Abstract
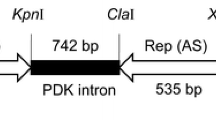
The coat protein (CP) gene of Tobacco streak virus (TSV) from sunflower (Helianthus annuus L.) was amplified, cloned and sequenced. A 421 bp fragment of the TSV coat protein gene was amplified and a gene construct encoding the hairpin RNA (hpRNA) of the TSV-CP sequence was made in the plasmid pHANNIBAL. The construct contains sense and antisense CP sequences flanking a 742 bp spacer sequence (Pdk intron) under the control of the constitutive CaMV35S promoter. A 3.6 kb Not I fragment containing the hpRNA cassette (TSV-CP) was isolated from pHANNIBAL and sub-cloned into the binary vector pART27. This chimeric gene construct was then mobilized into Agrobacterium tumefaciens strain LBA4404 via triparental mating using pRK2013 as a helper. Sunflower (cv. Co 4) and tobacco (cv. Petit Havana) plants were transformed with A. tumefaciens strain LBA4404 harbouring the hpRNA cassette and in vitro selection was performed with kanamycin. The integration of the transgene into the genome of the transgenic lines was confirmed by PCR analysis. Infectivity assays with TSV by mechanical sap inoculation demonstrated that both the sunflower and tobacco transgenic lines exhibited resistance to TSV infection and accumulated lower levels of TSV compared with non-transformed controls.
Similar content being viewed by others
Abbreviations
- CP:
-
coat protein
- PDR:
-
pathogen derived resistance
- TSV:
-
Tobacco streak virus
References
An, G.: Binary Ti vectors for plant transformation and promoter analysis. — Meth. Enzymol. 153: 292–305, 1987.
Bag, S., Singh, R.S., Jain, R.K.: Agrobacterium-mediated transformation of groundnut with coat protein gene of Tobacco streak virus. — Indian J. Virol. 18: 65–69, 2007.
Baulcombe, D.C.: Mechanisms of pathogen-derived resistance to viruses in transgenic plants. — Plant Cell 8: 1833–1844, 1996.
Baulcombe, D.C.: RNA silencing. — Curr. Biol. 12: R82–R84, 2002.
Bhat, A.I., Jain, R.K., Kumar, A., Ramiah, M., Varma, A.: Serological and coat protein sequence studies suggest that necrosis disease on sunflower in India is caused by strain of Tobacco streak ilarvirus. — Arch. Virol. 147: 651–658, 2002.
Bonfim, K., Faria, J.C., Nogueira, E.O.P.L., Mendes, E.A., Aragao, F.J.L.: RNAi-mediated resistance to Bean golden mosaic virus in genetically engineered common bean (Phaseolus vulgaris). — Mol. Plant Microbe Interact. 20: 717–726, 2007.
Chander Rao, S., Prasada Rao, R.D.V.J., Manojkumar, V., Raman, D.S., Raoof, M.A., Prasad, R.D.: First report of Tobacco streak virus infecting safflower in Maharashtra, India. — Plant Dis. 87: 1396, 2003.
Cupertino, F.O., Grogan, R.G., Petersen, L.J., Kimble, K.A.: Tobacco streak virus infection of tomato and some natural weed hosts in California. — Plant Dis. 68: 331–333, 1984.
Dijkstra, J.: Tobacco streak virus in sunflower (Helianthus annuus). — Neth. J. Plant Pathol. 89: 153–169, 1983.
Fagbenle, H.H., Ford, R.E.: Tobacco streak virus isolated from soybeans, Glycine max. — Phytopathology 60: 814–820, 1970.
Fahim, M., Ayala-Navarrete, L., Millar, A.A., Larkin, P.J.: Hairpin RNA derived from viral NIa gene confers immunity to Wheat streak mosaic virus infection in transgenic wheat plants. — Plant Biotechnol. J. 8: 821–834, 2010.
Ferreira, S.A., Pitz, K.Y., Manshardt, R., Zee, F., Fitch, M., Gonsalves, D.: Virus coat protein transgenic papaya provides practical control of Papaya ringspot virus in Hawaii. — Plant Dis. 86: 101–105, 2002.
Gleave, A.P.: A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. — Plant mol. Biol. 20: 1203–1207, 1992.
Goldbach, R., Bucher, E., Prins, M.: Resistance mechanisms to plant viruses: an overview. — Virus Res. 92: 207–212, 2003.
Gracia, O., Feldman, J.M.: Tobacco streak virus in pepper. — Phytopathol. Z. 80: 313–323, 1974.
Greber, R.S., Klose, M.J., Teakle, D.S., Milne, J.R.: High incidence of Tobacco streak virus in tobacco and its transmission by Microcephalothrips abdominalis and pollen from Ageratum houstonianum. — Plant Dis. 74: 450–452, 1991.
Gutha, L.R., Reddy, A.R.: Rice DREB1B promoter shows distinct stress-specific responses, and the overexpression of cDNA in tobacco confers improved abiotic and biotic stress tolerance. — Plant mol. Biol. 68: 533–555, 2008.
Horsch, R.B., Fry, J.E., Hoffman, N.L., Eichholtz, D., Rogers, S.G., Fraley, R.T.: A simple and general method for transferring genes into plants. — Science 227: 1229–1231, 1985.
Hull, R.: Matthews’ Plant Virology. 4th Ed. — Academic Press, San Diego 2002.
Jefferson, R.A., Kavanagh, T.A., Bevan, M.W.: GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. — EMBO J. 6: 3901–3907, 1987.
Jiang, F., Wu, B., Zhang, C., Song, Y., An, H., Zhu, C., Wen, F.: Special origin of stem sequence influence the resistance of hairpin expressing plants against PVY. — Biol. Plant. 55: 528–535, 2011.
Kaiser, W.J., Wyatt, S.D., Pesho, G.R.: Natural hosts and vectors of tobacco streak virus in Eastern Washington. — Phytopathology 72:1508-1512, 1982.
Kalantidis, K., Psaradakis, S., Tabler, M., Sagris, M.: The occurrence of CMV-specific short RNAs in transgenic tobacco expressing virus-derived double-stranded RNA is indicative of resistance to the virus. — Mol. Plant-Microbe Interact. 15: 826–833, 2002.
Krishnareddy, M., Devaraj, R.L., Jalali, S., Samuel, D.K.: Outbreak of Tobacco streak virus causing necrosis of cucumber (Cucumis sativus) and gherkin (Cucumis anguria) in India. — Plant Dis. 87:1264, 2003.
Lewi, D.M., Hopp, H.E., Escandon, A.S.: Sunflower (Helianthus annuus L.). — Meth. mol. Biol. 343: 291–297, 2006.
Lomonossoff, G.P.: Pathogen-derived resistance to plant viruses. — Annu. Rev. Phytopathol. 33: 323–343, 1995.
Murashige, T., Skoog, F.A.: A revised medium for rapid growth and bioassays with tobacco tissue cultures. — Physiol. Plant 15: 473–497, 1962.
Patil, B.L., Ogwok, E., Wagaba, H., Mohammed, I.U., Yadav, J.S., Bagewadi, B., Taylor, N.J., Kreuze, J.F., Maruthi, M.N., Alicai, T., Fauquet, C.M.: RNAi-mediated resistance to diverse isolates belonging to two virus species involved in cassava brown streak disease. — Mol. Plant Pathol. 12: 31–41, 2011.
Prasada Rao, R.D.V.J., Reddy, A.S., Chander Rao, A.S., Varaprasad, K.S., Thirumala Devi, K., Nagaraju, Muniyappa, V., Reddy, D.V.R.: Tobacco streak ilarvirus as a causal agent of sunflower necrosis disease in India. — J. Oilseeds Res. 17: 400–401, 2000.
Prasada Rao, R.D.V.J., Reddy, A.S., Reddy, S.V., Thirumala Devi, K., Chander Rao, S., Manoj Kumar, V., Subramanyam, K., Yellamanda Reddy, T., Nigam, S.N., Reddy, D.V.R.: The host range of Tobacco streak virus in India and transmission by thrips. — Ann. appl. Biol.142: 365–368, 2003.
Ramiah, M., Bhat, A.I., Jain, R.K., Pant, R.P., Ahlawat, Y.S., Prabhakar, K., Varma, A.: Isolation of an isometric virus causing sunflower necrosis disease in India. — Plant Dis. 85: 443, 2001.
Reddy, A.S., Prasada Rao, R.D.V.J., Thirumala Devi, K., Reddy, S.V., Mayo, M.A., Roberts, I., Satyanarayana, T., Subramaniam, K., Reddy, D.V.R.: Occurrence of Tobacco streak virus on peanut (Arachis hypogaea) in India. — Plant Dis. 86:173-178, 2002.
Salazar, L.F., Abad, J.A., Hooker, W.J.: Host range and properties of a strain of tobacco streak virus from potatoes. — Phytopathology 72: 1550–1554, 1982.
Sambrook, J., Russell, D.W.: Molecular Cloning: a Laboratory Manual. 3rd Ed. — Cold Spring Harbor Laboratory Press, Cold Spring Harbor -New York 2001.
Sanford, J.C., Johnston, S.A.: The concept of parasite-derived resistance-deriving resistance genes from the parasite’s own genome. — J. theor. Biol. 113: 395–405, 1985.
Schwind, N., Zwiebel, M., Itaya, A., Ding, B., Wang, M.B., Krczal, G., Wassenegger, M.: RNAi-mediated resistance to potato spindle tuber viroid in transgenic tomato expressing a viroid hairpin RNA construct. — Mol. Plant Pathol. 10: 459–469, 2009.
Sdoodee, R., Teakle, D.S.: Transmission of tobacco streak virus by thrips tabaci; a new method of plant virus transmission. — Plant Pathol. 36: 377–380, 1987.
Sharman, M., Persley, D.M., Thomas, J.E.: Distribution in Australia and seed transmission of Tobacco streak virus in Parthenium hysterophorus. — Plant Dis. 93: 708–712, 2009.
Shimizu, T., Nakazono-Nagaoka, E., Uehara-Ichiki, T., Sasaya, T., Omura, T.: Targeting specific genes for RNA interference is crucial to the development of strong resistance to rice stripe virus. — Plant Biotechnol. J. 9: 503–512, 2011.
Spiegel, S., Cohen, J.: Occurrence of tobacco streak virus in strawberries in Israel. — Plant Dis. 69: 448–449, 1985.
Tricoli, D.M., Carney, K.J., Russell, P.F., McMaster, J.R., Groff, D.W., Hadden, K.C., Himmel, P.T., Hubbard, J.P., Boeshore, M.L. and Quemada, H.D.: Field evaluation of transgenic squash containing single or multiple virus coat protein gene constructs for resistance to cucumber mosaic virus, watermelon mosaic virus 2, and zucchini yellow mosaic virus. — Bio/Technology 13: 1458–1465, 1995.
Tyagi, H., Rajasubramaniam, S., Rajam, M.V., Dasgupta, I.: RNA-interference in rice against rice Tungro bacilliform virus results in its decreased accumulation in inoculated rice plants. — Transgenic Res. 17: 897–904, 2008.
Van Dun, C.M.P., Overduin, B., Van Volten-Doting, L., Bol, J.F.: Transgenic tobacco expressing Tobacco streak virus or mutated Alfalfa mosaic virus coat protein does not crossprotect plants against alfalfa mosaic virus infection. — Virology 164: 389–398, 1988.
Van Vloten-Doting, L.: Coat protein is required for infectivity of Tobacco streak virus: biological equivalence of the coat proteins of tobacco streak and alfalfa mosaic viruses. — Virology 65: 215–225, 1975.
Wesley, S.V., Helliwell, C.A., Smith, N.A., Wang, M., Rouse, D.T., Liu, Q., Gooding, P.S., Singh, S.P., Abbott, D., Stoutjesdijk, P.A., Robinson, S.P., Gleave, A.P., Green, A.G., Waterhouse, P.M.: Construct design for efficient, effective and high-throughput gene silencing in plants. — Plant J. 27: 581–590, 2001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgements: This research was supported by the Department of Biotechnology, Government of India. We thank the CSIRO Plant Industry, Australia for providing the vector pHANNIBAL. Our thanks are also due to Dr. S. Winter, DSMZ, Germany for providing us the TSV antibody.
Rights and permissions
About this article
Cite this article
Pradeep, K., Satya, V.K., Selvapriya, M. et al. Engineering resistance against Tobacco streak virus (TSV) in sunflower and tobacco using RNA interference. Biol Plant 56, 735–741 (2012). https://doi.org/10.1007/s10535-012-0111-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10535-012-0111-5