Abstract
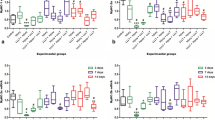
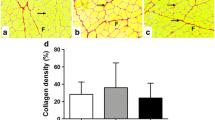
The aim of the present study was to determine the effects of low-level laser therapy (LLLT) on the homeostasis of reactive oxygen species (ROS) and expression of IGF-1 and TGF-β1 in the gastrocnemius muscles of rats following contusion. Muscle regeneration involves cell proliferation, migration, and differentiation and is regulated by growth factors. A growing body of evidence suggests that LLLT promotes skeletal muscle regeneration and accelerates tissue repair. Adult male Sprague-Dawley rats (n = 96) were randomly divided into three groups: control group (no lesion, untreated, n = 6), contusion group (n = 48), and contusion-plus-LLLT group (n = 42). Gallium aluminum arsenide (GaAlAs) laser irradiation (635 nm; beam spot, 0.4 cm2; output power, 7 mW; power density, 17.5 mW/cm2; 20 min) was administered to the gastrocnemius contusion for 20 min daily for 10 days. Muscle remodeling was evaluated at 0 h and 1, 2, 3, 7, 14, 21, and 28 days after injury. Hematoxylin and eosin and Van Gieson staining were used to evaluate regeneration and fibrosis; muscle superoxide dismutase (SOD) and malondialdehyde (MDA) were detected via biochemical methods; expression of transforming growth factor beta-1 (TGF-β1) and insulin-like growth factor-1 (IGF-1) were investigated via immunohistochemistry. The results showed that LLLT markedly promoted the regeneration of muscle and reduced scar formation. LLLT also significantly enhanced muscle SOD activity and significantly decreased muscle MDA levels 1, 2, and 3 days after injury. LLLT increased the expression of IGF-1 2, 3, and 7 days after injury and decreased the expression of IGF-1 21 and 28 days after injury. LLLT decreased the expression of TGF-β1 3 and 28 days after injury but increased expression at 7 and 14 days after injury. Our study showed that LLLT could modulate the homeostasis of ROS and of the growth factors IGF-1 and TGF-β1, which are known to play important roles in the repair process. This may constitute a new preventive approach to muscular fibrosis.





Similar content being viewed by others
References
Fillipin LI, Mauriz JL, Vedovelli K, Moreira AJ, Zettler CG, Lech O, Marroni NP, Gonzalez-Gallego J (2005) Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg Med 37(4):293–300. doi:10.1002/lsm.20225
Rizzi CF, Mauriz JL, Freitas Correa DS, Moreira AJ, Zettler CG, Filippin LI, Marroni NP, Gonzalez-Gallego J (2006) Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kappaB signaling pathway in traumatized muscle. Lasers Surg Med 38(7):704–713. doi:10.1002/lsm.20371
Nussbaum EL (1999) Low-intensity laser therapy for benign fibrotic lumps in the breast following reduction mammaplasty. Phys Ther 79(7):691–698
Katz TM, Glaich AS, Goldberg LH, Friedman PM (2010) 595-nm long pulsed dye laser and 1450-nm diode laser in combination with intralesional triamcinolone/5-fluorouracil for hypertrophic scarring following a phenol peel. J Am Acad Dermatol 62(6):1045–1049. doi:10.1016/j.jaad.2009.06.054
Cassuto DA, Scrimali L, Sirago P (2010) Treatment of hypertrophic scars and keloids with an LBO laser (532 nm) and silicone gel sheeting. J Cosmet Laser Ther 12(1):32–37. doi:10.3109/14764170903453846
Huard J, Li Y, Fu FH (2002) Muscle injuries and repair: current trends in research. J Bone Joint Surg Am 84-A(5):822–832
Cuzzocrea S, Thiemermann C, Salvemini D (2004) Potential therapeutic effect of antioxidant therapy in shock and inflammation. Curr Med Chem 11(9):1147–1162
Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina D, Settembre C, Gavina M, Pulze L, Giardino I, Pettoello-Mantovani M, D'Apolito M, Guido S, Masliah E, Spencer B, Quaratino S, Raia V, Ballabio A, Maiuri L (2010) Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat Cell Biol 12(9):863–875. doi:10.1038/ncb2090
De Minicis S, Seki E, Paik YH, Osterreicher CH, Kodama Y, Kluwe J, Torozzi L, Miyai K, Benedetti A, Schwabe RF, Brenner DA (2010) Role and cellular source of nicotinamide adenine dinucleotide phosphate oxidase in hepatic fibrosis. Hepatology 52(4):1420–1430. doi:10.1002/hep.23804
Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, Hebert RL (2010) Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299(6):F1348–F1358. doi:10.1152/ajprenal.00028.2010
Nabeebaccus A, Zhang M, Shah AM (2011) NADPH oxidases and cardiac remodelling. Heart Fail Rev 16(1):5–12. doi:10.1007/s10741-010-9186-2
Chan EC, Jiang F, Peshavariya HM, Dusting GJ (2009) Regulation of cell proliferation by NADPH oxidase-mediated signaling: potential roles in tissue repair, regenerative medicine and tissue engineering. Pharmacol Ther 122(2):97–108. doi:10.1016/j.pharmthera.2009.02.005
Smith C, Kruger MJ, Smith RM, Myburgh KH (2008) The inflammatory response to skeletal muscle injury: illuminating complexities. Sports Med 38(11):947–969. doi:10.2165/00007256-200838110-000055
Urish KL, Vella JB, Okada M, Deasy BM, Tobita K, Keller BB, Cao B, Piganelli JD, Huard J (2009) Antioxidant levels represent a major determinant in the regenerative capacity of muscle stem cells. Mol Biol Cell 20(1):509–520. doi:10.1091/mbc.E08-03-0274
Lubart R, Eichler M, Lavi R, Friedman H, Shainberg A (2005) Low-energy laser irradiation promotes cellular redox activity. Photomed Laser Surg 23(1):3–9. doi:10.1089/pho.2005.23.3
Fujimaki Y, Shimoyama T, Liu Q, Umeda T, Nakaji S, Sugawara K (2003) Low-level laser irradiation attenuates production of reactive oxygen species by human neutrophils. J Clin Laser Med Surg 21(3):165–170. doi:10.1089/104454703321895635
Avni D, Levkovitz S, Maltz L, Oron U (2005) Protection of skeletal muscles from ischemic injury: low-level laser therapy increases antioxidant activity. Photomed Laser Surg 23(3):273–277. doi:10.1089/pho.2005.23.273
Pelosi L, Giacinti C, Nardis C, Borsellino G, Rizzuto E, Nicoletti C, Wannenes F, Battistini L, Rosenthal N, Molinaro M, Musaro A (2007) Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. FASEB J 21(7):1393–1402. doi:10.1096/fj.06-7690com
Ten Broek RW, Grefte S, Von den Hoff JW (2010) Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cell Physiol 224(1):7–16. doi:10.1002/jcp.22127
Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J (2004) Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol 164(3):1007–1019
Cencetti FBC, Nincheri P, Donati C, Bruni P (2010) Transforming growth factor-beta1 induces transdifferentiation of myoblasts into myofibroblasts via up-regulation of sphingosine kinase-1/S1P3 axis. Mol Biol Cell 21(6):1111–1124
Minamoto VB, Grazziano CR, Salvini TF (1999) Effect of single and periodic contusion on the rat soleus muscle at different stages of regeneration. Anat Rec 254(2):281–287. doi:10.1002/(SICI)1097-0185(19990201)254:2<281::AID-AR14>3.0.CO;2-Z
Kami K, Masuhara M, Kashiba H, Kawai Y, Noguchi K, Senba E (1993) Changes of vinculin and extracellular matrix components following blunt trauma to rat skeletal muscle. Med Sci Sports Exerc 25(7):832–840
Crisco JJ, Jokl P, Heinen GT, Connell MD, Panjabi MM (1994) A muscle contusion injury model. Biomechanics, physiology, and histology. Am J Sports Med 22(5):702–710
Beiner JM, Jokl P, Cholewicki J, Panjabi MM (1999) The effect of anabolic steroids and corticosteroids on healing of muscle contusion injury. Am J Sports Med 27(1):2–9
McBrier NM, Neuberger T, Okita N, Webb A, Sharkey N (2009) Reliability and validity of a novel muscle contusion device. J Athl Train 44(3):275–278. doi:10.4085/1062-6050-44.3.275
Chan YS, Li Y, Foster W, Horaguchi T, Somogyi G, Fu FH, Huard J (2003) Antifibrotic effects of suramin in injured skeletal muscle after laceration. J Appl Physiol 95(2):771–780. doi:10.1152/japplphysiol.00915.2002
Hurme T, Kalimo H (1992) Activation of myogenic precursor cells after muscle injury. Med Sci Sports Exerc 24(2):197–205
Rao VP, Branzoli SE, Ricci D, Miyagi N, O'Brien T, Tazelaar HD, Russell SJ, McGregor CG (2007) Recombinant adenoviral gene transfer does not affect cardiac allograft vasculopathy. J Heart Lung Transplant 26(12):1281–1285. doi:10.1016/j.healun.2007.09.018
Patel S, Chung SH, White G, Bao S, Celermajer DS (2010) The "atheroprotective" mediators apolipoprotein A-I and Foxp3 are over-abundant in unstable carotid plaques. Int J Cardiol 145(2):183–187. doi:10.1016/j.ijcard.2009.05.024
Grounds MD (1999) Muscle regeneration: molecular aspects and therapeutic implications. Curr Opin Neurol 12(5):535–543
Shi X, Garry DJ (2006) Muscle stem cells in development, regeneration, and disease. Genes Dev 20(13):1692–1708. doi:10.1101/gad.1419406
Karu T (1998) The science of low-power laser therapy. Gordon and Breach Science Publishers, Amsterdam
Choi JE, Lee SS, Sunde DA, Huizar I, Haugk KL, Thannickal VJ, Vittal R, Plymate SR, Schnapp LM (2009) Insulin-like growth factor-I receptor blockade improves outcome in mouse model of lung injury. Am J Respir Crit Care Med 179(3):212–219. doi:10.1164/rccm.200802-228OC
Feghali-Bostwick CA (2005) IGF-I: mediator of fibrosis or carcinogenesis? Am J Physiol Lung Cell Mol Physiol 288(5):L803–L804. doi:10.1152/ajplung.00012.2005
Shen WLY, Zhu J, Schwendener R, Huard J (2008) Interaction between macrophages, TGF-beta1, and the COX-2 pathway during the inflammatory phase of skeletal muscle healing after injury. J Cell Physiol 214(2):405–412
Tidball JG (2005) Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288(2):R345–R353. doi:10.1152/ajpregu.00454.2004
Mesquita-Ferrari RA, Martins MD, Silva JA Jr, da Silva TD, Piovesan RF, Pavesi VC, Bussadori SK, Fernandes KP (2011) Effects of low-level laser therapy on expression of TNF-alpha and TGF-beta in skeletal muscle during the repair process. Lasers Med Sci 26(3):335–340. doi:10.1007/s10103-010-0850-5
Competing interests
The authors have declared that no competing interests exist.
Funding
This work was supported by the National Science Foundation of China (60878061)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 40 kb)
Rights and permissions
About this article
Cite this article
Luo, L., Sun, Z., Zhang, L. et al. Effects of low-level laser therapy on ROS homeostasis and expression of IGF-1 and TGF-β1 in skeletal muscle during the repair process. Lasers Med Sci 28, 725–734 (2013). https://doi.org/10.1007/s10103-012-1133-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1133-0