative therapy in supporting nerve regeneration and myelination. Cell Mol Neurobiol 26:1235–1252. doi:10.1007/s10571-006-9029-9
Khan M, Griebel R (1983) Acute spinal cord injury in the rat: comparison of three experimental techniques. Can J Neurol Sci 10:161–165
Kledal TN, Rosenkilde MM, Coulin F, Simmons G, Johnsen AH, Alouani S, Power CA, Luttichau HR, Gerstoft J, Clapham PR, Clark-Lewis I, Wells TN, Schwartz TW (1997) A broad-spectrum chemokine antagonist encoded by Kaposi’s sarcoma-associated herpesvirus. Science 277:1656–1659. doi:10.1126/science.277.5332.1656
Kunihara T, Sasaki S, Shiiya N, Ishikura H, Kawarada Y, Matsukawa A, Yasuda K (2000) Lazaroid reduces production of IL-8 and IL-1receptor antagonist in ischemic spinal cord injury. Ann Thorac Surg 69:792–798. doi:10.1016/S0003-4975(99)01413-7
Kuppens T, Herrebout W, Van Der Veken B, Corens D, De Groot A, Doyon J, Van Lommen G, Bultinck P (2006) Elucidation of the absolute configuration of JNJ-27553292, a CCR2 receptor antagonist, by vibrational circular dichroism analysis of two precursors. Chirality 18:609–620. doi:10.1002/chir.20297
Lee YL, Shih K, Bao P, Ghirnikar RS, Eng LF (2000) Cytokine chemokine expression in contused rat spinal cord. Neurochem Int 36:417–425. doi:10.1016/S0197-0186(99)00133-3
Li F, Zhang X, Gordon JR (2002) CXCL8((3–73))K11R/G31P antagonizes ligand binding to the neutrophil CXCR1 and CXCR2 receptors and cellular responses to CXCL8/IL-8. Biochem Biophys Res Commun 293:939–944. doi:10.1016/S0006-291X(02)00318-2
Ma M, Wei T, Boring L, Charo IF, Ransohoff RM, Jakeman LB (2002) Monocyte recruitment and myelin removal are delayed following spinal cord injury in mice with CCR2 chemokine receptor deletion. J Neurosci Res 68:691–702. doi:10.1002/jnr.10269
Marks K, Gulick RM (2004) New antiretroviral agents for the treatment of HIV infection. Curr HIV/AIDS Rep 1:82–88. doi:10.1007/s11904-004-0012-0
Martin SH, Bloedel JR (1973) Evaluation of experimental spinal cord injury using cortical evoked potentials. J Neurosurg 39:75–81
Miyazaki H, Patel V, Wang H, Edmunds RK, Gutkind JS, Yeudall WA (2006) Down-regulation of CXCL5 inhibits squamous carcinogenesis. Cancer Res 66:4279–4284. doi:10.1158/0008-5472.CAN-05-4398
Morokata T, Suzuki K, Masunaga Y, Taguchi K, Morihira K, Sato I, Fujii M, Takizawa S, Torii Y, Yamamoto N, Kaneko M, Yamada T, Takahashi K, Shimizu Y (2006) A novel, selective, and orally available antagonist for CC chemokine receptor 3. J Pharmacol Exp Ther 317:244–250. doi:10.1124/jpet.105.097048
Mueller CA, Schluesener HJ, Conrad S, Pietsch T, Schwab JM (2006) Spinal cord injury-induced expression of the immune-regulatory chemokine interleukin-16 caused by activated microglia/macrophages and CD8 + cells. J Neurosurg Spine 4:233–240. doi:10.3171/spi.2006.4.3.233
Nibbs RJ, Salcedo TW, Campbell JD, Yao XT, Li Y, Nardelli B, Olsen HS, Morris TS, Proudfoot AE, Patel VP, Graham GJ (2000) C-C chemokine receptor 3 antagonism by the beta-chemokine macrophage inflammatory protein 4, a property strongly enhanced by an amino-terminal alanine–methionine swap. J Immunol 164:1488–1497
Nicholson GC, Tennant RC, Carpenter DC, Sarau HM, Kon OM, Barnes PJ, Salmon M, Vessey RS, Tal-Singer R, Hansel TT (2007) A novel flow cytometric assay of human whole blood neutrophil and monocyte CD11b levels: upregulation by chemokines is related to receptor expression, comparison with neutrophil shape change, and effects of a chemokine receptor (CXCR2) antagonist. Pulm Pharmacol Ther 20:52–59. doi:10.1016/j.pupt.2005.11.009
Nystrom B, Berglind JE (1988) Spinal cord restitution following compression injuries in rats. Acta Neurol Scand 78:467–472. doi:10.1111/j.1600-0404.1988.tb03689.x
Palani A, Shapiro S, Clader JW, Greenlee WJ, Cox K, Strizki J, Endres M, Baroudy BM (2001) Discovery of 4-[(Z)-(4-bromophenyl)-(ethoxyimino)methyl]-1’-[(2,4-dimethyl-3-pyridinyl)carbonyl]-4’-methyl-1, 4’-bipiperidine N-oxide (SCH 351125): an orally bioavailable human CCR5 antagonist for the treatment of HIV infection. J Med Chem 44:3339–3342. doi:10.1021/jm015526o
Petkovic V, Moghini C, Paoletti S, Uguccioni M, Gerber B (2004) Eotaxin-3/CCL26 is a natural antagonist for CC chemokine receptors 1 and 5. A human chemokine with a regulatory role. J Biol Chem 279:23357–23363. doi:10.1074/jbc.M309283200
Popovich PG, Wei P, Stokes BT (1997) Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol 377:443–464. doi:10.1002/(SICI)1096-9861(19970120)377:3<443::AID-CNE10>;3.0.CO;2-S
Princen K, Hatse S, Vermeire K, Aquaro S, De Clercq E, Gerlach LO, Rosenkilde M, Schwartz TW, Skerlj R, Bridger G, Schols D (2004) Inhibition of human immunodeficiency virus replication by a dual CCR5/CXCR4 antagonist. J Virol 78:12996–13006. doi:10.1128/JVI.78.23.12996-13006.2004
Proudfoot AE, Power CA, Hoogewerf AJ, Montjovent MO, Borlat F, Offord RE, Wells TN (1996) Extension of recombinant human RANTES by the retention of the initiating methionine produces a potent antagonist. J Biol Chem 271:2599–2603. doi:10.1074/jbc.271.5.2599
Pruitt JR, Batt DG, Wacker DA, Bostrom LL, Booker SK, McLaughlin E, Houghton GC, Varnes JG, Christ DD, Covington M, Das AM, Davies P, Graden D, Kariv I, Orlovsky Y, Stowell NC, Vaddi KG, Wadman EA, Welch PK, Yeleswaram S, Solomon KA, Newton RC, Decicco CP, Carter PH, Ko SS (2007) CC chemokine receptor-3 (CCR3) antagonists: improving the selectivity of DPC168 by reducing central ring lipophilicity. Bioorg Med Chem Lett 17:2992–2997. doi:10.1016/j.bmcl.2007.03.065
Rappert A, Bechmann I, Pivneva T, Mahlo J, Biber K, Nolte C, Kovac AD, Gerard C, Boddeke HW, Nitsch R, Kettenmann H (2004) CXCR3-dependent microglial recruitment is essential for dendrite loss after brain lesion. J Neurosci 24:8500–8509. doi:10.1523/JNEUROSCI.2451-04.2004
Ravikumar R, Flora G, Geddes JW, Hennig B, Toborek M (2004) Nicotine attenuates oxidative stress, activation of redox-regulated transcription factors and induction of proinflammatory genes in compressive spinal cord trauma. Brain Res Mol Brain Res 124:188–198. doi:10.1016/j.molbrainres.2004.02.018
Renault-Mihara F, Okada S, Shibata S, Nakamura M, Toyama Y, Okano H (2008) Spinal cord injury: emerging beneficial role of reactive astrocytes’ migration. Int J Biochem Cell Biol 40:1649–1653. doi:10.1016/j.biocel.2008.03.009
Rice T, Larsen J, Rivest S, Yong VW (2007) Characterization of the early neuroinflammation after spinal cord injury in mice. J Neuropathol Exp Neurol 66:184–195. doi:10.1097/01.jnen.0000248552.07338.7f
Sabroe I, Peck MJ, Van Keulen BJ, Jorritsma A, Simmons G, Clapham PR, Williams TJ, Pease JE (2000) A small molecule antagonist of chemokine receptors CCR1 and CCR3. Potent inhibition of eosinophil function and CCR3-mediated HIV-1 entry. J Biol Chem 275:25985–25992. doi:10.1074/jbc.M908864199
Schnell L, Fearn S, Klassen H, Schwab ME, Perry VH (1999) Acute inflammatory responses to mechanical lesions in the CNS: differences between brain and spinal cord. Eur J NeuroSci 11:3648–3658. doi:10.1046/j.1460-9568.1999.00792.x
Schwab JM, Brechtel K, Mueller CA, Failli V, Kaps HP, Tuli SK, Schluesener HJ (2006) Experimental strategies to promote spinal cord regeneration—an integrative perspective. Prog Neurobiol 78:91–116. doi:10.1016/j.pneurobio.2005.12.004
Simmons G, Clapham PR, Picard L, Offord RE, Rosenkilde MM, Schwartz TW, Buser R, Wells TN, Proudfoot AE (1997) Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science 276:276–279. doi:10.1126/science.276.5310.276
Steinberger P, Andris-Widhopf J, Buhler B, Torbett BE, Barbas CF 3rd (2000a) Functional deletion of the CCR5 receptor by intracellular immunization produces cells that are refractory to CCR5-dependent HIV-1 infection and cell fusion. Proc Natl Acad Sci USA 97:805–810. doi:10.1073/pnas.97.2.805
Steinberger P, Sutton JK, Rader C, Elia M, Barbas CF 3rd (2000b) Generation and characterization of a recombinant human CCR5-specific antibody. A phage display approach for rabbit antibody humanization. J Biol Chem 275:36073–36078. doi:10.1074/jbc.M002765200
Stichel CC, Muller HW (1998) The CNS lesion scar: new vistas on an old regeneration barrier. Cell Tissue Res 294:1–9. doi:10.1007/s004410051151
Stokes BT, Noyes DH, Behrmann DL (1992) An electromechanical spinal injury technique with dynamic sensitivity. J Neurotrauma 9:187–195
Takenaga M, Ohta Y, Tokura Y, Hamaguchi A, Nakamura M, Okano H, Igarashi R (2006) Lecithinized superoxide dismutase (PC-SOD) improved spinal cord injury-induced motor dysfunction through suppression of oxidative stress and enhancement of neurotrophic factor production. J Control Release 110:283–289. doi:10.1016/j.jconrel.2005.10.022
Tamamura H, Imai M, Ishihara T, Masuda M, Funakoshi H, Oyake H, Murakami T, Arakaki R, Nakashima H, Otaka A, Ibuka T, Waki M, Matsumoto A, Yamamoto N, Fujii N (1998) Pharmacophore identification of a chemokine receptor (CXCR4) antagonist, T22 ([Tyr(5, 12), Lys7]-polyphemusin II), which specifically blocks T cell-line-tropic HIV-1 infection. Bioorg Med Chem 6:1033–1041. doi:10.1016/S0968-0896(98)00061-3
Tang BL, Low CB (2007) Genetic manipulation of neural stem cells for transplantation into the injured spinal cord. Cell Mol Neurobiol 27:75–85. doi:10.1007/s10571-006-9119-8
Tarlov IM, Klinger H, Vitale S (1953) Spinal cord compression studies. I. Experimental techniques to produce acute and gradual compression. AMA Arch Neurol Psychiatry 70:813–819
Tator CH (1998) Biology of neurological recovery and functional restoration after spinal cord injury. Neurosurgery 42:696–707. doi:10.1097/00006123-199804000-00007
Tator CH, Deecke L (1973) Studies of the treatment and pathophysiology of acute spinal cord injury in primates. Paraplegia 10:344–345
Tonai T, Shiba K, Taketani Y, Ohmoto Y, Murata K, Muraguchi M, Ohsaki H, Takeda E, Nishisho T (2001) A neutrophil elastase inhibitor (ONO-5046) reduces neurologic damage after spinal cord injury in rats. J Neurochem 78:1064–1072. doi:10.1046/j.1471-4159.2001.00488.x
Tremblay CL, Giguel F, Chou TC, Dong H, Takashima K, Hirsch MS (2005a) TAK-652, a novel CCR5 inhibitor, has favourable drug interactions with other antiretrovirals in vitro. Antivir Ther 10:967–968
Tremblay CL, Giguel F, Guan Y, Chou TC, Takashima K, Hirsch MS (2005b) TAK-220, a novel small-molecule CCR5 antagonist, has favorable anti-human immunodeficiency virus interactions with other antiretrovirals in vitro. Antimicrob Agents Chemother 49:3483–3485. doi:10.1128/AAC.49.8.3483-3485.2005
Ubogu EE, Cossoy MB, Ransohoff RM (2006) The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci 27:48–55. doi:10.1016/j.tips.2005.11.002
Vanicky I, Urdzikova L, Saganova K, Cizkova D, Galik J (2001) A simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. J Neurotrauma 18:1399–1407. doi:10.1089/08977150152725687
Walser TC, Rifat S, Ma X, Kundu N, Ward C, Goloubeva O, Johnson MG, Medina JC, Collins TL, Fulton AM (2006) Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer. Cancer Res 66:7701–7707. doi:10.1158/0008-5472.CAN-06-0709
Watson C, Jenkinson S, Kazmierski W, Kenakin T (2005) The CCR5 receptor-based mechanism of action of 873140, a potent allosteric noncompetitive HIV entry inhibitor. Mol Pharmacol 67:1268–1282. doi:10.1124/mol.104.008565
White JR, Lee JM, Young PR, Hertzberg RP, Jurewicz AJ, Chaikin MA, Widdowson K, Foley JJ, Martin LD, Griswold DE, Sarau HM (1998) Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J Biol Chem 273:10095–10098. doi:10.1074/jbc.273.17.10095
White JR, Lee JM, Dede K, Imburgia CS, Jurewicz AJ, Chan G, Fornwald JA, Dhanak D, Christmann LT, Darcy MG, Widdowson KL, Foley JJ, Schmidt DB, Sarau HM (2000) Identification of potent, selective non-peptide CC chemokine receptor-3 antagonist that inhibits eotaxin-, eotaxin-2-, and monocyte chemotactic protein-4-induced eosinophil migration. J Biol Chem 275:36626–36631. doi:10.1074/jbc.M006613200
Wu X, Fan J, Wang X, Zhou J, Qiu S, Yu Y, Liu Y, Tang Z (2007) Downregulation of CCR1 inhibits human hepatocellular carcinoma cell invasion. Biochem Biophys Res Commun 355:866–871. doi:10.1016/j.bbrc.2007.01.199
Yang L, Butora G, Jiao RX, Pasternak A, Zhou C, Parsons WH, Mills SG, Vicario PP, Ayala JM, Cascieri MA, MacCoss M (2007) Discovery of 3-piperidinyl-1-cyclopentanecarboxamide as a novel scaffold for highly potent CC chemokine receptor 2 antagonists. J Med Chem 50:2609–2611. doi:10.1021/jm070166b
Acknowledgment
We thank Professor Martin Maršala, M.D. (Anesthesiology Research Laboratory, University of California, USA) for critically reading the manuscripts and for his useful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
P. Gál and P. Kravčuková contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gál, P., Kravčuková, P., Mokrý, M. et al. Chemokines as Possible Targets in Modulation of the Secondary Damage After Acute Spinal Cord Injury: A Review. Cell Mol Neurobiol 29, 1025–1035 (2009). https://doi.org/10.1007/s10571-009-9392-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-009-9392-4